Research - (2021) Volume 9, Issue 1
The Influence of Canal Taper on the Efficacy of Activated EDTA on the Smear Layer Removal from the Mesial Molar Canals (An In vitro study)
Maad Aqeel Mahmood1*, Adel F Ibraheem2 and Saif Alarab A Mohmmed
*Correspondence: Maad Aqeel Mahmood, Department of Restorative and Aesthetic Dentistry, College of Dentistry, University of Baghdad, Iraq, Email:
Abstract
Objectives: The aim of this study is to investigate the effect of canal taper on the efficacy of the sonically activated EDTA to remove the smear layer from the mesial canals of molar teeth.
Material and method: 32 freshly extracted maxillary and mandibular molar teeth are collected, mesio-buccal canals with curvature less than 33% are selected. The samples then will be divided into two groups according to the taper of the preparation taper (n=16).
Group (A): Were prepared with Size 25/.06 of OC, 2Shape (TS2), then subdivided into two subgroups (n=8), based on the final irrigation protocol as following: (A1 with EDTA), (A2 with EDTA and sonic activation.
Group (B): Were prepared with Size 25/.04 of OC, 2Shape (TS1), then subdivided into two subgroups (n=8), based on the final irrigation protocol as following: (B1 with EDTA), (B2 with EDTA and sonic activation.
Standardized canal preparation for all the samples was done as following: 16 canals (Group A) were enlarged to an apical size 25/.06 of OC, 2Shape (TS2), and the other 16 canals (Group B) were enlarged to an apical size of 25/.04 of OC, 2Shape (TS1), instruments were driven with low-speed rotary hand piece at 350 rpm, and 2 Ncm controlled-torque. Then 1.5 ml of 17% EDTA will be used as a final irrigation solution without any type of agitation for both of groups A1 and B1, 1.5 ml of 17% EDTA with sonic activation for 1 minute using Endo Activator (Dentsply, Maillefer, Switzerland). Then all samples were longitudinally cut, and the residual smear layer were examined under scanning electron microscope SEM (Te scan, Vega III, Czech Republic), then the images analyzed according to the scale that was defined by Hulsmann.
Results: The descriptive statistics of the study showed that the mean of smear layer removal in Group A1and Group A2 were (1.37), (1.33) respectively, and in Group B1 and Group B2 were (1.45), (1.08) respectively. Mann-Whitney U test was performed to investigate any significant difference among the main groups; the level of significance is 0.05. The test demonstrated no significant difference between group A and B at all the thirds for both tested subgroups.
Conclusion: There was no influence of file taper on its cleaning efficiency when all the other factors are controlled.
Keywords
Canal taper, Endo activator, 2Shape, EDTA
Introduction
Apical periodontitis, an inflammatory process around the apical part of a tooth root, is primarily a sequel to microbial infection of the pulp space of teeth and is an exceptionally common problem [1]. Removing the vital and or dead pulpal tissue and reducing any irritants that may remain within the root canal system is considered the first goal in any routine endodontic treatment. Hence, root canals cleaning and sanitization are fundamental to attain successful root canal treatmen [2,3].
The three main subsequent steps in any endodontic therapy are:
Access opening preparation of the canal.
Chemo-mechanical preparation to the entire canal space.
Three-dimensional obturation of the canal [4]. It is familiar that the entire root canal system cannot be sufficiently instrumented the peripherally located anatomies (apical third, isthmuses, lateral canals) as they remain unreached while the instrument is rotating or reciprocating along its axis [5]. The unreached peripheral regions can only be approached by introducing irrigant into the canal [6].
The complex anatomy and morphology of accessory canals, lateral canals and isthmuses could remarkably minimize the total disposal of any debris from the root canal system [7]. Therefore, It has been suggested that debridement of the relevant areas is improved by the application of a final irrigation protocol after the chemo-mechanical canal preparation with sufficient amount of chemically active irrigant solutions [8].The irrigation solutions play a role in lubrication, disinfection and cleaning aiding in the eradication of the debrided tissues produced by the root canal shaping procedure and offsetting the bacterial loud to optimize the biomechanical root canal system preparation [9]. Nowadays, chemical solutions such as ethylenediaminetetraacetic acid (EDTA) and sodium hypochlorite (NaOCl) are suggested for the final irrigation to remove the residual organic and inorganic smear layer [10]. Therefore, many attempts are focused on increasing the efficacy of irrigation solution penetration and its interaction with the root canal system [11] as they can improve the smear layer removal. Yet very few studies have examined the influence of canal taper on activated EDTA and its effect on smear layer removal.
It seems that more studies on the protocol of improving the efficiency of debris removal by EDTA are still needed for better understanding of such matter.
Materials and Methods
A total of 32 freshly extracted human maxillary and mandibular molar teeth were collected. The inclusion criterion is the mesial roots with the following criteria:
Fully formed apex.
No internal resorption.
Canals curvature less than 33%.
Absence of root decay.
No previous root canal treatment.
Sound roots without any fractures or visible cracks.
Diagnostic x-ray were taken to confirm these criteria, the samples were collected from local health care centers and private clinics ,the collected teeth were rinsed with water, any remnants of soft tissue and calculus were cleaned, any carious lesions was removed and teeth restored with composite fillings and then stored in closed container containing distilled water [12]. Samples length were set to 16mm measuring from the apex to the midpoint of the crown middle third by partially decorwning them using two-sided diamond disc bur mounted on straight hand piece under water coolant [13]. High speed round bur under water coolant had been used to establish an access opening and enough coronal chamber space were checked using periodontal prob. Working length for each root was determined using k file size 10 (Dentsoly, Maillefer, Switzerland) by observing the appearance of the file apically then subtracting 0.5 mm to prevent any violation to the apical constriction. The degree of root canal curvature obtained using the method described by Schneider for determining canal curvature using only one parameter to define the angle [14]. To standardize the x ray images, a triangle piece of sponge was used to hold the tooth and the x-ray film at a fixed distance from each other and from the x-ray tube, the same sponge was used for all the sample teeth to standardize the x-Ray images and readings. Canals with curvature over 33* were excluded.
The samples had been randomly divided into two main groups (n=16) according to root canal preparation files (Ts1, Ts2), and then each main group was further divided into two subgroups (n=8) according to the used final irrigation protocol as following:
Group A (n=16)
Subgroup A1 (n=8): EDTA
Subgroup A2 (n=8): EDTA+Endoactivator
Group B (n=16)
Subgroup B1 (n=8): EDTA
Subgroup B2 (n=8): EDTA+Endoactivator
To standardization and to ensure closed canal system during irrigation, all teeth were embedded in a plastic tube filled with heavy body silicon (Protesil, Italy) to about 3mm of the crown. Dental surveyor was used to align the long axis of teeth perpendicular to the horizontal plane of the silicon block (1 C). Teeth was marked at 3 mm apical to the coronal end using digital vernier, Figure (1 A), this mark will guide were to stop during teeth embedding. A small hole was made at the plastic tube bottom to allow air escaped during silicon insertion and teeth embedding, also this hole will ease the block removal from the tube after silicon setting.
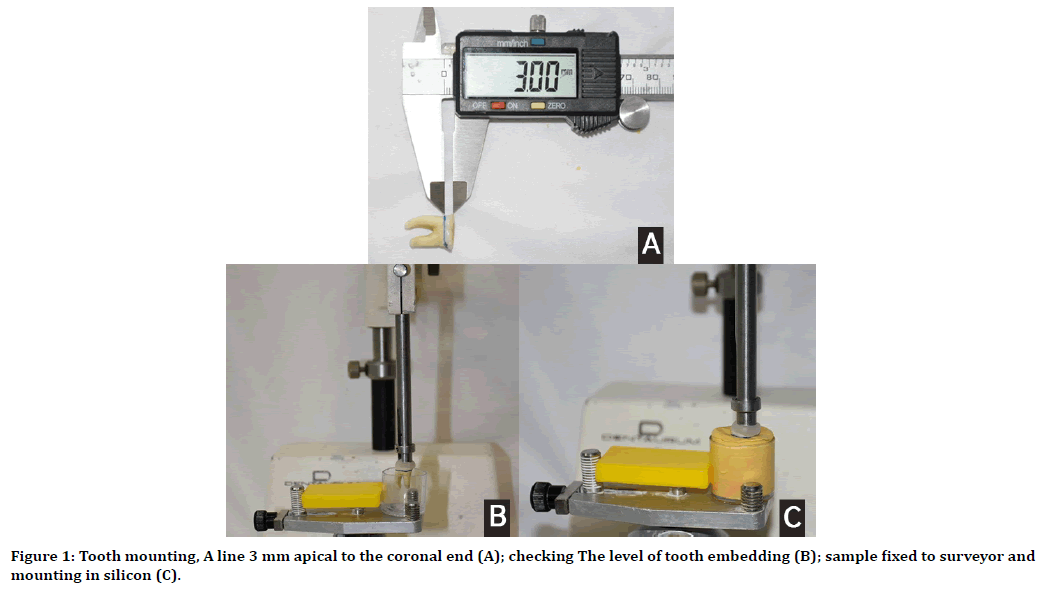
Figure 1. Tooth mounting, A line 3 mm apical to the coronal end (A); checking The level of tooth embedding (B); sample fixed to surveyor and mounting in silicon (C).
The coronal chamber sealed by Teflon and fixed to the surveyor arm using wax, this will prevent any contamination by the wax from reaching the root canals, also before embedding of the teeth, a pre-check to ensure the level of vertical insertion was done with empty plastic tube (1 B).
After the silicon being set, the specimens were then removed from the plastic tube and fixed to the bench as a standard root canal preparation position to avoid any error due to sample movement during instrumentation. At this research, the mesio-buccal canals were instrumented manually by using #10 and #15 K files (Dentsoly, Maillefer, Switzerland) until the latterwas loose, the canal was irrigated by using 1 ml of 5.25% NaOCl (Cerkamed, Poland) during initial instrumentation.
After initial negotiation and establishing of patency; canal preparation had been done as following:
-(Group A) (n=16) canals were enlarged to an apical size 25/06 of OC, 2Shape (TS2) (Micro Mega, France)
-(Group B) (n=16) canals were enlarged to an apical size 25/04 of OC, 2Shape (TS1) (Micro Mega, France)
Instrumentation dynamics and protocol for TS1 and TS2 files:
Progressive movement in three waves (3 up-anddown movements) with upward circumferential filing movement.
Insert the rotating instrument into the root canal until a resistance can be felt. Perform a circumferential brushing movement when feeling the resistance to eliminate the primary constraints. Remove the file from the root canal, clean the canal and irrigate the root canal using 1 ml of 5.25% NaOCl (Cerkamed, Poland) then continue the progressive downward movement.
Two to three cycles are usually sufficient to reach the working length.
Instruments were driven with low-speed rotary hand piece X-Smart IQ® Cordless Motor (DentsplySirona, USA), at 350 rpm, and 2 Ncm controlled-torque. Each sample was instrumented by a new file not only to make the instrumentation safer but also to make it more standardized. Finally, all the above-mentioned groups were flushed using 5 ml distilled water [15], the canals were dried using the same corresponding size paper points.
Following completion of canals preparation, the canals of Subgroup A1/B1 (EDTA), were finally irrigated by 1.5 ml of 17% EDTA (Vista dental products, USA), without any type of agitation [16], the canals of Subgroup A2/B2 (EDTA+EndoActivator), were finally irrigated by 1.5 ml of 17% EDTA [16] with thirty seconds of sonic activation in 2-3 mm strokes using EndoActivator (Dentsply, Maillefer, Switzerland) 2 mm short from the working length (Ruddle 2008). For all the above mentioned irrigation solutions, a 27 gauge side vented needle attached to 5 ml disposable syringe was used, a new needle was used for each sample for the purpose of standardization. The needle was inserted down till 2 mm from the apex and the solution was manually pressed at constant speed. Then, the canals were flushed with 5 ml distilled water [15] and dried with the corresponding paper point.
While holding the peripheries of each root by a pair of pillars, the roots were longitudinally grooved by using a diamond disc, on the lingual and buccal surfaces both to prevent further contamination and to facilitate postinstrumentation vertical splitting with a chisel [15]. It was necessary to prevent penetration of the disc into the canal space to avoid the risk of creating artificial debris. Then, a chisel was used to split each root into two halves with conserving and coding the half that contained the most visible part of the apex.If any specimen show an irregular cleavage or grooves penetration into the canal, after splitting procedure, it would have been discarded and replaced by a new one. Marchesanet 2008 protocol in fixation and dehydration were used. First, the samples were immersed in 2.5% buffered solution of glutaraldehyde (EOBA CHEMIE PVT, India) and 0.1 ml of (pH=7.4) sodium cacodylate (BDH Chemicals Ltd, England) at 4°C and leave it for 12 hr., then rinse them for 3 min. with distilled water, then soak them for 1 hr. in distilled water. After that ascending graded ethyl alcohol baths were used to to dehydrate the samples in the following order: 25% (20 min.), 50% (20 min.), 75% (20 min.), 95% (30 min.), and finaly with 100% (60 min.). After that they left for 24 hr. to dry, then were fixed on aluminum stubs after metallized with a layer of gold Then the samples were examined using SEM (TESCAN, Vega III, Czech Republic) at coronal, middle, and apical third then observed under 3000x magnification for smear layer evaluation.
Two separated trained persons blindly evaluate SEM images for smear layer level with a five scores index according to the scale that was defined by Hulsmann et al. [17] the examination images were analyzed as the following:
Absence of smear layer, all dentinal tubules open.
Little amount of smear layer, most dentinal tubules open.
Most of the root canal wall is covered by a homogenous smear layer, few dentinal tubules open.
The whole root canal wall is covered by a homogenous smear layer, no open dentinal tubules.
The whole root canal wall is covered by heavy, non-homogenous smear layer.
Below are samples of sem images of each subgroup:
Results
Table 1 shows the results of the descriptive statistics for all samples groups, which included the mean, the standard deviation, the standard error, and the minimum and maximum values.
| Group | Ts2 ( A) | Ts1 ( B) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Third | A | M | C | A | M | C | ||||||
| Sub Group | (A1) EDTA | (A2) EDTA +Sonic | (A1) EDTA | (A2) EDTA +Sonic | (A1) EDTA | (A2) EDTA +Sonic | (B1) EDTA | (B2) EDTA +Sonic | (B1) EDTA | (B2) EDTA +Sonic | (B1) EDTA | (B2) EDTA+Sonic |
| N. | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Mean | 1.38 | 1.63 | 1.5 | 1.25 | 1.25 | 1.13 | 1.88 | 1.25 | 1.63 | 1 | 1.13 | 1 |
| Sd. | 0.52 | 0.52 | 0.53 | 0.46 | 0.46 | 0.35 | 0.64 | 0.46 | 0.52 | 0 | 0.35 | 0 |
| Min. | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Max. | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 2 | 2 | 1 | 2 | 1 |
Table 1: Descriptive statistics for all groups.
Table 1 shows that the highest mean of smear layer within group A was the coronal area of subgroup A2 scoring (1.63), whereas the coronal area of subgroup A2 had the lowest mean of smear layer level scoring (1.00). For group B, the apical area of subgroup B1 had the highest mean of smear layer level scoring (1.88), whereas both the middle and the coronal and middle areas of subgroup B2 had the lowest mean of smear layer level scoring (1.00).
As shown in table 2, to see whether these differences in means of smear layer scores for subgroups was significant or not, Mann Whitney U test was performed to investigate any significant difference among the main groups; the level of significance is 0.05. The test shows no significant difference between the corresponding subgroups at each main group whatever the position of scoring. Table 3 shows a comparison of the differences among each subgroup smear layer score level means at each third within each main group.
| Groups | EDTA | EDTA +Sonic | ||||
|---|---|---|---|---|---|---|
| Tests | Mann-Whitney U | P. value | Significance | Mann-Whitney U | P Value | Significance |
| Third | ||||||
| A | 18.5 | 0.112 | NS | 20 | 0.143 | NS |
| M | 28 | 0.626 | NS | 24 | 0.143 | NS |
| C | 28 | 0.535 | NS | 28 | 0.317 | NS |
Table 2: Mann-Whitney U between the main groups.
| Main group | Root third | Kruskal Wallis | Mann-Whitney U |
|---|---|---|---|
| EDTA Vs. EDTA+Endoactivator | |||
| Ts2 (A) | A | 0 | 0.317 |
| M | 0 | 0.333 | |
| C | 0 | 0.535 | |
| Ts1 (B) | A | 0 | 0.045 |
| M | 0 | 0.009 | |
| C | 0 | 0.312 |
Table 3: Kruskal Wallis and Mann-Whitney U tests of smear layer within each tested group.
Apical area
There was a significant difference (P≤0.05), in the mean levels of smear layer between subgroup (B1) and (B2) as subgroup (B2) (EDTA+ EndoActivator) shows lower mean of smear layer level.
Middle area
There was a significant difference (P≤0.05), in the mean levels of smear layer between subgroup (B1) and (B2) as subgroup (B2) (EDTA+ EndoActivator) shows lower mean of smear layer level.
Coronal area
There was no significant difference (P>0.05), in the mean levels of smear layer between both subgroups within each main groups.
Discussion
Cleaning and shaping of the root canal system play a key role in successful endodontic treatment [18]. Initially, root canals are usually cleaned by mechanical instrumentation, in which rotary nickel–titanium files and/or hand stainless-steel files are used to remove the large bulk of the canal content leading to generate large number of debris and smear layer [19]. The components of the smear layer include remnants of ground dentin, odontoblastic processes, and pulp tissue in addition to bacteria and microbes that are present in infected teeth [20]. Clinician opinions regarding the smear layer are controversial [21]. However, it has been concluded that in the presence of smear layer, the root canal filling undergoes both leakage and compromised sealing efficiency [22]. The currently suggested procedure to eliminate the organic and inorganic components of the smear layer is by finally irrigating the root canal with a chelating agent, like NaOCl and EDTA [2].
The efficiency of cleaning is usually assessed by using SEM, which helps in examining the whole area of the canal. The images of SEM are analyzed by utilizing a specific numeric score to evaluate the amount of smear layer. The main drawback of utilizing SEM is that it provides a two-dimensional assessment of the walls of the canals [23].
The influence of canal taper
This study shows that there was no significant difference in smear layer removal efficiency between group A(Ts2) and B(Ts1) at all the thirds, which reveals that the file taper shows no effect on the amount of smear layer removal when all the other factors are controlled. The small size of the used files (25) might contribute to this finding and this in total agreement with the study done by Akhlaghi et al. who reported that the smear layer removal efficacies of files size 25.04 and 25.06 were not significantly different [24]. On the other hand, Akhlaghi et al found that there was a significant difference between files size 30.04 and 30.06 on smear layer removal [24], these findings could be explained by the increased volume, more advance penetration, and the better flushing ability of the irrigation solution due to the increased size/taper of the file [25]. Like Akhlaghi et al. Srirekha et al. found that 30.06 files size resulted in better irrigation penetration than 30.04 files size without mentioning how the smear layer removal was affected by this irrigation penetration [26].
The influence of sonic activation of edta on smear layer removal
This study shows that in comparison to distilled water, the introduction of EDTA as a final irrigation solution demonstrated a considerable improvement in smear layer removal at all the canal thirds of both main groups. This finding agrees with many of previous studies as in 2010, Uroz et al reported that using 17% EDTA solution as a final irrigant might contributed to the most effective smear layer removal, and without using this solution, the smear layer was seen to cover the coronal, middle, and apical thirds of the root canal surface [27].
Similarly, Ahmetoglu et al. suggested that to remove the smear layer, EDTA solution should be used for the final irrigation of the root canal, regardless of the used irrigation technique [28]. Furthermore, it was indicated that, disregarding the irrigation technique, using NaOCl alone failed to remove the smear layer whereas combining NaOCl and EDTA resulted in partial or complete elimination of the smear layer [28]. The superior role of the NaOCl/EDTA combination solution versus NaOCl alone was also suggested.
The results of this study support the findings of other previous studies and highlight the key role of chelating or acid solutions in removing the smear layer during root canal preparation [29].
The influence of sonic activation on smear layer removal
In group A (Ts2), sonic activation of EDTA with EndoActivator exhibited slight improvement in smear layer removal at all the thirds, and the difference was statistically insignificant, these findings agree with the results of previous studies which concluded that the smear layer removal ability of NaOCl/EDTA could not be enhanced by EndoActivator [27].
In group B (Ts1), sonic activation of EDTA with EndoActivator demonstrated a statistically significant improvement in smear layer removal at all the thirds. The high efficiency of Endoactivator in eliminating the smear layer was also suggested in a study by Khaord et al, which ranked the irrigation techniques according to their efficacy in smear layer elimination [30]. Khaord et al. reported that Endoactivator was the most efficient technique, which was followed by passive ultrasonic irrigation, manual dynamic activation, and simple syringe irrigation respectively [30]. Similarly, Karade et al. stated that EndoActivator was more beneficial in removing the smear layer than simple syringe irrigation alone [31], which can be explained by the improvement in irrigation output due to the clearer intacanal fluid produced by sonic agitation [32].
In 2018, a clinical trial by Shalan and Al-Huwaizi revealed that EndoActivator did not efficiently remove the smear layer, particularly at the apical areas of the root canal [33]. This limited efficiency was explained by the limited available space for the tip of the EndoActivator to agitate the irrigation solution [33].
In this study, the difference in the EndoActivator efficacy between the main groups can be explained by the larger taper in group A compared with group B, 6% and 4% respectively. In group A, sonic activation effect was less significant as the larger taper allowed for larger volume and better penetration of the irrigation solution at all the thirds despite the sonic activation while in group B, sonic activation effect was more noticeable as it overcame the lesser volume and penetration of the irrigation solution due to smaller taper.
Conclusion
There were no differences of file taper on its cleaning efficiency when all the other factors are controlled.
References
- Figdor D. Microbial aetiology of endodontic treatment failure and pathogenic properties of selected species. Aust Endo J 2014; 30:11-14.
- Haapasalo M, Endal U, Zandi H, et al. Eradication of endodontic infection by instrumentation and irrigation solutions. Endodontic Topics. 2005; 10:77-102.
- Ballal NV, Moorkoth S, Mala K, et al. Evaluation of chemical interactions of maleic acid with sodium hypochlorite and chlorhexidine gluconate. J endodont 2011; 37:1402-1405.
- Cohen S, Burns RC, Walton R, et al. Pathways of the pulp. Learning 1998; 30:10.
- Peters, Ove A, Andres Laib, et al. Changes in root canal geometry after preparation assessed by high-resolution computed tomography. J Endod 2011; 27:1-6.
- Macedo RG, Robinson JP, Verhaagen B, et al. A novel methodology providing insights into removal of biofilm‐mimicking hydrogel from lateral morphological features of the root canal during irrigation procedures. Inter Endod J 2014; 47:1040-1051.
- Wu MK, Wesselink PR. A primary observation on the preparation and obturation of oval canals. Int Endod J 2011; 34:137-141.
- Ballal NV, Kandian S, Mala K, et al. Comparison of the efficacy of maleic acid and ethylenediaminetetraacetic acid in smear layer removal from instrumented human root canal: A scanning electron microscopic study. J Endodont 2009; 35:1573-1576.
- Siqueira J, Alves JF, Versiani FR, et al. Correlative bacteriologic and micro–computed tomographic analysis of mandibular molar mesial canals prepared by self adjusting file, reciproc, and twisted file systems. J Endod 2013; 39:1044-1050.
- Vasconcelos BCD, Luna-Cruz SM, De-Deus G, et al. Cleaning ability of chlorhexidine gel and sodium hypochlorite associated or not with EDTA as root canal irrigants: A scanning electron microscopy study. J Applied Oral Sci 2007; 15:387-391.
- Druttman ACS, Stock CJR. An in vitro comparison of ultrasonic and conventional methods of irrigant replacement. Int Endod J 1989; 22:174-178.
- da Silva LAB, Sanguino ACM, Rocha CT, et al. Scanning electron microscopic preliminary study of the efficacy of smear clear and EDTA for smear layer removal after root canal instrumentation in permanent teeth. J Endod 2008; 34:1541-1544.
- Dagna A, Gastaldo G, Beltrami R, et al. F360 and F6 skytaper: SEM evaluation of cleaning efficiency. Annali Stomatologia 2015; 6:69.
- Schneider Sam W. A comparison of canal preparations in straight and curved root canals. Oral Surg Oral Med Oral Pathol 1971; 32:271-275.
- Jimna MM, Ashwini TS, Sowmya HK, et al. Comparison and evaluation of two reciprocating root canal instruments on removal of smear layer by using two irrigants at apical one-third of the root canal-an ex vivo-scanning electron microscopic study. J Conserv Dent 2017; 20:451.
- Guo X, Miao H, Li L, et al. Efficacy of four different irrigation techniques combined with 60 C 3% sodium hypochlorite and 17% EDTA in smear layer removal. BMC Oral Health 2014; 14:1-6.
- Hülsmann M, Rümmelin C, Schäfers F. Root canal cleanliness after preparation with different endodontic handpieces and hand instruments: a comparative SEM investigation. J Endodont 1997; 23:301-306.
- Hales JJ, Jackson CR, Everett AP, et al. Treatment protocol for the management of a sodium hypochlorite accident during endodontic therapy. Gen Dent 2001; 49:278-281.
- Jeon IS, Spångberg LS, Yoon TC, et al. Smear layer production by 3 rotary reamers with different cutting blade designs in straight root canals: a scanning electron microscopic study. Oral Sur Oral Med Oral Pathol Oral Radiol Endodontol 2003; 96:601-607.
- Dotto SR, Travassos RM, De Oliveira EP, et al. Evaluation of ethylenediaminetetraacetic acid (EDTA) solution and gel for smear layer removal. Australian Endodont J 2007; 33:62-65.
- Hata G, Hayami S, Weine FS. Effectiveness of oxidative potential water as a root canal irrigant. Inter Endod J 2001; 34:308-317.
- Lui JN, Kuah HG, Chen NN. Effect of EDTA with and without surfactants or ultrasonics on removal of smear layer. J Endodont 2007; 33:472-475.
- Topçuoğlu HS, Akti A, Düzgün S, et al. Effectiveness of different irrigation procedures for removal of dentin debris from a simulated internal resorption cavity. Int J Artificial Organs 2015; 38:165-169.
- Akhlaghi NM, Dadresanfar B, Darmiani S, et al. Effect of master apical file size and taper on irrigation and cleaning of the apical third of curved canals. J Dentistry 2014; 11:188.
- Shuping, George B, Ørstavik D, et al. Reduction of intracanal bacteria using nickel-titanium rotary instrumentation and various medications. J Endod 2000; 26:751-755.
- Srirekha AP, Shrivastava R, Vijay A, et al. Effect of apical preparation size and taper on irrigant penetration in apical third of root canal using two different endodontic needles: An In Vivo study. J Dent Oral Biol 2017; 2:1083.
- Uroz-Torres D, González-Rodríguez MP, Ferrer-Luque CM. Effectiveness of the endoactivator system in removing the smear layer after root canal instrumentation. J Endodont 2010; 36:308-311.
- Ahmetoglu F, Keles A, Yalcin M, et al. Effectiveness of different irrigation systems on smear layer removal: A scanning electron microscopic study. Euro J Dent 2014; 8:53.
- Mancini M, Armellin E, Casaglia A, et al. A comparative study of smear layer removal and erosion in apical intraradicular dentine with three irrigating solutions: A scanning electron microscopy evaluation. J Endodont 2009; 35:900-903.
- Khaord P, Amin A, Shah MB, et al. Effectiveness of different irrigation techniques on smear layer removal in apical thirds of mesial root canals of permanent mandibular first molar: A scanning electron microscopic study. J Conservative Dent 2015; 18:321.
- Karade P, Johnson A, Baeten J, et al. Smear Layer Removal Efficacy Using Endoactivator And Endoultra Activation Systems: An Ex Vivo SEM analysis. Compendium of continuing education in dentistry (Jamesburg, NJ: 1995). 2018; 39:9-12.
- Mancini M, Cerroni L, Iorio L, et al. FESEM evaluation of smear layer removal using different irrigant activation methods (EndoActivator, EndoVac, PUI and LAI). An in vitro study. Clin Oral Investigations 2018; 22:993-999.
- Shalan LA, Al-huwaizi HF. Cleaning efficiency of root canal after irrigation with new irrigation technique: A scanning electron microscopic study. Iran Endod J 2018; 13:102.
Author Info
Maad Aqeel Mahmood1*, Adel F Ibraheem2 and Saif Alarab A Mohmmed
1Department of Restorative and Aesthetic Dentistry, College of Dentistry, University of Baghdad, Iraq2Head of Conservative and Esthetic Dentistry Department, College of Dentistry, University of Baghdad, Iraq
3Iraq
Citation: Maad Aqeel Mahmood, Adel F Ibraheem, The Influence of Canal Taper on the Efficacy of Activated EDTA on the Smear Layer Removal from the Mesial Molar Canals (An in vitro study), J Res Med Dent Sci, 2021, 9 (1): 192-199.
Received: 01-Dec-2020 Accepted: 23-Dec-2020
