Research Article - (2022) Volume 10, Issue 8
The Optical Biosensor was used to Determine Antibiotic Mics against H. pylori.
lIsraa ML*, Saqari and Layla MH Al-Ameri
*Correspondence: lIsraa ML, Department of Oral Medicine, College of Dentistry, University of Baghdad, Baghdad, Iraq, Email:
Abstract
Active Helicobacter pylori infection always causes active chronic gastritis. In most people, this can be clinically overlooked throughout life, but in a significant minority, gastro duodenal disease, gastric cancer, and Mucosa Associated Lymphoma Tissue (MALT) Antibiotic resistance will emerge as a potential threat over the next few decades. This is a global phenomenon in which globalization acts as a catalyst. The most common technique currently used to detect antibiotics is biosensors. Optical biosensors based on multi-mode no core fibre are an interesting option for the detection of H. pylori by many analysts because they offer several advantages, such as high sensitivity, direct and real time measurements. The Biosensor which depends on the inline Mach-Zehnder interferometer is a breakthrough in the identification on of H. pylori by measuring the bacteria in the BHI broth turbidity stabilizer and comparing the results with the traditional biochemical tests. This review will introduce some of the developments in the field of optical biosensors, focusing on applications with innovations to address the challenges associated with H. pylori bacteria detection.
Keywords
H. pylori, Optical biosensors, Antibiotic, Analytes, MICIntroduction
Gram-negative spiral micro aerobic bacterium Helicobacter pylori, which infects the human stomach, was first reported by Warren and Marshall in 1983 [1]. Helicobacter pylori (H. pylori) are an important cause in the etiology of gastric duodenal disease, including gastric ulcers, Mucosa-associated lymphoid lymphoma, and gastric adenocarcinoma. About 15% of the world's population is infected with H. pylori. [2] The gastric mucosa is well protected from bacterial infections. But H. pylori adapt to mucus and remain attached to epithelial cells, avoiding an immune response and persistent colonization in the stomach. It is not well understood why the immune system is unable to get rid of H. pylori infection. In addition, the mechanisms that control the induction and maintenance of chronic inflammation induced by H. Pylori are only partially understood [3]. Patients with stomach ulcers, a family history of gastric cancer, or dyspepsia are all examples of those who need therapy [4]. Pylori were classified as a Group 1 carcinogen for stomach cancer by the International Agency for Research on Cancer in 1994 [5]. When Clarithromycin's resistance to it has recently led to its widespread usage in the pharmaceutical business. World Health Organization (WHO) is a high priority disease in the need of innovative ideas. Drugs that fight bacteria Clarithromycin resistance has increased since 1998 [6]. Subsequent declines in the success rate of Clarithromycin based triple therapy were indirect signs of increased tolerance [7]. There have been several studies that have shown modest levels of antibiotic resistance in the absence of prior treatment with particular medicines. Clarithromycin, and in this case, the triple treatment is excellent in efficacy and effectiveness (90%) [8]. A link between antibiotic use and resistance to Clarithromycin and Levofloxacin has recently been demonstrated in Europe [9]. With the prevalence of Clarithromycin and Metronidazole resistant H. pylori strains, eradication rates with triple therapy are below 80% worldwide. In contrast to clarithromycin or metronidazole, H. pylori resistance to Amoxicillin (AMO) remains rare. AMO is H. It is also unique in that its bactericidal activity against pylori is time-dependent (68 hours after administration) and can be enhanced by increasing the maximum gastric pH (>6.5) using a high-dose Proton Pump Inhibitor (PPI) [10]. Due to the high level of treatment failure of standard triple therapies (proton pump inhibitors, Amoxicillin, and Clarithromycin) these days, some new treatment options are needed [11]. Reliable and rapid diagnosis of H. pylori infection before and after eradication therapy is of great clinical importance for the monitoring and treatment of gastro duodenal disease [12]. Found that H. pylori could invade and replicate in epithelial cells. Thus, H. pylori can be considered an intracellular microorganism, and this has an impact on its biological life cycle and its resistance to antibiotics [13]. Diagnosis of H. pylori infection is generally "invasive", which requires endoscopy to sample gastric tissue and mucus, or "only blood, respiration, urine, saliva, and stool samples." It is classified as "non-invasive" [14]. Non-invasive methods are the best test for many patients, as invasive methods can cause pain and psychological distress in patients. Among the non-invasive methods developed, the biosensor provides accuracy for both initial diagnosis and confirmation of eradication. It is one of the most reliable tests for diagnosing Helicobacter pylori infection. Biosensors typically recognize bio molecules such as nucleic acids, proteins, and cells that are associated with the disease. This is made possible by three key components: the biologically sensitive element, the detector element, and the reader [15]. Biosensors use a semi-quantitative approach, making them a very practical solution for large-scale detection of antibiotics [16]. However, because bacterial infections are now an important issue for human health, pathogen detection has become one of the most suitable targets for these devices.
Aims: To identify Helicobacter pylori bacteria and MIC level for antibiotics that affect H. pylori isolated from the clinical specimens before developing a new form of antibiotic resistance, optically by using the new technique of optical biosensor.
MNCM optical biosensor features for bacterial detection
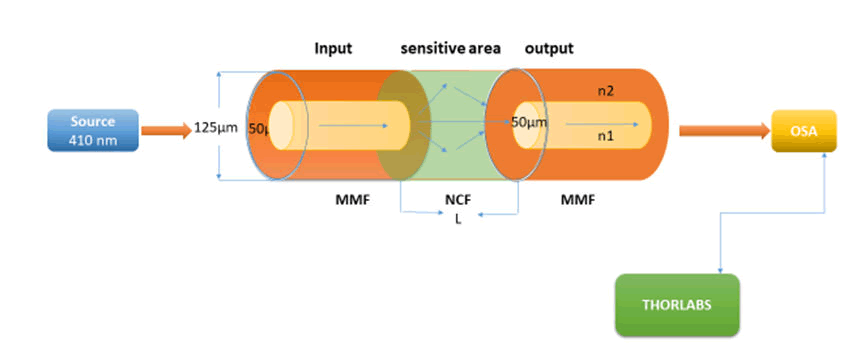
This study presents a facile, rapid, and effective method for the fabrication of optical fibre biosensors. A section of the core less fibre (type FC) is spliced between two multi-mode fibres (type FC) to construct a Multi Mode-Coreless-Multi Mode (MNCM) fibre structure as shown in Figure 1. We take full advantage of the transmission characteristics of the core less fibre. Sensors with a length of core less optical fibres of 3 cm were constructed. After a comprehensive comparison, we chose the MNCM optical fibre sensor with a core less fibre length of 3 cm for the detection of H. Pylori and antibiotics based on an inline Mach-Zehnder interferometer [17]. Ppropagating wavelength along the inline MZI creates a superposition pattern. The separation between two peaks in a two-mode interferometer is defined as:
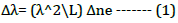
Where λ is the wavelength, L represents the MZ interferometer length and â??ne is the effective differences in the refractive index between two arms of inline MZI [18].
After activation and incubated the bacterial cells for 18 h directly, a measurement of turbidity by the Mcfarland device (0.5) was done and the structure laser biosensor of the multimode–core-multimode structure was built for detection of H. Pylori and antibiotics sensitivity to determine the Minimum Inhibition Concentration (MIC) as shown in Figure 1. Where a diode laser with 410 nm as wavelength act as a source of light where it matches the absorption peak of H. Pylori, and the multimode-core-multimode optical fibre is connected to the source of light and connected to the OSA from the other hand for getting data that show in computer.
Figure 1: Multi-mode-no core-optical bbiosensor scheme.
Materials and Methods
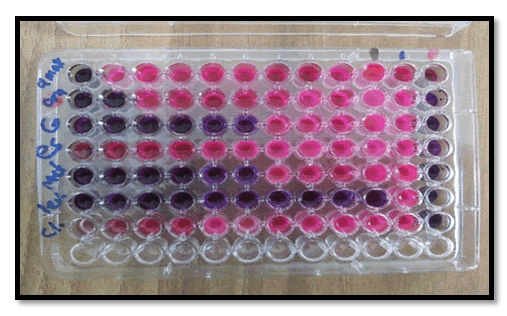
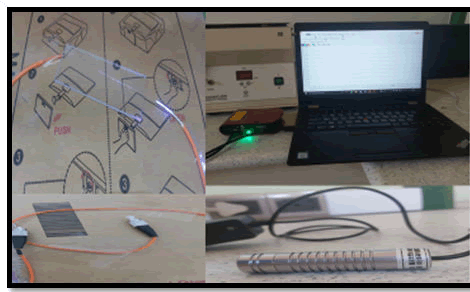
Samples were taken from patients before PPI ingestion of antibiotic therapy and directed endoscopy, and at least two simultaneous gastric mucosal biopsies were obtained approximately 3 cm from each patient-guided gastric duodenum. (One from the sinus and the other from the patient's stomach). Endoscopy (OGD) of the upper esophagus (OGD) under the supervision of an endoscopist with standard biopsy forceps using xylose as local anaesthesia assesses the degree of clinical condition and placed in the appropriate transport media. Ideally, H. pylori Within 6 hours of isolation and identification, the biopsy was first ground with 0.5 ml burusera broth with an electric homogenizer. The suspension was then plated on a BHI broth medium. Columbia Blood Agar (10 mg/ml) enriched with 10% human blood and supplemented with Vancomycin (10 mg/ml), Ceftaroline (5 mg/ml), Trimethoprim (5 mg-/ml) and action (100 mg/ml). After 24 hours in Himedia), the plates were incubated in a slightly aerobic atmosphere (glass containing GasPaks) for 2 days. Suspected H. pylori colonies were identified by the presence of urease, catalase, and oxidase activity. The strain was frozen in Brucella broth containing 25% glycerol at 270°C and stored on dry ice. that's all [22] The antibiotics tested were Amoxicillin, Clarithromycin, and Metronidazole since the drugs used in the trial were either Amoxicillin (1 g) plus clarithromycin (500 mg) omeprazole (20 mg) or metronidazole (400 mg) Gentamicin (40 mg/ml) esomeprazole (40) The appropriate stock was prepared from each antibiotic using the equation (C1V1=C2V2), MIC assay was performed using a microtiter plate as shown in Figure 2. All samples of H. pylori used were turbidity stabilized (0.5) by using the McFarland device and All antibiotics used are not absorbed in wavelength 410 nm. (Taken into account) After incubating the microtiter plate for 24 h, the results were stained with Resazurin (blue dye) to build the BIOSENSOR; the optical fibres were cut using a (fibre cleaver X-50C), splicing optical fibre multi-mode-core less by (Fusion Splicer S-16). The samples were examined by the biosensor as shown in Figure 3 core less acting as sensing area for detecting H. Pylori bacteria with and without antibiotics. The curve was extracted, then the most virulent sample was taken, for which a MIC was made and examined by the laser biosensor, with the measurement of changes with each antibiotic.
Figure 2: MIC\Microtiter plate.
Figure 3: Optical biosensor structure.
Results and Discussion
The conventional method for detecting bacteria
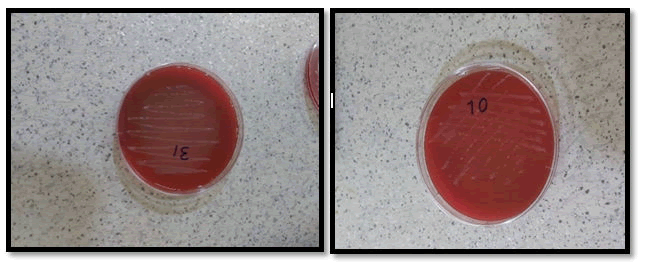
The results obtained in this work were performed from gastric biopsy samples were collected from patients (males and females) who suffered from two or more dyspeptic symptoms including (epigastric pain, anorexia, weight loss, acidity, heartburn, nausea, and vomiting), the patient`s ages ranged from 15-85 years, they were referred to the gastroenterology center in Medical City Hospital in Baghdad. Gastric biopsies specimens of them were taken only from the patient's group to evaluate the prevalence of H. pylori infection. Diagnoses of Peptic Ulcer Diseases (PUD) were established under the consultation of the supervision endoscopist and with the help of the hospital medical staff based on clinical symptoms and test investigation (culture and serology) guiding Oesophageal Gastro duodenal Endoscopy (OGD). Ppositivity for H. pylori was taken as a golden standard method in the current study as shown in Figure 4 (Table 1).
Figure 4: Morphology of H. pylori colonies on colombia medium after two days of incubation under optimal condition.
| Gender | Age mean ± SE of mean (Years) | Probability |
|---|---|---|
| Males | 39.44 ± 1.84 | P>0.05 |
| Females | 43.60 ± 2.32 | |
| Total | 41.45 ± 1.48 |
Table 1: Biopsy positivity* endoscopic clinical findings* gender.
PCR (polymerase chain reaction)
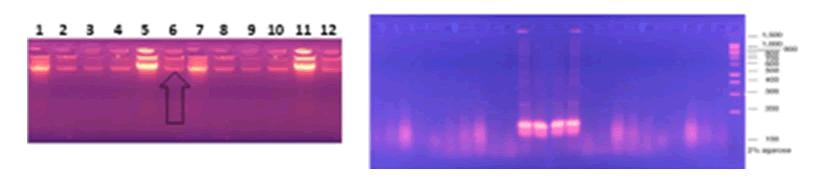
Mainly Polymerase Chain Reaction (PCR) Molecular technology based on DNA amplification by assay. Pathogen detection using PCR is a sensitive method, but it is expensive and time consuming. The polymerase chain reaction technique is the most common molecular method used to detect the presence of the urease gene. This work was designed to test for the presence of the Ure A gene, which encodes urease, in H. pylori isolated from individuals with PUD, utilizing PCR techniques using particular primers for the Ure A gene. The results investigated that two of the twelve H. pylori isolates were positive for urease production (33.3%) Because the enzyme urease contains 5 subunits and Ure A is one of them for more confirmation PCR test was detected of those isolates to examined the Ure A gene, The findings revealed that the Ure A gene included the needed amplicon to achieve the specificity of the primers, in terms of the sensitivity of PCR on DNA taken from a pure culture of H. pylori, isolates Figure 5 show PCR for the Ure A gene.
Figure 5: PCR Analysis Subjecting in Gel Electrophoresis Staining with ETBR to Detect PCR Amplification of Ure A Gene From twelve H. pylori Isolates.
| PCR | Gender | Probability | |
|---|---|---|---|
| Males No. (%) | Females No. (%) | ||
| Positive | 0 (0.0) | 2 (33.3) | P>0.05 |
| Negative | 6 (100.0) | 4 (66.7) | |
| Total | 6 (100.0) | 6 (100.0) | |
Table 2: Biopsy positivity* PCR* gender.
Biosensor method for detection of bacteria results
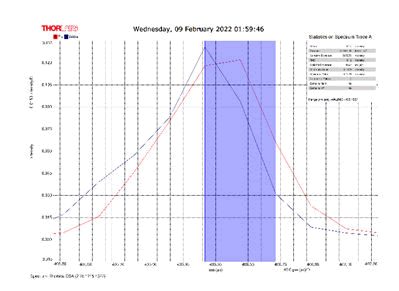
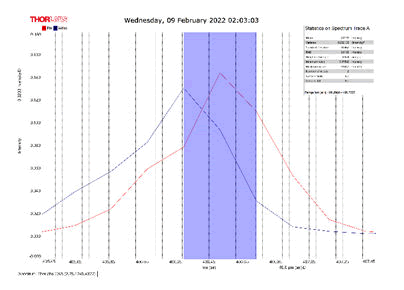
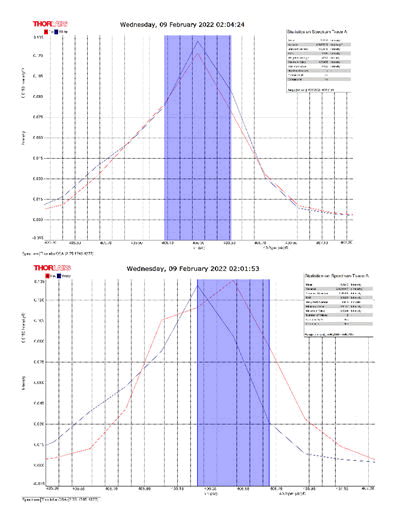
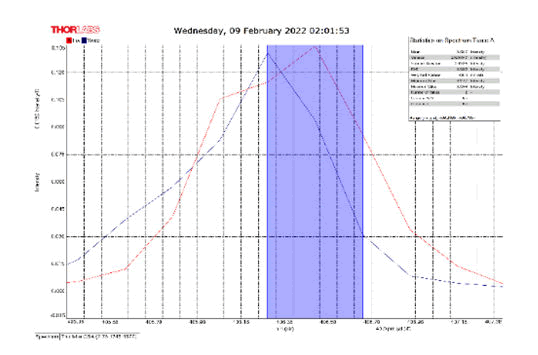
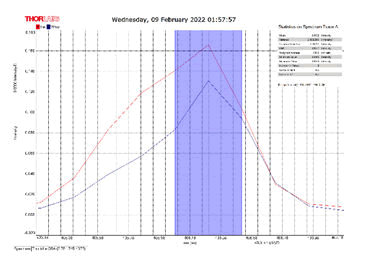
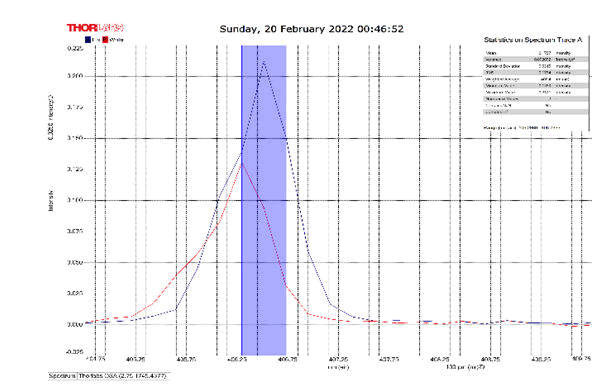
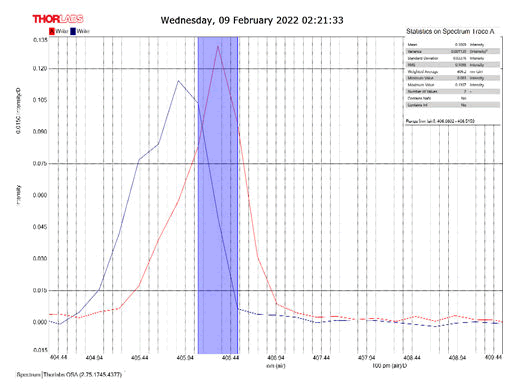
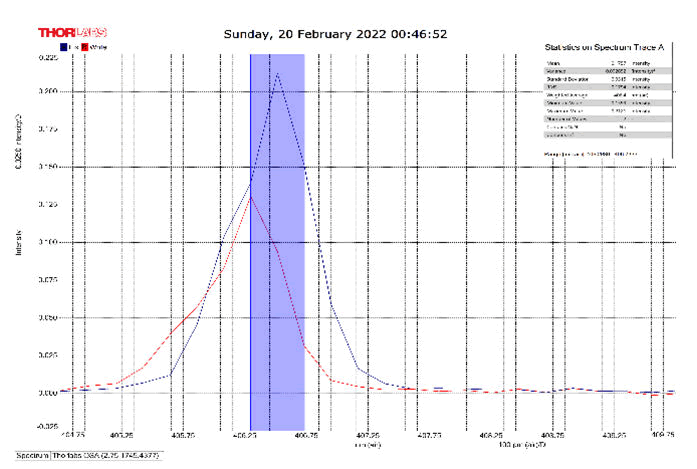
After activating the samples for 24 h, Droplets (50 μm) Broth media are added to the sensor area (no core), The bio-sensor was built based on the inline Mach-Zhinder interference, where the light enters from the source and is reflected from the first splicing area, a part of the light goes to the no core area and the other part of the light passes from the Multi-mode area to the no core then transmitted out. The second splicing area is collimated light which comes out of the Multi-mode area. Effect of antibiotics on H. Pylori how similar effects to those of MIC in the microtiter plate, where the highest peak was for Levofloxacin as shown in Figure 11, which proved its effectiveness in killing H. pylori bacteria, followed by gentamicin and metronidazole equally as shown in Figures 8,9 then omeprazole and amoxicillin as shown in Figures 10,13, and finally, we note the lowest effect of clarithromycin and esomeprazole as shown in Figures 7,6 The intensity of transmitted light is decreased with increase the concentration of H. Pylori as shown in Figure 12 where the more antibiotics affect the H. Pylori which cause a decrease of the concentration of bacteria increasing the intensity of transmitted light and increase the transmitted wavelength [19]. Table 3 shows bio statistical analysis of the effect of antibiotics on H. Pylori which examined optically by the biosensor.
| Antibiotics | culture | mean | variance | Standard deviation | wavelength |
|---|---|---|---|---|---|
| Omeprazole | - | 0.153 | 0.00032 | 0.017 | 406.0-406.5 nm |
| Esomeprazole | - | 0.12 | 8.702 | 0.0029 | 406.2-406.7 nm |
| gentamicin | - | 0.103 | 0.0007 | 0.026 | 406.0-406.5 nm |
| Amoxicillin | - | 0.066 | 3.385 | 0.0058 | 405.8-406.2 nm |
| Levofloxacin | - | 0.175 | 0.0026 | 0.051 | 406.2-406.7 nm |
| Metronidazole | - | 0.124 | 0.00019 | 0.013 | 406.2-406.7 nm |
| Clarithromycin | - | 0.111 | 0.002 | 0.046 | 406.2-406.7 nm |
| Sample 0.5 | positive | 0.106 | 0.001 | 0.033 | 406.3 nm |
| source | - | 0.099 | 0.00044 | 0.021 | 405.9 nm |
Table 3: Biostatistical analysis of antibiotics effect on H. pylori examined optically.
Figure 6: Esomeprazole and sample intensity peak in MNCMF.
Figure 7: Clarithromycin and Sample Intensity Peak in MNCMF.
Figure 8: Gentamicin and Sample Intensity Peak in MNCMF.
Figure 9: Metronidazole and Sample Intensity Peak in MNCMF.
Figure 10: Omeprazole and Sample Intensity Peak in MNCMF.
Figure 11: Levofloxacin and Sample Intensity Peak in MNCMF.
Figure 12: (0.5) Turbidity, Sample Intensity Peak in MNCMF with source.
Figure 13: Amoxicillin and Sample Intensity Peak in MNCMF.
Conclusion
Gastric biopsy samples were collected from patients who suffered from two or more dyspeptic symptoms including (epigastric pain, anorexia, weight loss, acidity, heartburn, nausea, and vomiting) M-NO-M bio-sensor was built based on the inline Mach-Zhinder interference. The intensity of transmitted light is decreased with an increase in the concentration of H. Pylori. Effect of antibiotics shows similar effects to those of MIC in the microtiter plate when examined by optical Biosensor.
Reference
- FerreccioC, RollanA, HarrisPR, et al. Gastric cancer is related to early Helicobacter pylori infection in a high-prevalence country. Cancer Epidemiol Biomarkers Prev 2007; 16:662–667.
- Allaker RP, YoungKA, HardieJM, et al. Prevalence of Helicobacter pylori at oral and gastrointestinal sites in children: Evidence for possible oral-to-oral transmission. J Med Microbiol 2002; 51:312–317.
- BujandaL, Nyssen OP, VairaD, et al. Antibiotic resistance prevalence and trends in patients infected with helicobacter pylori in the period 2013–2020: Results of the european registry on h. pylori management (hp-eureg). Antibiotics 2021; 10:1058.
- EisigJN, SilvaFM, Barbuti RC, et al. Resistencia do Helicobacter pylori aos antibioticos no Brasil: A claritromicina ainda e umu7a boa opcao. Arq Gastroenterol 2011; 48:261–264.
- ValenzuelaM, CerdaO, Toledo Overview on chemotaxis and acid resistance in Helicobacter pylori. Biol Res 2003; 36:429–436.
- Lopes AI, Quiding-JarbrinkM, PalhaA, et al. Cytokine expression in paediatric Helicobacter pylori infection. Clin Diagn Lab Immunol 2005; 12:994–1002.
- YangDC, BlairKM, TaylorJA, et al. A Genome-Wide Helicobacter pylori Morphology Screen Uncovers a Membrane-Spanning Helical Cell Shape Complex. J Bacteriol 2019; 201:00724-18.
- RimbaraE, SuzukiM, MatsuiH, et al. Isolation and characterization of Helicobacter suis from human stomach. Proc Natl Acad Sci 2021; 118:1–8.
- KaoCY, SheuBS, WuHelicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis. Biomed J 2016; 39:14–23.
- RamisMolecular methods for detection of Helicobacter pylori infection: Could they be the gold standard? J Bras Pato e Med Lab 2017; 53:4.
[Crossref]
- TanihNF, OkeleyeBI, NaidooN, et al. Marked susceptibility of South African Helicobacter pylori strains to ciprofloxacin and amoxicillin: Clinical implications. S Afr Med J 2010; 100:49–52.
- MegraudF, LehnN, LindT, et al. Antimicrobial Susceptibility Testing of Helicobacter pylori in a Large Multicenter Trialâ?¯: the MACH 2 Study. Antimicrob Agents Chemother 1999; 43:2747–2752.
- Megraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev 2007; 20:280–322.
- Cavallaro LG, EganB, MorainCO, et al. Treatment of Helicobacter pylori Infection. Helicobacter 2006; 11:36–39.
[Crossref]
- Hernandez C, SerranoC, EinismanH, et al. Peptic ulcer disease in helicobacter pylori-infected children: Clinical findings and mucosal immune response. J Pediatr Gastroenterol Nutr 2014; 59:773–778.
- MungrooNA, Neethirajan S. Biosensors for the detection of antibiotics in poultry industry-A Review. Biosensors 2014; 4:472–493.
- Zhang L, Luo H, Sun Q, et al. Microfiber-Based Inline Mach–Zehnder Interferometer for Dual-Parameter Measurement. IEEE Photonics J 2015; 7:1-8.
- Salman, Ali N, J Tahe HJr, et al. Tapered Splicing Points SMF-PCF-SMF Structure based on Mach-Zehnder interferometer for Enhanced Refractive Index Sensing. Iraqi J Laser 2017; 16:19-24.
- Khalid S, Mohammed ABL, H Al-Ameri. Institute of Laser for Postgraduate Studies Assessment of some hormone level variations using optical sensor. 2021;21.
Author Info
lIsraa ML*, Saqari and Layla MH Al-Ameri
Department of Oral Medicine, College of Dentistry, University of Baghdad, Baghdad, IraqReceived: 03-Jun-2022, Manuscript No. JRMDS-22-57152; , Pre QC No. JRMDS-22-57152; Editor assigned: 07-Jun-2022, Pre QC No. JRMDS-22-57152; Reviewed: 21-Jun-2022, QC No. JRMDS-22-57152; Revised: 04-Aug-2022, Manuscript No. JRMDS-22-57152; Published: 16-Aug-2022