Review - (2022) Volume 10, Issue 1
Treatment of Obstructive Sleep Apnea with Orthodontic Oral appliances: Systematic Review
Nasir H AlHamlan1*, Lujain A AlGhrairy1, Waad E AlSaadi1, Khaled W AlBawardi2, Rana A AlOlaiq2 and Afnan T AlZomaili2
*Correspondence: Nasir H AlHamlan, DMD, King Abdulaziz Medical City in National Guard, Riyadh, Saudi Arabia, Email:
Abstract
Objective: This systematic review study aims to determine the treatment success in patients with obstructive sleep apnea syndrome in different orthodontic treatment method. Methods: A systematic search to identify all relevant randomized control trials was conducted in PubMed databases. A supplemental manual search was performed by reviewing the reference lists of the related articles. The key words used to conduct the research were; obstructive sleep apnoea, oral appliance, orthodontic therapy, snoring, treatment success. Study selected to be included is in English language, within twenty past years. No exclusions were made based on ethnicity, age, or gender. Results: In terms of MAD comparing to inactive control devices, four RCT studies conclude that the mandibular advancement splint (MAS) resulted in significant improvements in AHI and Oxygen Desaturation Index (p<0.001). Moreover, two studies designed to compare oral appliance against no treatment. It concludes that all treated subjects had significantly lower apnea index, AHI (p<0.001) and hypopnea index values (p<0.001), whereas in untreated control subjects these values remained almost unchanged. Additionally, five studies compared one of the MRA with another design of MRA. One of these studies concludes that both devices (MAS and TSD) had a similar efficacy in AHI reduction, yet, improvements in snoring, quality of sleep and better compliance were reported by the patients for MAS than TSD. Another study results in significant reduction in AHI, AI and improvement in ESS and SS in both SILENT NITE and a one-piece Monoblock appliance despite the patients' preference for Monoblock appliance. Furthermore, a study was conducted to determine if the design of MRA can affect the end treatment result of OSA. Despite similar outcome of both appliances, there was a significant preference of the minimal coverage of teeth and palate MRA design. Similarly, A custom-made MRA is statistically more effective in the management of OSA and also in patients’ preference and compliance. Finally, in evaluating the effectiveness of MAS to control SDB in children, there were an overall clinical reduction of AHI, snoring time with active MAS wearing, and improvement of quality of life and behavior with active MAS than sham MAS. Conclusion: Many studies resulting in an overall improvement of AHI, hypopnea index values, snoring reduction, quality of sleep, quality of life and neuro-cognitive functions. These outcomes intensify the importance of multidisciplinary management of OSAS. Other important health outcomes related to OSAS.
Keywords
Sleep, Apnea, Orthodontic, Appliance
Introduction
The specialty of orthodontics is not only limited to just moving teeth, and the management of sleep apnea has witnessed this. As such, there is an increase interest in the role of the orthodontist in screening for obstructive sleep apnea syndrome (OSAS). According to the American Academy of Sleep Medicine Task Force, obstructive sleep apnea is “the complete interruption of airflow to the airway for at least 10 seconds. If the anatomical obstacle or the functional change leads to complete prevention of the inspiratory flow, the patient experiences oxygen desaturation and microarousals”, which is defined as hypopnea [1,2]. Over the past thirty years many types of abnormal breathing during sleep have been described as abnormal or/ difficulty in breathing, but not as apnea [3]. OSAS are characterized by an absence of oral and nasal airflow despite persistent inspiratory efforts [4]. OSAS patients may present with certain symptoms including snoring, witnessed apnea, and choking or/gasping during sleep. Also, parents or caregivers may describe that the child sleeps in unusual positions, for example having the neck hyperextended or with the head hanging off the side of the bed, in addition to appearing very restless with frequent position changes during sleep. Additionally, some children with OSAS may present with sleepiness; those who previously had discontinued daytime napping may resume daily or frequent naps [2,3]. Estimating the prevalence of OSAS in adults vary in the literature, it is commonly thought to involve up to 4% of middle-aged men and 2% of adult women. Its prevalence increases with age until approximately around the seventh and eighth decades of life; it is more frequent in men and postmenopausal women [3,5]. In children, OSAS prevalence is 10% equally distributed between males and females. Moreover, it has been estimated that OSAS is strongly associated with childhood obesity, [body mass index [BMI] ≥ 30 kg/m2], up to 60% [6,7], which in turns has led to an increase in awareness of pediatric sleep disorders such as obesity-related OSAS. Timely diagnosis and management of pediatric OSAS may prevent associated comorbidities and considered the key to prevent future complications [1,7]. Moreover, there is an evidence in the literature supported that OSAS has a hereditary component [5-11]. Upon reviewing the literature, it has been found that some genetic syndromes, specifically craniofacial anomalies, are associated with an increased risk of OSAS. Also, certain craniofacial morphologies may predispose to OSAS such as retrognathia, long and narrow faces, dolichocephalic facial type, narrow and deep palate, steep mandibular plane angle, anterior open bite, midface deficiency, and lower hyoid position. Thus, it should be noted, that the strength between the relationship of these craniofacial morphologies and the development of OSAS is not well established [12-14]. Many types of interventions, mainly medical intervention, have been used to treat of OSAS such as adenotonsillectomy (AT) that resulted in improvements in the quality of life, behaviour, attention and in cognitive abilities [4,15]. Quality of life in children with OSA has been shown to improve after AT. However, the treatment of OSAS is not only limited to AT. In November 2017, the Board of Trustees of the American Association of Orthodontists (AAO) tasked a panel of medical and dental experts in sleep medicine and dental sleep medicine to create guidelines implicating the role of orthodontic specialty in the management of OSAS. Following these guidelines upon reviewing the recent studies, it has been suggested that after the diagnosis of OSAS by a physician, a patient may be referred to (or back to) an orthodontist to be treated by oral appliances such as advancing oral appliances and tongue retaining devices, which usually effective options for OSAS [16,17]. Also a study conducted by (Pirelli, et al, 2004), where OSAS was treated with a rapid palatal expander (RPE) showed that 9 out of 10 patients demonstrated reduced in symptoms [18]. Furthermore, several other studies have shown the efficacy of using RPE in treating pediatric OSAS [19-23]. However, according to the systematic review which suggested that the most popular mode of treatment for OSAS in orthodontic is the forward advancement of the mandible increases and stabilizes the oropharyngeal and/or hypo pharyngeal airway space [24]. So, the aim of the study is to review the literature to determine the treatment success in patients with obstructive sleep apnea syndrome using orthodontic treatment.
Method
Eligibility criteria
The eligibility criteria used in this paper included five criteria. The first was the selected population, and it was the patients diagnosed with obstructive sleep apnoea. The second was for the type of intervention used in these cases, which was treatment with oral appliances. The third was for the comparison method used in these studies, and it was the treatment versus control (placebo or inactive appliance or with another oral appliance) comparison. The fourth was for the type of outcome, which was the efficacy of oral appliance for treatment of obstructive sleep apnoea. The last and fifth criteria were for the study design, and it included randomized clinical trials (RCTs) only.
Information sources and search strategy
The main Source used in this paper was PubMed, and it is considered one of the largest databases for medical articles. The second source was the references of the selected articles. A systematic search was done in PubMed using multiple key words, which were; obstructive sleep apnoea, oral appliance, orthodontic therapy, snoring, and treatment success. The number of articles found using these key words was, excluding duplicates, 993 articles. Then filters were applied to the search to further exclude unnecessary articles. These filters were; Randomized Controlled Trial from the article type filter, from year 2000 – present day from the publication date filter, and lastly Humans from the species filter. The number of articles reduced to 174 after applying the filters. In the next step, the full text availability of the articles was checked, and there were 168 articles with available full text. Following the previous step, the articles were screened for eligibility and the impact factor of the publishing journal, and the number of the articles that passed the screening was 57. After further exclusion based on the inclusion and exclusion criteria, the final number of included articles was 12.
Selection criteria
Inclusion criteria
• Randomized controlled trials.
• Written in English.
• Studies done on humans.
• Full text articles.
Exclusion criteria
• Cases that were not treated with oral appliances.
• Articles that were published before 2000 A.D.
• No exclusions were made based on ethnicity, age, or gender.
Study selection
Study selection was done by two reviewers, following a systematic process. The eligibility of selected article was checked through the title, and abstract of these articles. The PRISMA flow diagram (Figure 1) further explains the process of the study selection.
Data collection process
The data was extracted by two reviewers, and then it was combined and compared for accuracy. Disagreements were resolved by a third reviewer.
Data items
For each of the selected trial, the data was collected on (Table 1): (1) First author and publication year; (2) Study design; (3) Follow-up period; (4) Sample size; (5) Type and number of subjects; (6) Methods (clinical examination, Cephalometric analyses and dental cast measurements); (7) Intervention; (8) Control; (9) Outcomes.
Risk of bias in individual studies
Two of the authors independently evaluated the risk of bias in the included studies, while following the recommendations of The Cochrane Risk of Bias criteria. Disagreements were resolved by a third reviewer.
Results
Study selection
A flow diagram illustrating the selection process is outlined in figure 1. A total of 117 articles were selected using the search strategy specified before. Of these, 61 were excluded. After reviewing the titles and/or abstracts, remaining 26 articles of possible interest, 11 studies finally met all the inclusion criteria and was included in this review.
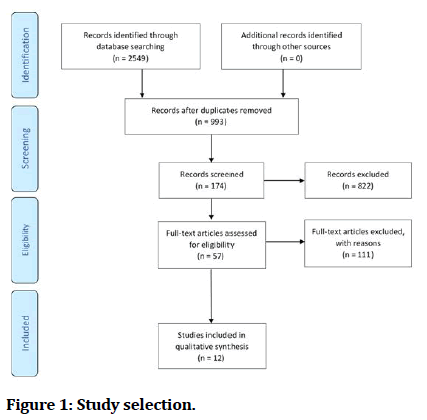
Figure 1: Study selection.
Study characteristics
found in Table 1. Included articles were all in English language and all of them were conducted after the year 2000.
| Study | Study design | Observation period | Sample Size | Participants | Methods | Intervention | Comparison/control | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Zhou [25] |
A randomised titrated crossover study | 6-month periods | 16 | Sex: (13 men and three women) |
Two different types of oral appliances were tested in each patient, a one- piece Monoblock and the SILENT NITE® -Each for 3-months periods separated by a 2- week wash-out period in between |
Monoblock | The SILENT NITE® | The monoblock and SILENT NITE® appliances reduced Apnoea Hypopnoea Indexed at, (AHI)from 26.38 ± 4.13 to 7.58 ± 2.28 (P < 0001) and 8.87 ± 2.88 (P < 0001), respectively. |
| Age: The age range of the patients varied from 26.3 to 55.4 years (average 45.23) |
The Monoblock appliance was statistically more efficient in reducing AHI and Apnoea Indexed at, (AI) than the SILENT NITE® |
|||||||
| Inclusion criteria | ||||||||
| Diagnosed with mild to moderate OSAHS |
||||||||
| More than 20 teeth present | ||||||||
| Free from caries, periodontal disease, temporomandibular pain or movement limitations. |
||||||||
| Villa [26] |
Randomized Controlled Study | 6 months period | 32 | Sex: 20 males Age: (age range, 4 to 10 years; mean age, 7.1 ± 2.6 years) |
A group of 19 subjects was randomly assigned to a 6-mo trial of an oral appliance; the remainder acted as control subjects. | 19 subjects undergo a 6-mo trial of a personalized oral appliance. |
13 subjects didn’t undergo any therapy | Poly-somnography after the trial showed that treated subjects all had significantly lower apnea Indexed at, (p 0.001) and hypopnea Indexed at, values (p 0.001) than before the trial, whereas in untreated con- trol subjects these values remained almost unchanged. |
| Inclusion criteria | ||||||||
| Had an apnea Indexed at, (AI) of â?¥ 1 event/hr of sleep during diagnostic polysomnography |
||||||||
| Quinnell [27] |
A crossover randomised controlled trial | 6 weeks of treatment with three non- adjustable MADs and 4 weeks no treatment |
90 | Sex: Age: above 18 |
6 weeks of treatment with three non- adjustable MADs: self-moulded (SleepPro 1; SP1); semi-bespoke (SleepPro 2; SP2); fully-bespoke MAD (bMAD); and 4 weeks no treatment. |
62 | 21 no treatment | All three MADs significantly decreased the AHI against no treatment by 26% for the SP1, 33% for the SP2 and 36% for the bMAD |
| Inclusion criteria | SP1:21 | |||||||
| Mild to moderate OSAHS con- firmed by respiratory polysomnography |
SP2:21 | |||||||
| bMAD:20 | ||||||||
| Mehata [28] | A Randomized, Controlled Study | 28 | Sex: (22 men and six women) | Patients underwent three polysomnographs with either a control oral plate, which did not advance the mandible (A), or MAS (B), 1 wk apart, in either the ABB or BAA sequence. |
Group I (sequence ABB) | Group II (BAA sequence) | Subjective improvements with the MAS were reported by the majority of patients (96%). | |
| Inclusion criteria | ||||||||
| The presence of at least two symptoms of OSA , and evidence of OSA on polysomnography, with an apnea /hypopnea Indexed at, (AHI) > 10/h. |
||||||||
| Johnston [29] |
a randomized clinical trial | 4â??6 weeks for each appliance | 21 | Sex: (17 males and four females) |
Subjects were provided with a maxillary placebo appliance and a MAA for 4â??6 weeks each, in a randomized order. |
MAA | Maxillary placebo Appliance |
Among the remaining 20 subjects, the MAA produced significantly lower AHI and ODI values than the placebo. |
| Age: adult Inclusion criteria: Those with an hourly rate of 10 or more desaturations |
||||||||
| Johal [30] |
A randomized crossover trial | 3-month period of ready-made or custom-made MRD, with an intervening washout period of 2 weeks, prior to crossover. | 25 | Sex: Age: adults (> 18 years) |
Patients were randomly assigned to receive either a 3-month period of ready-made or custom-made MRD, with an intervening washout period of 2 weeks, prior to crossover.ents Study of the efficacy of an oral appliance vs an intraoral placebo |
ready-made MRD | custom-made MRD | The MRDc achieved a complete treatment response in 64% of participants, compared with 24% with the MRDr (p < 0.001). |
| Inclusion criteria | ||||||||
| Diagnosis of mild-moderate OSA | ||||||||
| Marklund [31] |
A Randomized Clinical Trial | 4-month follow-up | 96 | Sex: Age: aged 20 to 70 years |
Study of the efficacy of an oral appliance vs an intraoral placebo |
Oral appliance group | Placebo device group | An AHI lower than 5 was recorded in 49% of the patients using the oral appliance and in 11% of the patients using the placebo device (P = .001) |
| Inclusion criteria | ||||||||
| Patients With mild to moderate sleep apnea |
||||||||
| Idris [32] |
A randomized crossover clinical trial | Each appliance was worn for three weeks and treatment periods were separated by a two-week washout. |
18 | Sex: Age: age range from 8 to 12 years |
Each participant wore an Active and a Sham MAS appliance overnight for three weeks. Treatment periods were separated by a two-week washout period. |
Active Mandibular Advancement Appliance |
Sham Mandibular Advancement Appliance |
Compared to the Sham MAS, the wearing of the Active MAS resulted in a significant reduction in overall AHI; p 0.002) and supine AHI; p < 0.001). |
| Inclusion criteria | ||||||||
| Parental report of loud snoring for three or more nights per week. |
||||||||
| Gotsopoulos [33] |
A Randomized, Controlled Trial | 4 weeks for treatment for each appliance with 1 week -washout period | 73 | Sex: 59 men and 14 women | Patients received 4 weeks of treatment MAS and a control device with an intervention 1 week -washout | Mandibular advancement splint | Inactive oral appliance | There was a highly significant reduction in both the reported frequency and intensity of snoring with the MAS compared with the control device (p 0.0001) |
| Age: middle -aged | ||||||||
| Inclusion Criteria |
||||||||
| Moderate and severe OSA | ||||||||
| Overweight | ||||||||
| Deane [34] | A Randomized Controlled Trial | 4 weeks for each device with 1-week washout period | 27 | Sex: (20 males, 7 female) | The patients had an 8-week acclimatization period (4 weeks with each device), with 1-week washout periods |
Mandibular advancement splint | Tongue Stabilizing Device | A decrease in AHI was recorded for 91% of the patients when using MAS and 77% of the patients when using TSD. |
| Age: age > 20 years |
||||||||
| Inclusion criteria | ||||||||
| Apnea hypopnea Indexed at, (AHI) > 10 per hour. |
||||||||
| Bishop [35] |
A randomized crossover study | 24 | Inclusion Criteria |
Subjects in arms K and T were compared with respect to the variables measured at baseline. |
The Klearway | The TAP3. | For both appliances, the Epworth Sleepiness Scale (ESS), and Sleep Apnea Quality of Life Indexed at, (SAQLI) were significantly (pâ?¤ 0.05) lower than the baseline |
|
| Diagnosed with obstructive sleep apnea by polysomnography |
Table 1: Summary of study characteristics of included trials.
In most studies [25-31] only patients with mild to moderate OSA or asymptomatic snores were included. One study [32] includes those with moderate and severe OSA.
Four studies [28,29,31,33] compared oral appliance with inactive placebo appliance while two studies [26,27] compared oral appliance against no treatment. Five of the included articles [ 25,30,32,34,35] compared between two different oral appliances for the treatment of OSA.
The duration of the included studies in this review was variable and ranged from approximately 3 months [31] to a mean follow up of 6 months [25,26].
Synthesis of results
Studies comparing MADs with inactive control OAs
Four RCT studies compared a MAD with inactive control devices. The control appliances were designed not to advance the mandible. Three randomized controlled studies [28,29,33] observed a significant reduction between baseline and follow-up apnoea/hypopnoea index (AHI). In a study [28] the mandibular advancement splint (MAS) resulted in significant improvements in AHI (30 ± 2/h versus 14 ± 2/h, p, 0.0001). Similarly [29], found the Mandibular Advancement Appliance (MAA) produced significantly lower AHI and Oxygen Desaturation Index (ODI) values than the placebo as well as [31] reported an AHI lower than 5 was recorded in 49%of the patients using the oral appliance. A study done by [33] observed highly significant reduction in both the reported frequency and intensity of snoring with the MAS compared with the control device.
Studies comparing oral appliance against no treatment
Two studies compared oral appliance against no treatment [26,27]. Villa et al [26] found that treated subjects all had significantly lower apnea index (p 0.001) and hypopnea index values (p 0.001) than before the trial, whereas in untreated control subjects these values remained almost unchanged. Likewise, Quinnell et al, 2014 [27] reported All three MADs that used in the study significantly decreased the AHI against no treatment.
Studies comparing between two different oral appliances
Five studies compared one of the MRA with another design of MRA. Deane et al. [34] evaluated the efficacy between MAS and TSD in the treatment of OSA. In terms of AHI reduction, both devices had a similar efficacy, yet, improvements in snoring, quality of sleep and better compliance were reported by the patients for MAS than TSD [34]. A study done by Zhou et al. [25] which compared the effectiveness of the SILENT NITE and a one-piece monoblock in managment of OSAHS. A significant reduction in AHI and AI, also increased patient preference with the monoblock appliance, however, both appliances had an improvement in ESS and SS [25]. Another study was conducted by Bishop et al. [35] to determine if the design of MRA can affect the end treatment result of OSA. Despite similar outcome of both appliances, there was a significant preference of the minimal coverage of teeth and palate MRA design [35]. According to Johal et al. [31] custom-made MRA is statistically more effective in the management of OSA and also in patients’ preference and compliance. Idris et al. [32] evaluated the effectiveness of MAS to control SDB in children. Overall clinical reduction of AHI and snoring time with active MAS wearing, moreover, reported improvement of quality of life and behavior with active MAS than sham MAS [32].
Discussion
As the study aims to determine the success of removable orthodontic appliances such as, MAA or MRA, Tongue Stabilizing Device. Three groups of studies were included to determine such success, the first study comparing MADs with inactive control OAs. The second study, comparing oral appliance against no treatment. The third one, comparing between two different oral appliances.
When comparing MADs with inactive control OAs, objective treatment outcome is crucial to be determined so the result can be obtained with no bias. Yet only three studies mentioned the treatment outcome clearly such as in Mehata et al. [28], Johnston et al. [29], Gotsopoulos et al. [33]. However, in Marklund et al. [31] the reduction in AHI was mentioned with indication of success or failure of such treatment. Moreover, in Mehata et al. [28] using MAS resulted in significant reduction in the mean of AHI (53%) when compared with the control. Two AHI cut-offs were measured one at 10/h which resulted in complete response in 54% and the other at 15/h which resulted in complete response in 75%. Where in Johnston et al. [29], a cut-off at 10/h or less resulted in reduction in 33% which is considered low.
Two studies comparing oral appliances against no treatment were included were both have resulted in favouring MADs against no treatment patients. However, in Villa et al. [26] the cut-off for AHI was not mentioned yet a 50% reduction in AHI was considered successful treatment. Also, in Quinnell et al. [27] AHI cut-off was not mentioned, yet the three MADs used resulted in great reduction of the AHI. Both studies have proven that MADs are clinically effective in treatment of OSA. Though, patient compliance plays a vital role in determining such success.
Deane et al. [34] evaluated the efficacy between MAS and TSD in the treatment of OSA. In terms of AHI reduction, both devices had a similar efficacy, yet, improvements in snoring, quality of sleep and better compliance were reported by the patients for MAS than TSD [34].
The MAS has abundance supporting literature supporting the treatment of OSP, particularly those with mild to moderate OSA [34]. Decreasing of AHI is the primary outcome of this study [34].
Additionally, the arousal index decreased significantly with MAS and TSD [34]. TSD appliance is non-adjustable; patient should squeeze the bulb and protruded the tongue into the appliance with differing force [34]. This stretching posture of the soft tissue may cause uncomfortable situation [34]. So this manner required ensuring patient safety by clinical supervision [34]. It found that MAS more comfortable and easier than the TSD, although patients achieved the same response to treatment with both MAS and TSD [34]. It has been demonstrated in other studies that quality of sleep was improved with oral appliance therapy [34]. In this study all patients reported improvement in quality of sleep with MAS, compared with 45% with TSD [34]. This could be due to the better compliance with MAS which is comparable to other studies [36-38]. There is no literature to assess compliance with TSD yet, but it can be assumed that like MAS [34]. The compliance rates are likely to decrease relative to the length of follow-up with TSD [34].
Both MAS and TSD have side effects and each appliance producing a different type and severity of problems [34]. The main concerns with MSD were jaw discomfort and dryness of mouth which were largely mild in nature [34]. With TSD appliance, the main side effects were reported as excess salivation, dryness of mouth, and soft tissue irritation varied from mild to severe in nature [34]. Several patients described a temporary tingling sensation, minor ulceration of the lingual frenum also occurred [34]. Lingual frenum inhibited the ability to protrude the tongue into the TSD, that in turn affected compliance [34]. Swallowing was more difficult with TSD due to the vertical mouth opening [34]. The reduction of AHI in supine sleep and slight reduction in REM sleep may have been influenced by the side effects, in particular excess salivation, these side effects were severe enough to prevent nearly half of the sample continuing with the TSD which might be the cause of decreasing the compliance with follow up, however there was no statistically significant difference between MAS and TSD [34].
Another prospective crossover study compared the two different types of MAAs in the treatment for mild to moderate OSAHS concluded that, both appliances were efficient in improving AHI in patients with mild to moderate OSAHS [25]. The monoblock appliance was statistically more efficient in reducing AHI and Apnoea Index (AI) than the SILENT NITE® (GlideWell Laboratories). The scores on Epworth’s Sleepiness Scale (ESS) and Snoring Scale (SS) were improved significantly by both appliances. The upper airway spaces showed significant enlargement by both mandibular advancement appliances (MAAs), while no significant differences were found between the two appliances. This result is consistent with some of the previous studies where they compare two different MAAs appliance. In another prospective study, the SILENT NITE®* and the Karwetzky activator were compared and both appliances reduced the mean Respiratory Disturbance Index (RDI) and AI. The Karwetzky activator was significantly more efficient than the SILENT NITE®*. This could be related to the vertical mandibular movement variations of the two appliances [25]. Moreover, Ghazal et al. compared the Herbst-like IST® appliance‡ with the monoblock appliance TAPTM§ in a randomised prospective study [25]. The IST® appliance‡ was assumed to be less effective than the TAPTM§ in the short-term comparison. This study revealed that the short-term acceptance of one-piece appliance is relatively higher than that of the two-piece SILENT NITE®*, where the comfort is main concern of patients when wearing appliances. The retention form has a major contribution to the comfort of patients, which is reduced in the SILENT NITE as the force transfers to the soft tissue. One of the reasons for lower preference rate is detachment of the appliance during mandible advancement. However, the monoblock appliance is retained on teeth by clasps, which translates into minimised pressure on the soft tissue and superior comfort for patients. There were no statistic differences between monoblock and SILENT NITE® appliances, most probably because of the identical vertical and horizontal advancements of both appliances, therefore eliminating the hypothesis that the reduction of the pharyngeal airway could be the reason for the efficiency differences of the appliances. The movement freedom of the twopiece appliance was considered an advantage, but it was obvious that the structure cannot provide a solid consistent airway enlargement. This fact may constitute an explanation of the results in our study [25].
Moreover, a study conducted by Bishop et al. [35] aimed to determine if the design of mandibular repositioning appliance (MRA) can affect the end treatment result of OSA [35]. No statistically significant difference was found between the two appliances of different designs. Clear majority of subjects preferred an appliance that is less bulky and covers fewer surfaces of the mouth. So, when selecting or designing an appliance, bulk of the aapliance should be minimized, allow treatable protrusion and should provide some increase in the vertical space between the maxillary and mandibular teeth [35].
According to Johal et al, [30] MRD could lower the baseline AHI and ODI values, whilst there was a significant difference in their relative effectiveness in its different designs. Custom-made MRA is more effective in the management of OSA and also in patients’ preference and compliance than ready-made [30]. Both designs of MRD achieved a reduction in reported daytime sleepiness, which was similar in magnitude to previous reports [39,40]. Excessive daytime sleepiness persisted less in custom-made than ready-made MRDs. Quinnell et al. [40] and Johal et al. [30] reported that, custom-made MRD achieved greatest improvement in terms of both generic (SF-36) and specific (FOSQ) measures, which in term improve the quality of life. The potential benefits of a custom-made MRD, with incremental advancement, permitting better adaptation to the device may explain the more favourable results [30]. MRD therapy is an entirely patient-dependent treatment and its success has to be patient comfort and consequent use. In Vanderveken et al. [39] and Johal et al [30] studies, patients expressing preference more favour to MRDc as compared to MRDr. In contrast, in the Quinnell et al. study [40], 96% of patients reported minor adverse events, which related predominantly to discomfort. However, this may reflect the short duration of the study, that lead to insufficient time for adaptation [31,40,41]. In spite of that, Patients found the ready-made MRD difficult to tolerate too [30]. Ready-made MRDs unfortunately are limited in their design, by the very fact that the manufacturer is attempting to cater to the needs of a very diverse population, with inherent differences in the size of their jaws and ability to protrude their mandible [30]. Nevertheless, Patients and sleep physicians are recommending or purchasing the ready-made MRD devices, not least because they are easily available from multiple sources and relatively cheap. Thus, clinical use and patients were instructed to “follow the manufacturer’s instructions” with regards to the readymade MRD and provided a custom-made MRD by a dental professional, in order to highlight both the clinical and patient centered aspects of their use [30].
Idris et al. [32] evaluated the effectiveness of MAS to control SDB in children with an Active or a Sham MAS. This randomized crossover trial concludes that wearing of the Active MAS resulted in a significant reduction in overall AHI, supine AHI and improved the ratings of quality of life [33]. In addition, mean snoring time per night was shorter with the Active MAS than with the Sham MAS. In fact, the AHI showed a tendency to increase after treatment when using the Sham MAS. This may be due to the inevitable bite opening of 0.5–1mm with the Sham MAS (upper and lower Hawley retainers), that caused by retention clasps on posterior teeth. Acrylic base plates that cover the palate and the lingual side of the mandibular dental arch and may occupy some of the tongue space and push the tongue to a slightly backward position could by a possible cause. Moreover, some participant’s suffered from the common cold during at the end of one treatment period, it might cause increase in the evaluated AHI compared to base records [33]. Positive behavioural changes after adenotonsillectomy, a resolution of obstruction in the upper airway, which in agreement with positive behavioural changes when using MAS in this study [41].
Conclusion
The last decade has been a breakthrough with pioneer studies resulting in an overall improvement of patient care and underlining the importance of multidisciplinary management of OSAS. Considering the limited number of included studies “12 published studies were selected for this systematic review’’, the presented orthodontic treatments is found to be effective in managing snoring and OSAS. Thus, the respective results suggested that the correction of craniofacial structure disharmony to the optimal conditions; which in turns led to diminish snoring and OSAS. Other important health outcomes related to OSAS, such as quality of life, Neuro-cognitive function and cardiovascular health have not yet been systematically addressed and no conclusion on orthodontic treatments should be taken in this regard. The challenge to educate healthcare practitioners, and health organizations to investigate the impact of integrating dentistry and medicine at educational and operational levels on determining the medical necessity of the conditions at the time of treatment.Orthodontic treatment of OSAS guidelines cannot be extrapolated and generalized from this systematic review and meta-analysis. In the future, more studies should be conducted with larger sample size and with specific inclusion and exclusion criteria
Conflicts of Interest
The authors do not have any conflicts of interest to disclose.
References
- Quan SF, Gillin JC, Littner MR, et al. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep 1999; 22:662-89.
- Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events: Deliberations of the sleep apnea definitions task force of the American academy of sleep medicine. J Clin Sleep Med 2012; 8:597-619.
- Gould GA, Whyte KF, Rhind GB, et al. The sleep hypopnea syndrome. Am Rev Respir Dis 1988; 137:895-8.
- Uliel S, Sivan Y. Normal polysomnographic values for children and adolescents. Am J Respir Crit Care Med 2002; 165:262A.
- Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. New England J Med 1993; 328:1230-5.
- Erler T, Paditz E. Obstructive sleep apnea syndrome in children. Treatments Respirat Med 2004; 3:107-22.
- Massicotte C, Al-Saleh S, Witmans M, et al. The utility of a portable sleep monitor to diagnose sleep-disordered breathing in a pediatric population. Canadian Respirat J 2014; 21:31-5.
- Lim J, Lasserson TJ, Fleetham J, et al. Oral appliances for obstructive sleep apnoea. Cochrane Database Syst Rev 2006; CD004435.
- Sateia MJ. Neuropsychological impairment and quality of life in obstructive sleep apnea. Clin Chest Med 2003; 24:249-59.
- Alchanatis M, Zias N, Deligiorgis N, et al. Sleep apnea-related cognitive deficits and intelligence: An implication of cognitive reserve theory. J Sleep Res 2005; 14:69â??75.
- Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: Cross-sectional results of the sleep heart health study. Am J Respir Crit CareMed 2001; 163:19â??25.
- Guilleminault C, Li KK, Khramtsov A, et al. Sleep disordered breathing: Surgical outcomes in prepubertal children. Laryngoscope 2004; 114:132-7.
- Zonato AI, Martinho FL, Bittencourt LR, et al. Head and neck physical examination: Comparison between nonapneic and obstructive sleep apnea patients. Laryngoscope 2005; 115:1030-4.
- Jamieson A, Guilleminault C, Partinen M, et al. Obstructive sleep apneic patients have craniomandibular abnormalities. Sleep 1986; 9:469-77.
- Flanary VA. Long-term effect of adenotonsillectomy on quality of life in pediatric patients. Laryngoscope 2003; 113:1639-44.
- Mansukhani MP, Kolla BP, Ramar K. International classification of sleep disorders 2 and American academy of sleep medicine practice parameters for central sleep apnea. Sleep Med Clin 2014; 9:1-1.
- Friedman M. 16 Friedman tongue position and the staging of obstructive sleep apnea/hypopnea syndrome. Sleep Apnea Snoring E-Book: Surgical and Non-Surgical Therapy 2008.
- Pirelli P, Saponara M, Guilleminault C. Rapid maxillary expansion in children with obstructive sleep apnea syndrome. Sleep 2004; 27:761-6.
- Guilleminault C, Li KK. Maxillomandibular Expansion for the treatment of sleep-disordered breathing: Preliminary result. Laryngoscope 2004; 114:893-6.
- Timms DJ, Effect of RME on respiration. Rev Assoc Dent Mexicana 1990; 4:179â??180.
- Timms DJ. The effect of rapid maxillary expansion on nasal airway resistance. Br J Orthod 1986; 13:221-8.
- De Felippe NL, Da Silveira AC, Viana G, et al. Relationship between rapid maxillary expansion and nasal cavity size and airway resistance: short-and long-term effects. Am J Orthod Dentofac Orthop 2008; 134:370-82.
- Guilleminault C, Monteyrol PJ, Huynh NT, et al. Adeno-tonsillectomy and rapid maxillary distraction in pre-pubertal children, a pilot study. Sleep Breathing 2011; 15:173-7.
- Lim J, Lasserson TJ, Fleetham J, et al. Oral appliances for obstructive sleep apnoea. Cochrane Database Syst Rev 2006.
- Zhou J, Liu Y. A randomised titrated crossover study comparing two oral appliances in the treatment for mild to moderate obstructive sleep apnoea/hypopnoea syndrome. J Oral Rehab 2012; 39:914-922.
- Villa M, Bernkopf E, Pagani J, et al. Randomized controlled study of an oral jaw-positioning appliance for the treatment of obstructive sleep apnea in children with malocclusion. Am J Respir Crit Care Med 2002; 165:123-127.
- Quinnell T, Bennett M, Jordan J, et al. A crossover randomised controlled trial of oral mandibular advancement devices for obstructive sleep apnoea-hypopnoea (TOMADO). Thorax 2014; 69:938-945.
- Mehta A, Qian J, Petocz P, et al. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med 2001; 163:1457-1461.
- Johnston C. Mandibular advancement appliances and obstructive sleep apnoea: A randomized clinical trial. Eur J Orthod 2002; 24:251-262.
- Johal A, Haria P, Manek S, et al. Ready-made versus custom-made mandibular repositioning devices in sleep apnea: A randomized clinical trial. J Clin Sleep Med 2017; 13:175-182.
- Marklund M, Carlberg B, Forsgren L, et al. Oral Appliance therapy in patients with daytime sleepiness and snoring or mild to moderate sleep apnea. Internal Med 2015; 175:1278.
- Idris G, Galland B, Robertson C, et al. Mandibular advancement appliances for sleep-disordered breathing in children: A randomized crossover clinical trial. J Dent 2018; 71:9-17.
- Gotsopoulos H, Chen C, Qian J, et al. Oral appliance therapy improves symptoms in obstructive sleep apnea. Am J Respir Crit Care Med 2002; 166:743-748.
- Deane S, Cistulli P, Ng A, et al. Comparison of mandibular advancement splint and tongue stabilizing device in obstructive sleep apnea: A randomized controlled trial. Sleep 2009; 32:648-653.
- Bishop B, Verrett R, Girvan T. A randomized crossover study comparing two mandibular repositioning appliances for treatment of obstructive sleep apnea. Sleep Breathing 2013; 18:125-131.
- Mehta A, Qian J, Petocz P, et al. A randomized controlled study of a mandibular advancement splint for obstructive sleep apnoea. Am J Respir Crit Care Med 2001; 163:1457â??61.
- Ferguson KA, Cartwright R, Rogers R, et al. Oral appliances for snoring and obstructive sleep apnea: A review. Sleep 2006; 29:244â??62.
- Schmidt-Nowara W, Lowe AA, Wiegand L, et al. Oral appliances for the treatment of snoring and obstructive sleep apnea: A review. Sleep 1995; 18:501â??10.
- Vanderveken OM, Devolder A, Marklund M, et al. Comparison of a custommade and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med 2008; 178:197â??202.
- Quinnell TG, Bennett M, Jordan J, et al. A crossover randomised controlled trial of oral mandibular advancement devices for obstructive sleep apnoeahypopnoea (TOMADO). Thorax 2014; 69:938â??945.
- Galland BC, Dawes PJ, Tripp EG, et al. Changes in behavior and attentional capacity after adenotonsillectomy. Pediatr Res 2006; 59:711-6.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar. Cross Ref
Indexed at, Google Scholar, crosss ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Nasir H AlHamlan1*, Lujain A AlGhrairy1, Waad E AlSaadi1, Khaled W AlBawardi2, Rana A AlOlaiq2 and Afnan T AlZomaili2
1DMD, King Abdulaziz Medical City in National Guard, Riyadh, Saudi Arabia2College of Dentistry, King Saudi bin Abdulaziz University for Health Science, Riyadh, Saudi Arabia
Citation: Nasir H AlHamlan, Lujain A AlGhrairy, Waad E AlSaadi, Khaled W AlBawardi, Rana A AlOlaiq, Afnan T AlZomaili,Treatment of Obstructive Sleep Apnea with Orthodontic Oral appliances: Systematic Review , J Res Med Dent Sci, 2022, 10(1): 499-508
Received: 06-Dec-2021, Manuscript No. JRMDS-21-48890; , Pre QC No. JRMDS-21-48890 (PQ); Editor assigned: 08-Dec-2021, Pre QC No. JRMDS-21-48890 (PQ); Reviewed: 22-Dec-2021, QC No. JRMDS-21-48890; Revised: 27-Dec-2021, Manuscript No. JRMDS-21-48890 (R); Published: 03-Jan-2022
