Research - (2019) Volume 7, Issue 5
The Role of Epithelial Mesenchymal Transition Process in Inflammatory Gingival Hyperplasia
Lina Ibtesam Khalid*, Saif S Saliem and Bashar Hamid Abdullah
*Correspondence: Lina Ibtesam Khalid, Department of Periodontics and Oral Pathology, College of Dentistry, University of Baghdad, Iraq, Email:
Abstract
Background: Gingival enlargement, the currently accepted terminology or an increase in the size of the gingiva. Local and systemic factors influence the gingival conditions of the patient. These factors result in a spectrum of diseases that can be developmental, reactive and inflammatory to neoplastic. Inflammatory gingival enlargement is the most common one; it is an inflammatory restraint to local irritant correlating with the gingiva.
Material and method: The study involved 15 tissue blocks of inflammatory gingival hyperplasia taken from the archives of oral pathology, laboratory of oral diagnosis department, collage of dentistry/university of Baghdad. Immunohistochemical expression of Vimentin, E-Chdaherin and α-SMA was assessed.
Results: Vimentin and E-Chadherin immunoreactivity of the connective tissue fibroblasts showed 100% positivity, while Alphasmooth muscle actin staining was mostly seen in the endothelial lined blood vessels with a few myofibroblast with in the connective tissue being stained positive. In this article we sought to investigate if the Epithelial mesenchymal transition theory participitated in the advancement of this benign lesion.
Keywords
Gingival hyperplasia, Traditional treatment, Cytoplasmic expression, Myofibroblast
Introduction
Inflammatory gingival hyperplasia is an inflammatory restraint to local irritant correlating with the gingiva; the irritant could be microbial like plaque and calculus. Clinically present as deep red or bluish, considerably friable and fine with smooth glossy surface and commonly bleed easily [1]. Histologically, inflammatory gingival enlargement characterized by thicking of the epithelium with increased volume of the connective tissue with different degree of inflammation and fibrosis [2]. Gingival overgrowth usually treated with traditional periodontal treatment such as scaling and root planning, but if it include significant fibrotic component that don't respond to the traditional treatment so it will be treated by surgical removal of the excess tissue [3].
EMT process is one of the diverse theories attempted to explain the mechanism of advancement of this benign lesion. EMT is a process in which epithelial cells migrate in to the connective tissue and transdifferentiate into fibroblast-like cells, this occurs as the epithelial cell-cell and cell- extracellular matrix interactions are destabilized [4]. In this regard, Vimentin is the most abundant and highly conserved type III intermediate filaments protein. It is mainly expressed in cells of mesenchymal origin and it is often used as a marker for epithelial mesenchymal transition. Vimentin has an important role in adhesion and cell-cell interaction through their association with hemidesmosome and desmosome [5]. E-Cadherin is considered as a prototypical epithelial marker of EMT. The reduction in epithelial expression of E-Cadherin also called the Cadherin switch has been known to promote EMT by facilitating weakening of the intercellular junctions and promoting movement of epithelial cells towards the connective tissue [6]. Alpha smooth muscle actin positive myofibroblast have been demonstrated in type 2 EMT [7].
Materials and Methods
Study samples
The study involved 15 tissue blocks of inflammatory gingival hyperplasia were obtained from the archives of oral pathology, laboratory of oral diagnosis department, collage of dentistry/university of Baghdad from 1971 to 2018 after approval of the ethical committee. The specimen was taken surgically from different age range patient (from 13–60) years old, suffering from gingival hyperplasia caused by plaque accumulation as a result to poor oral hygiene.
Four micron serial sections for Immunohistochemistry were performed for each tissue blocks and staining was done according to the manufactures instructions using three primary antibodies (Table 1); Manufactured by Abcam (Cambridge, UK) and modified from manufactures datasheets in an assay dependent dilution.
| Antibody | Source | Isotype | Specific reactivity | Dilution |
|---|---|---|---|---|
| Polyclonal anti-vimentin antibody | ab45939 | IgG | Mouse, Rat, Rabbit, Human | 1:700 |
| Polyclonal anti-E-Chadherin antibody | ab15148 | IgG | Human, Pig | 1:50 |
| Polyclonal anti-Alpha-smooth muscle actin antibody | ab5694 | IgG | Mouse, Rat, Human | 1:100 |
Table 1: Characteristics of the antibody used for the Immunohistochemical study.
Detection was carried out using Rabbit specific HRP/DAP Detection kit (Abcam, ab236469; 30 ml) that used for the detection of all primary antibodies. Negative controls were carried out on consecutive sections with omitting of the primary antibody resulting in no detectable staining.
Evaluation
Slides were observed and registrated with a light microscope (Olympus CH). All slides were scanned at low power (10x) to select 5 representative fields, visualized and scored with a 400x objective; a mean positive percentage was recorded and an experienced pathologists evaluated the slides independently with statistical correlation to assure acceptable agreement, the percentage of positive cells was scored semi quantitatively as shown in Table 2.
| Markers | Score (1) | Score (2) | Score (3) | Score (4) |
|---|---|---|---|---|
| Vimentin | Scattered spotty Staining | up to 25% of Cells positive | 25% to 50% cells positive | more than 50% of cells positive |
| α-SMA | Scattered spotty Staining | up to 25% of cells positive | 25% to 50% cells positive | more than 50% of cells positive |
| E Cadherin | <10% positive cells | 10 to 20% positive cells | >20 to 50% positive cells | >50% positive cells |
Table 2: Scoring system of the study markers.
The data analyzed using Statistical Package for Social Sciences (SPSS) version 25. The data presented as mean, standard deviation and ranges. Categorical data presented by percentages. Independent t-test (two tailed) was used to compare the continuous variables between study groups accordingly. Chi square test was used to assess the association between markers score and study groups.
Results
Immunohistochemical analysis
Table 3 shows the pattern of distribution of the study markers in samples analyzed. Regarding Vimentin; all samples of inflammatory gingival hyperplasia expressed vimentin, 6 out of 15 was score (3) and the other 9 samples score was 4. Figure 1 shows positive cytoplasmic expression of vimentin in the fibroblast cells of connective tissue (CT).
| Markers | Inflammatory gingival hyperplasia | Mean ± SD |
|---|---|---|
| Vimentin | ||
| Score (3) | 6 (42.9) | 52.26% ± 10.66% |
| Score (4) | 9 (52.9) | |
| E Cadherin | ||
| Score (3) | 9 (52.9) | 46.3% ± 12.4% |
| Score (4) | 6 (46.2) | |
| Alpha-SMA | ||
| Score (1) | 9 (39.1) | 23.4% ± 8.2% |
| Score (2) | 6(75.0) | |
Table 3: Distribution of markers in study samples.
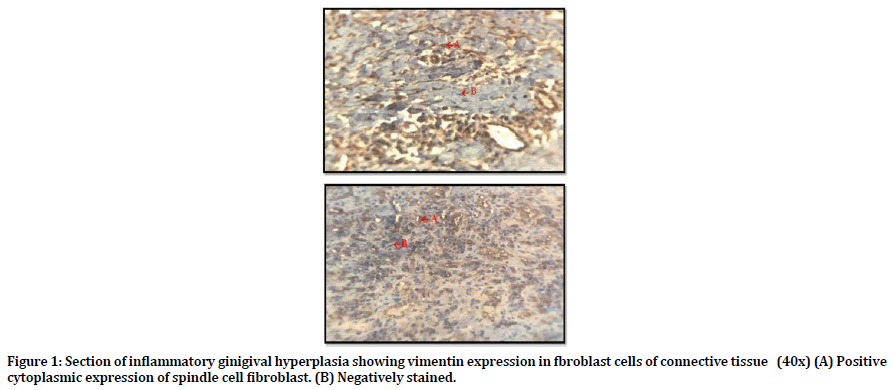
Figure 1. Section of inflammatory ginigival hyperplasia showing vimentin expression in fbroblast cells of connective tissue (40x) (A) Positive cytoplasmic expression of spindle cell fibroblast. (B) Negatively stained.
Regarding the the E-Cadherin; all samples of inflammatory gingival hyperplasia expressed E-Cadherin, 9 out of 15 was score (3) and 6 out of 15 score (4). Figure 2 shows positive cytoplasmic and membranous expression of E-Cadherin in fibroblast cells of CT and in the epithelial surface which use as internal control for E-Chadherin.
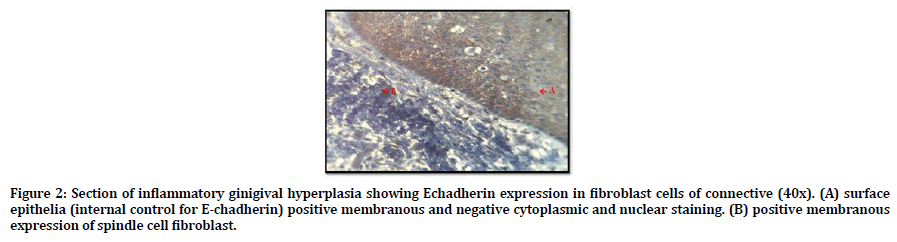
Figure 2. Section of inflammatory ginigival hyperplasia showing Echadherin expression in fibroblast cells of connective (40x). (A) surface epithelia (internal control for E-chadherin) positive membranous and negative cytoplasmic and nuclear staining. (B) positive membranous expression of spindle cell fibroblast.
Regarding the α-SMA; all samples of inflammatory gingival hyperplasia weakly expressed α-SMA, 9 out of 15 was score (1) and 6 out of 15 score (2). Figure 3 shows positive cytoplasmic expression of α-SMA in myofibroblast cell of the CT.
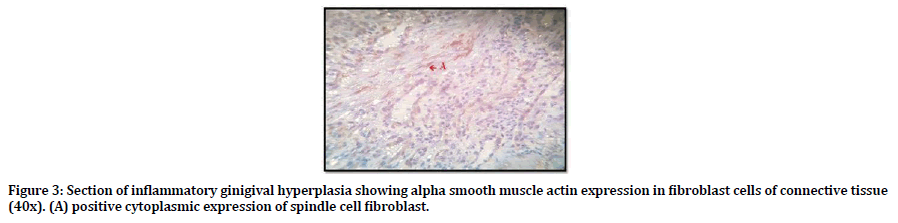
Figure 3. Section of inflammatory ginigival hyperplasia showing alpha smooth muscle actin expression in fibroblast cells of connective tissue (40x). (A) positive cytoplasmic expression of spindle cell fibroblast.
Discussion
The present study was carried out in an effort to determine if EMT operates in the pathogenesis of inflammatory gingival hyperplasia to serve as a source of fibroblasts. To confirm this mechanism, we investigate the Immunohistochemical expression of three specific markers assessing EMT mechanism namely α-SMA, Vimentin and E-Cadherin. Alpha-SMA is a putative myofibroblast marker. Since myofibroblasts are implicated in EMT induced fibrosis we sought to analyse α – SMA expression in the samples.
As mentioned previously, the mean number of myofibroblast positive alpha-sma was; 23.4% in samples analyzed, as the Alpha-sma is one of the cytoskeletal biomarkers for the epithelial mesenchymal transition process [8] so first of all this study suggested that EMT process may be participated at least partly in pathogenesis of inflammatory gingival hyperplasia and this result was in accordance with [9,10].
Regarding the weak expression of Alpha-sma in the study group which is the weakest expression compared with other two markers of this study (Vimentin and E Chadherin) this can be explained based on the concept that in the granulation tissue, fibroblast cells progressively become the majority population and takes myofibroblast phenotype, including expression of Alphaactin [11] then these myofibroblast can long remain silent or disappear by apoptosis after wound healing. This probably the situation that occurred in case of gingival inflammation when the number of Alpha-sma positive cells was very low if not reduced to cells from the blood vessel wall [9].
E-Cadherin is required for the maintenance of normal intercellular adhesion and barrier integrity in oral tissues [12]. Vimentin is one of the most familiar members of intermediate filaments (IFs), as it is the major IF protein in mesenchymal cells and it is frequently used as a developmental marker of cells and tissues [13].
As mentioned previously; the mean number of vimentin& E-Chadherin positive fibroblast were 51.43% & 48.56%, respectively, so as these two markers are biomarkers for EMT, it is suggested that EMT process may be involved at least partly in the pathogenesis of inflammatory gingival hyperplasia. Similar result reported by [8,14,15] but it disagree with [12] who claimed that vimentin should not be considered as a marker of type 2 EMT in the setting of fibrosis.
Tumor growth factor beta one (TGF-B1) has emerged as a potent inducer for EMT, not only through SMAD pathway, but also through pathway not mediated by SMAD, so increased expression and activation of TGF-B1 in inflammatory gingival hyperplasia (14) promote an epithelial cell plasticity that may progress to EMT [15].
Regarding Vimentin; Previous study on renal fibrosis provide an evidence that fibroblast can form locally by EMT during the pathological stress of tissue fibrosis, in renal fibrogenesis about 36% of new fibroblasts come from local EMT, about 14-15% from bone marrow and the rest from local proliferation. This finding reinforce the notion that EMT play arole in the genesis of fibroblast during organ fibrosis in adult tissues [10,16]. And as vimentin is expressed in mesenchymal cells, so connective tissue fibroblast can be used as internal control for vimentin [17]. Therefore as a result to the implication of EMT process in the pathogenesis of inflammatory gingival hyperplasia, this will lead to the overproduction of connective tissue fibroblast which is vimentin positive.
Regarding E-Chadherin; Arora and other researchers try to investigate if the epithelial mesenchymal transition process operates in Cyclosporin A induced gingival overgrowth. They demonstrated that when primary human gingival epithelial cells were treated with TGF-B1 it induced reduction in barrier function as evidenced by reduced electrical resistance and paracellular permeability and simulantanously decreasing the expression of cell surface E-Chadherin [15]. Jeopardized E-Chadherin expression could alter the cell phenotype from epithelial to fibroblast with spindle shape morphology [17]. Okada H and coworkers have shown that the epithelial cells migrate from the epithelial layer, travel through the basement membrane and accumulate in the interstititium of the tissue; here they eventually get rid of their epithelial markers and gain a fully fibroblastic phenotype [18,19]. Mousa and co-workers have shown that TGF-B1 was higher in inflammatory gingival hyperplasia compared to normal gingiva [14].
Conclusions
Epithelial mesenchymal transition process in inflammatory gingival hyperplaia may be driven by elevated level of TGF-B1.
References
- Agrawal AA. Gingival enlargements: Differential diagnosis and review of literature. World J Clin Cases 2015; 3:779–788.
- Trackman PC, Kantarci A. Connective tissue metabolism and gingival Overgrowth. Crit Rev Oral Biol Med 2004; 15:165-175.
- Newman MG, Takei H, Klokkevold PR, et al. Carranza clinical periodontology. 9th Edn. Philadelphia: Saunders 1996; 754.
- Boyer B, Valles AM, Edme N. Induction and regulation of epithelial mesenchymal transitions. Biochem Pharmacol 2000; 60:91–99.
- Dave JM, Bayless KJ. Vimentin as an Integral regulator of cell adhesion and endothelial sprouting. Microcirculation 2014; 21:333–344.
- Huber MA, Kraut N, Beug H. Molecular requirements for epithelial mesenchymal transition during tumor progression. Curr Opin Cell Biol 2005; 17:548–58.
- Zeisberg EM, Potenta S, Xie L, et al. Discovery of endothelial to mesnchymal transition as a source for carcinoma associated fibroblasts. Cancer Res 2007; 67:10123–10128.
- Ivaska J, Pallari HM, Nevo J, et al. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp Cell Res 313:2050–2062.
- Zeisberg M, Neilson EG. Biomarkers of epithelial mesesnchymal transitions. J Clin Invest 119:1429-1437.
- Pascu EI, Pisoschi CG, Andrei AM, et al. Heterogeneity of collagen secreting cells in gingival fibrosis: An immunohistochemical assessment and a review of the literature. Rom J Morphol Embryol 2015; 56:49-56.
- Arora H, Madapusi BT, Ramamurti A, et al. Immunohistochemical localization of epithelial mesenchymal transition markers in cyclosporine a induced gingival overgrowth. J Clin Diagn Res 2016; 10:48–52.
- Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen 2005; 13:7-12.
- Fujita T, Hayashida K, Shiba H, et al. The expressions of claudin-1 and E-cadherin in junctional epithelium. J Periodontal Res 2010; 45:579–582.
- Ivaska J, Pallari HM, Nevo J, et al. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp Cell Res 2007; 313:2050–2062.
- Mousa AO, Saliem SS, Abdullah BH, et al. Immunohistochemical expression of transforming growth factor beta-1 and interferon gamma in hereditary gingival fibromatosis in comparison to inflammatory gingival overgrowth and clinically healthy gingiva. MSC Thesis. Collage of Dentistry, University of Bagdad 2018.
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial mesenchymal transition. Nat Rev Mol Cell Biol 2014; 15:178-196.
- Sume SS, Kantarci A, Lee A, et al. Epithelial to mesenchymal transition in gingival overgrowth. Am J Pathol 2010; 177:208–218.
- Reichert M, Muller T, Hunziker W. The PDZ domains of zonula occludens induce an epithelial to mesenchymal transition of madin- darby canine kidney I cells. Evidence for a role of β-catenin/Tcf/Lef signaling. J Biol Chem 2000; 275:9492–500.
- Okada H, Strutz F, Danoff TM, et al. Possible mechanisms of renal fibrosis. Contrib Nephrol 1996; 118:147–54.
Author Info
Lina Ibtesam Khalid*, Saif S Saliem and Bashar Hamid Abdullah
Department of Periodontics and Oral Pathology, College of Dentistry, University of Baghdad, IraqCitation: Lina Ibtesam Khalid, Saif S Saliem, Bashar Hamid Abdullah, The Role of Epithelial Mesenchymal Transition Process in Inflammatory Gingival Hyperplasia, J Res Med Dent Sci, 2019, 7(5):80-84.
Received: 16-Sep-2019 Accepted: 01-Oct-2019
