Research - (2020) Advances in Dental Surgery
The Photothermal Effect of 940nm Diode Laser on Enterococcus Faecalis Biofilm in Infected Root Canal
Mustafa M Buraihi* and Salah A Alkurtas
*Correspondence: Mustafa M Buraihi, Department of Biomedical Applications, Institute of Laser for Postgraduate Studies, University of Baghdad, Iraq, Email:
Abstract
Background: The complete eradication of root canal pathogens (especially E. faecalis) is still dilemma for endodontists, however with the advanced laser technology newer disinfection protocols proposed to be effective for routine endodontic treatment. Aim: The aim behind this study is to test the efficiency of 940nm diode laser in destroying E. faecalis biofilm with/without the combination of NaoCl within root canals. Materials and methods: Forty extracted permanent single-rooted teeth were prepared, autoclaved and inoculated with E. faecalis culture then incubated for two weeks. After the incubation period, the specimens divided randomly in 4 groups; group A (n=10) control group, group B (n=10) irradiated with laser, group C (n=10) treated with NaoCl, and group D (n=10) treated with combination of NaoCl and laser radiation. Bacterial sampling done by inserting paper points inside the canals then plated on blood agar media to count CFU. Results: Dunnett’s T3 test revealed highly significant difference among the groups (p=0.000, for groups C&D p=0.02) with exception for that between groups A and B which was significant difference (p=0.013). However, the combination protocol yielded the highest bacterial killing (95.5% CFU/ml reduction). Conclusion: The photothermal effect of the 940 nm wavelength has weak bactericidal effect on E. faecalis biofilm. However, when combined with 5.25% NaoCl a good disinfection results achieved with short irrigation time.
Keywords
Biofilm, E. faecalis, Diode laser, Root canal
Introduction
The success of any non-surgical endodontic treatment relies primarily on the capability of cleaning and disinfecting the root canal system efficiently [1]. However, the deep invasion of microorganisms to the tubular system, the presence of smear layer which formed during mechanical instrumentation together with the complex anatomy of the root canal seem to be the greater obstacles for successful root canal disinfection [2]. The disinfection procedure usually done by mechanical action which achieved by instrumentation with files and by chemical action of the irrigation solutions [3]. Different instrumentation techniques showed their efficiency regarding decrease the total bacterial load inside the canal; however in vitro studies reveled their failure in complete bacterial elimination [4].
Sodium hypochlorite, chlorohexidine gluconate and ethylenediaminetetraacetic acid (EDTA) are the mostly used root canal irrigant during routine endodontic treatment yet none of these chemicals can be considered as an ideal irrigation solution [5]. Due to the effect of surface tension these irrigant solutions cannot penetrate dentin for more than 100 micrometer and as wellknown that pathogenic bacteria have the ability to invade deep into dentin for approximately 1000 μm making the chemical irrigant inefficient against these deep seated microorganisms [6].
The apical third of the root canal has its own challenge to the irrigation process and the important issue there is the balance between safety and effectiveness [7]. The diffusion of the irrigant solution through dentinal tissue generally slow and probably depends on some factors such as irrigant concentration and temperature [8].
From the difficulties it is obvious not all root canal treatments (RCT) could be succeeding, instead there is a failure rate of 14- 16% in initial RCT [9,10].
Hence, to retreat these failed cases successfully the persistent microorganisms should be eliminated during the biomechanical instrumentation and their existence after cleaning and shaping phase has a great impact on the success rate of these cases [11]. In fact, complete elimination of all microorganisms within the root canal prior to the obturation is exceedingly difficult and complicated [12]. And that's why additional disinfection methods to the conventional treatment protocols should be imposed to overcome the shortage in eliminating the bacterial biofilm inside root canals [13].
Enterococci (especially E. faecalis), streptococci with some fastidious anaerobes (Example: Propionibacterium species, and P. alactolyticus are the most abundant genera collected from endodontically treated teeth with persistent apical periodontitis. Studies revealed that E. faecalis present in about 90% of treated teeth with persistent infection suggesting its responsibility for the recurrent root canal infection [15].
E. faecalis is gram positive facultative anaerobic bacteria and found as a part of normal flora in human mouth. Studies revealed that E. faecalis can survives root canal treatment and withstand the tough endodontically treated canal's environment. Its ability to invade dentinal tubules and sometimes penetrates deep inside these tubules enabling the microorganisms to escape away from the action of instrumentation and the bactericidal effect of the irrigant solution. Another property for this bacterium is its ability to compose biofilm within the root canal, this biofilm increases the bacterial resistance against antimicrobials and environmental stress. Moreover, this bacterium resists calcium hydroxide and this may be due to the active proton pump which enables this microorganism to withstand high pH [16,17].
Laser technology has been used increasingly in non-surgical root canal treatment in the last years. Its high power and unique coherent rays make it useful in root canal cleaning and disinfection phase in combination with chemical solutions. Laser shown to be effective in activation of root canal irrigant by producing mechanical disturbances [18].
Because of the small size, portability, low price, and wide range of applications in oral medicine the 940 nm diode laser is widely used in dental clinics. Its radiations produce bactericidal photothermal disruptive effect that can destroy bacterial cell's wall [19]. The 940 nm wavelength has low absorption coefficients in water (about μa=0.04–0.05 cm−1) so it has poor absorption in water and hydroxyapatite which are the main constituents of dentin and this property give the 940nm laser beam deep penetration depth inside dentin (over 1000 μm) thus killing distant bacteria [19,20].
Aim of study
To evaluate the bactericidal efficiency of 940 nm diode laser with and without 5.25% sodium hypochlorite against E. faecalis biofilm.
Materials and Methods
Preparation of teeth specimens
In this study 40 extracted human single rooted teeth having single canal were used. All the teeth have approximately the same dimensions with their apices completely formed and did not undergo root canal treatment before. After collecting the teeth, root surface debridement was the first step in their preparation. After their debridement, all teeth were rinsed in 5.25 NaoCl (Chlorax d 5.25%, Cerkamed, Poland) for 30 minutes then soaked in normal saline till the next preparation day.
To obtain uniform working length all teeth specimens sectioned at their cervical areas so that they have 15 mm working length. Root canal patency for each specimen was assessed with K-files (Dentsply Maillefer, Switzerland) #10, #15, and #20. The root canals were prepared by Protaper file system SX-S1-S2-F1-F2-F3 (Dentsply Maillefer, Switzerland).
Irrigation was performed after each file by injecting 3 ml of 5.25% sodium hypochlorite using a 30-gauge irrigation needle (Sinalident, China). The irrigation needle was placed at 2 mm short from the apices of the canals with a delivery rate fixed at 3 ml/min so that every canal had 10 minutes total irrigation time.
The next step after instrumentation with files was injecting 2ml of 17% EDTA (EDTA 17%, PD, Australia) in each canal for 3 minutes followed by a final irrigation with 5.25% NaoCl for 3 minutes. Both solutions (EDTA, NaoCl) were activated for 30 second with an ultrasonic tip. A final wash with sterile water was made to all the canals then dried with paper points.
After completing the cleaning and shaping, the apical foramens were sealed with composite resin fillings (Brilliant Everglow, Coltene, Switzerland) then all the specimens were autoclaved for 20 minutes at 121ºC under 15 psi pressure. After sterilization, each specimen placed in 2 ml of sterile Luria-Bertani broth (LB broth) contained in eppendorf tube and incubated for 48 hours at 37°C. Daily screening for the tubes revealed no turbidity in the broth.
Experimental root canals contamination
All teeth specimens were contaminated with Enterococcus faecalis bacteria isolated from infected root canals and identified by vitek (Vitek 2 comnpact, BioMérieux, France) by inoculating each one with 20 μl (using micropipette) of e,facalis in LB broth. The bacterial suspension diluted spectrophotometrically to match the turbidity of McFarland 0.5 scale, then the canals orifices dried and sealed with light cured temporary fillings (Temp it, Spident, South Korea) then placed into test tubes each one contains 2ml of LB broth and incubated at 37°C under anaerobic conditions for two weeks. The broth was changed every three days.
Laser parameters selection
In root canal disinfection continuous and pulsed emission modes are used by clinicians. For diode laser the common and effective powers used are 1 ,1.5 and 2 watts (CW) and the exposure time limited within a range of 5-10 seconds [5-7].
As laser radiation elevates root surface temperature, a pilot study was made to test how much these powers could elevate the temperature on the external root surface to ensure that our power still safe for the periodontal tissues and within the biological limit.
The method applied in this pilot study was that a tooth prepared just like the 40 teeth specimens used in this research and placed in stone mold then connected to a thermocouple wire at the thinnest surface in the root (the mesial root surface). The thermocouple wire connected to a highly accurate thermometer (AMPROBE TMD®-56, Everett, WA, USA) with basic accuracy of 0.1% and gives temperature record in every one second.
The thermometer connected to a computer via USB cable to record temperature changes. the procedure started by inserting laser fiber (size 200μm) inside root canal reaching up to one millimeter under the apex then applying laser beam with a helicoid motion in downward and upward directions for 5 seconds exposure followed by 10 seconds resting interval for four times.
The powers tested were 1, 1.3, and 1.5 watt. The maximum temperature elevation gained with those powers were 4.6, 6.7, and 7.6 degrees respectively. As well known the maximum temperature elevation tolerable by the periodontal tissues should be lower than 7 degrees so that 1.5 watt was excluded and 1.3 watt was the power used in this research as it still within normal temperature limit [21-23].
Experimental specimens’ disinfection
After two weeks of anaerobic incubation the teeth specimens brought out from the incubater and soaked in CHX solution for two minutes then washed with sterile water then randomly divided into four groups each one contains 10 teeth specimens. The groups are: group A (control group) the specimens received no treatment, group B (laser group) its specimens received only laser radiation, group C (NaoCl group) its specimens treated with 5.25% sodium haypochlorite, and group D (combination group) its teeth received both laser and NaoCl together.
Group B (n=10): Diode laser
After elimination of temporary fillings, tooth specimens in laser group were disinfected by the diode laser with an endodontic fiber tip (ezTip Endo, 20 mm/200 μm) at an outpot power of 1.3 watt at continuous emission mode (CW). Each canal received 0.1 ml of sterile water then irradiated with 5 seconds laser exposure four times, each exposure followed by ten seconds interval. The laser tip was inserted directly into the root canal one millimeter beneath the working length and moved in a helicoid pattern downward and upward. After lasing the specimens, each specimen received 0.1 ml of sterile water then sealed with temporary restoration and incubated for 24 hours.
Group c (n=10): NaoCl
Tooth specimens were disinfected by irrigation with 3ml of 5.25% sodium hypochlorite for 3 minutes. The procedure started with introducing a 30-gauge irrigation needle passively inside the canals up to two millimeters under the apices then injecting 3 ml of the NaoCl solution. After irrigating the ten samples, all the canals were dried with sterile paper points than 0.1 ml of sterile water was introduced into each canal and sealed coronary with temporary filling and incubated for 24 hr.
Group D (n=10): Combination
The disinfection protocol for the specimens in this groups consisted of the combination of sodium hypochlorite and laser radiation in two steps (i.e. introducing NaoCl then applying laser). First The irrigation with NaoCl was like that in group C. then after 3 minutes the sodium hypochlorite remained inside the canals and laser irradiation performed directly inside each canal as in group B. After that, a sterile water introduced in all specimens and coronary sealed and incubated for 24 hours.
Determination of bacterial count
After the experimental disinfection all the treated specimens incubated for 24 hours then in the next day the ten specimens of the control group (group A) together with the 30 disinfected specimens brough out and their temporary fillings were removed then refilled with 0.1 ml of sterile water to serve as transport media. Then sampling from the root canals consisted of inserting sterile K-File #25 inside each canal with circumferential filing for 30 seconds to disrupt the bacterial biofilm and collecting dentin chips. Then 40 sterile paper points #F3 introduced inside the root canals collecting the transport media along with the swimming dentin chips. Then each paper point was placed inside Eppendorf tube containing 2 ml of sterile LB broth then vortexed for 60 seconds then incubated for 24 hours.at 37°C under anaerobic conditions.
After incubation, 0.5 ml was taken from each Eppendorf tube and undergoes tenfold serial dilution then inoculated onto blood agar dishe and incubated for 24 hr. After the incubation period, bacterial growth was seen represented as colony forming units (CFU). The final step was counting those CFU and transforming these numbers into actual counts per ml of the original specimen's broth by using the formula:
''No. of CFU X Dilution factor=No. of CFU/ml'' [24,25].
Then comparisons between the mean of group A and the means of other disinfected groups were made to determine the bacterial colonies reduction in each experimental group.
Statistical analysis
Data of the study were analyzed using oneway analysis of variance (ANOVA) model to compare the mean CFUs/ml among the groups. Then multiple comparisons of mean CFU/ml between groups were made using Dunnett’s T3 post hoc test. P value<0.05 was considered statistically significant. The statistical analysis was performed using SPSS for windows (SPSS INC, Chicago, Il, USA) version 26.0.0.
Results
Bacteriological evaluation
Specimens in all groups showed bacterial growth after 24 hours with exception for one specimen in group C (sodium hypochlorite) which exhibited no growth on the blood agar media. CFUs numbers showed below in Table 1.
| Control group | Laser group | 5.25% NaoCl | Combination |
|---|---|---|---|
| 25000 | 12200 | 7400 | 700 |
| 28700 | 19800 | 4200 | 500 |
| 12700 | 18400 | 10000 | 1100 |
| 23600 | 16000 | 9500 | 900 |
| 29500 | 17300 | 0 | 800 |
| 22000 | 21100 | 7000 | 1200 |
| 24500 | 18100 | 6700 | 1800 |
| 22700 | 19500 | 8000 | 1700 |
| 26100 | 17900 | 10700 | 1300 |
| 28000 | 18700 | 4900 | 1100 |
Table 1: The number of CFUs/ml in all specimens after tenfold serial dilution.
From the Table 2 and Figure 1 comparisons between the mean of control group with those of the treated groups can be made to assess the bacterial reduction in each treated group. Group D has the greatest bacterial reduction as 95.5% of bacteria were died followed by group C (71.9% bacterial reduction) and group B (26.7% bacterial reduction) respectively. It’s clear that the powerful bactericidal effect against E. Faecalis biofilm achieved by combining NaOCl and laser radiation inside infected root canal. Statistical test of CFU among groups using Oneway Analysis of Variance (ANOVA) revealed highly significant difference as p=0.00.
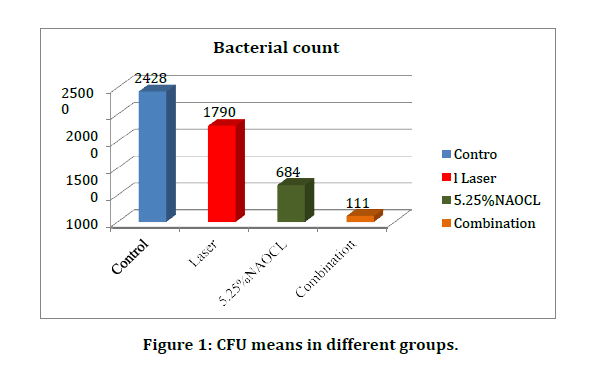
Figure 1: CFU means in different groups.
| CFU | |||||
|---|---|---|---|---|---|
| Groups | Mean | ± SD | ± SE | Minimum | Maximum |
| Control | 24280 | 4793.004 | 1515.681 | 12700 | 29500 |
| Laser | 17900 | 2444.949 | 773.161 | 12200 | 21100 |
| 5.25% Naocl | 6840 | 3183.01 | 1006.556 | 0 | 10700 |
| Combination | 1110 | 414.863 | 131.191 | 500 | 1800 |
Table 2: Descriptive statistics foe CFUs/ml among groups.
Multiple comparisons of CFU/ml between groups were made using Dunnett’s T3 post hoc test (Table 3). All comparisons revealed highly significant differences except between control and laser groups, the finding is significant p=0.013.
| Multiple Comparisons | ||||
|---|---|---|---|---|
| Dependent Variable: CFU | ||||
| Dunnett’s T3 post hoc test | ||||
| Groups | Groups | Mean Difference | P value | |
| Laser | 6380 | 0.013 | Sig. | |
| Control | 5.25%NAOCL | 17440 | 0 | HS |
| Combination | 23170 | 0 | ||
| Laser | 5.25%NAOCL | 11060 | 0 | |
| Combination | 16790 | 0 | ||
| 5.25% NAOCL | Combination | 5730 | 0.002 | |
Table 3: Multiple comparisons of CFU between groups using (Dunnett’s T3 post hoc test).
Diagnosis
This invitro study was conducted to test and evaluate the bactericidal effect of 940nm semiconductor laser alone and in combination with 5.25% sodium hypochlorite on root canals infected with E. faecalis biofilm. Due to its well tolerance to physical stresses, temperature changes, and biomechanical instrumentation E. Faecalis was chosen in this study [26]. 20-23% of patients with endodontic failure after one year of treatment were attributed to the presence of E. faecalis inside their treated root canals [27]. The conventional root canal irrigant fail to eradicate this bacterium from the tubular system completely as E. faecalis penetrates deep inside dentin for more than 1000 μm thus additional disinfection methods should be imposed to enhance bacterial killing within infected root canals [28].
The disruptive photothermal effect of the diode laser can destroy distant microorganisms in dentinal tubules. However, its bactericidal effect on gram-negative bacteria is more than that on gram- positive bacteria which may relate to cell wall structural characteristics (i.e. cell wall pigmentation) [29].
In the present study, we tested the antibacterial effect of laser and 5.25% NaoCl separately and combined. The 1.3 watt of 940nm laser radiation showed shriveled antibacterial effect on the bacterial biofilm when used without NaoCl (group B) as there was only 26.7% CFU reduction. This weak result agrees with Ozkocak et al. as he reported a weak antibacterial effect of 940nm diode laser on E. faecalis biofilm cultured inside extracted central incisors [30].
Udart et al. tested bactericidal effect of 940nm wavelength on E. faecalis biofilm. His study found that this wavelength exhibits very weak antibacterial effect on the bacterial biofilm and he concluded that the bactericidal effect of the 940nm laser comes from its thermal effect and for this effect to be powerful enough to disinfect the root canal it must elevate root canal dentin temperature for about 70°C and that could destroys the periodontal tissues [31]. Many concentrations of NaOCl are used regarding root canal bacterial disinfection (e.g. 3%, 5%, 5.25%, 6%, and 8%) however many studies proved that 5.25% gives the best antibacterial effect on pathogenic bacteria (including E. faecalis) of infected root canal with tolerable toxicity [32,33]. In this study sodium hypochlorite showed moderate bactericidal effect on E. faecalis biofilm at 3 minutes irrigation time as there was about 71.9% CFU reduction in group C. Heating up NaoCl to 50c before introducing inside the canal shown to increase the bactericidal effect of this solution [34]. The best results achieved in our study when 5.25% NaoCl combined with laser radiation within the root canal as there was 95.5% bacterial reduction in group D. This result supported by Castelo P et al. Who studied the antibacterial effect of the combination of 940nm diode laser and NaoCl on E. faecalis biofilm inside extracted human teeth and found that the best bacterial killing achieved in the combination protocol [35]? According to Olivi G the local increment of temperature produced by near infrared laser could activate irrigation solution and due to poor absorption by water it could not initiate cavitation’s [36].
Conclusion
In conclusion, the 940nm diode laser radiation has mild antibacterial effect on E. faecalis biofilm if applied alone inside root canal as its photonic energy absorbed by gram-negative bacteria with poor absorption by gram- positive bacteria. However, when combined with 5.25% sodium hypochlorite it boosts the bactericidal effect thus enhances root canal disinfection.
References
- Childer H. Cleaning and shaping the root canal. Dent ClinN Am 1974; 18:269–296
- Torabinejad M, Handysides R, Khademi AA, et al. Clinical implications of the smear layer in endodontics: A review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002; 94:658–666.
- Alfredo E, Marchesan MA, Sousa-Neto MD, et al. Temperature variation at the external root surface during 980-nm diode laser irradiation in the root canal. J Dent 2008; 36:529–534.
- Dalton BC, Orstavik D, Phillips C, et al. Bacterial reduction with nickel-titanium rotary instrumen-tation. J Endod 1998; 24:763–767.
- Rdinola-Zapata R, Bramante CM, Garcia RB, et al. The antimicro-bial effect of new and conventional endodontic irrigants on intra-orally infected dentin. Acta Odontol Scand 2013; 71:424– 431.
- Berutti E, Marini R, Angeretti A. Penetration ability of dif-ferent irrigants into dentinal tubules. J Endod 1997; 23:725–727.
- Haapasalo M, Shen Y, Wang Z, Gao Y. Irrigation in endodontics. Br Dent J 2014; 216:299 303.
- Van der Sluis LW. Endodontics in motion: New concepts, materials and techniques 3. The role of irrigant during root canal treatment. Ned Tijdschr Tandheelkd 2015; 122:533–538.
- Ng YL, Mann V, Rahbaran S, et al. Outcome of primary root canal treatment: systematic review of the literature–part2: Influence of clinical factors. Int Endod J 2008; 41:6-31.
- Song M, Kim HC, Lee W, et al. Analysis of the cause of failure in nonsurgical endodontic treatment by microscopic inspection during endodontic microsurgery. J Endodont 2011; 37:1516–1519.
- Farzaneh M, Abitbol S, Lawrence HP, et al. Treatment outcome in endodontics-the Toronto Study. Phase II: Initial treatment. J Endod 2004;30:302–9.
- Orstavik D, Haapasalo M. Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Endod Dent Traumatol 1990 ;6:142–149.
- Castelo-Baz P, Martín-Biedma B, Ruíz-Piñón M, et al. Combined Sodium Hypochlorite and 940 nm diode laser treatment against mature E. faecalis biofilms in vitro. J Lasers Med Sci 2012; 3:116-121.
- Siqueira JF. Aetiology of root canal treatment failure: why well- treated teeth can fail. Int Endod J 2001; 34:1-10.
- Stephen C. Pathways of the Pulp 11th Edn 2014.
- Sohrabi K, Sooratgar A, Zolfagharnasab K, et al. Antibacterial Activity of diode laser and sodium hypochlorite in Enterococcus Faecalis-Contaminated root canals. Iran Endod J 2016; 11: 8-12.
- Forghani M, Afshari E, Parisay I, et al. (2017). Effect of a passive sonic irrigation system on elimination of Enterococcus faecalis from root canal systems of primary teeth, using different concentrations of sodium hypochlorite: An in vitro evaluation. J Dent Res 2017; 11:177–182.
- Bago JI, Anic I. The use of lasers in disinfection and cleanliness of root canals: A review. Acta Stomatologica Croatica 2014; 15:6-15.
- Tilakchand M, Singh NN, Yeli MM, et al. Evaluation of the antibacterial efficacy of EZLASE diode LASER on the infected root canal system: An in vivo study. J Conserv Dent 2018; 21:306–310.
- Martins MR, Franzen R, Depraet F, et al. Rationale for using a double-wavelength (940 nm+2780 nm) laser in endodontics: Literature overview and proof-of-concept. Lasers Dent Sci 2018; 2:29–41.
- Gutknecht N, Kaiser F, Hassan A, et al. Long-term clinical evaluation of endodontically treated teeth by Nd: YAG lasers. J Clin Laser Med Surg 1996; 14:7-11.
- Al-Zand SA, Al-Maliky MA, Mahmood AS, et al. Temperature elevation investigations on the external root surface during irradiation with 940 nm diode laser in root canal treatment. Saudi Endod J 2018; 8:14-18.
- Gutknecht N, Franzen R, Meister J, et al. Temperature evolution on human teeth root surface after diode laser assisted endodontic treatment. Lasers in Medical Science, 2005; 20:99– 103.
- Ajith Kumar M. Colony forming unit. Bio resource technical resources in biotechnology 2011.
- Karaman R. Calculating the colony forming unit bacteria 2016.
- Fabricius L, Dahlen G, Sundqvist G, et al. Influence of residual bacteria on periapical tissue healing after chemomechanical treatment and root filling of experimentally infected monkey teeth. Eur J Oral Sci 2006; 114:278–285.
- Ng YL, Mann V, Rahbaran S, et al. Outcome of primary root canal treatment: systematic reviewof the literature–part 1. Effects of study characteristics on probability of success. Int Endod J 2007; 40:921–939.
- Vatkar NA, Hegde V, Sathe S. Vitality of Enterococcus faecalis inside dentinal tubules after five root canal disinfection methods. J Conserv Dent 2016; 19:445-449.
- Marchesan MA, Brugnera A, Ozorio JE, et al. Effect of 980-nanometer diode laser on root canal permeability after dentin treatment with different chemical solutions. J Endod 2008; 34:721–724.
- Ozkocak I, Gokturk H, Şay C et al. Antibacterial efficiency of different irrigation solutions, lasers and photodynamic therapy with indocyanine green in root canals infected by Enterococcus Faecalis. Meandros Med Dent J 2018; 19:289-295.
- Udart M, Stock K, Graser R, et al. Inactivation of bacteria by high-power 940nm laser irradiation. Med Laser App 2011; 26:166–171.
- Ghivari SB, Bhattacharya H, Bhat KG, et al. Antimicrobial activity of root canal irrigants against biofilm forming pathogens-An in vitro study. J Conserv Dent 2017; 20:147-151.
- Luddin N, Ahmed HM. The antibacterial activity of sodium hypochlorite and chlorhexidine against Enterococcus faecalis: A review on agar diffusion and direct contact methods. J Conserv Dent 2013; 16:9–16.
- Iandolo A, Amato M, Dagna A, et al. Intracanal heating of sodium hypochlorite: Scanning electron microscope evaluation of root canal walls. J Conserv Dent 2018; 21:569-573.
- Castelo-Baz P, Martín-Biedma B, Ruíz-Piñón M, et al. Combined sodium hypochlorite and 940 nm diode laser treatment against mature E. Faecalis biofilms invitro. J Lasers Med Sci 2012; 3:116-121.
- Olivi G. Laser use in endodontics: Evolution from direct laser irradiation to laser-activated irrigation. J Laser Dent 2013; 21:58–71.
Author Info
Mustafa M Buraihi* and Salah A Alkurtas
Department of Biomedical Applications, Institute of Laser for Postgraduate Studies, University of Baghdad, IraqCitation: Mustafa M Buraihi, Salah A Alkurtas, The Photothermal Effect of 940nm Diode Laser on Enterococcus Faecalis Biofilm in Infected Root Canal, J Res Med Dent Sci, 2020, 8 (7): 480-486.
Received: 29-Oct-2020 Accepted: 19-Nov-2020 Published: 26-Nov-2020
