Research - (2020) Volume 8, Issue 4
The Effects of Commiphora Myrrh Mouthwash Verses Chlorhexidine on Dental Plaque and Gingivitis: A Comparative Study
Reem A Alotaibi, Salwa Aldahlawi and Fatimah M Alyami*
*Correspondence: Fatimah M Alyami, Department of Basic and Clinical Oral Sciences, Faculty of Dentistry, l-Abdia campus, 21955, Makkah, Saudi Arabia, Email:
Abstract
Introduction: Plaque induced gingivitis is a precursor to periodontitis. Mechanical plaque control alone is insufficient to eliminate dental plaque. Herbal mouthwash can adjunct self-performed plaque control, but clinical effectiveness needs to be evaluated.
Aims: To evaluate the effectiveness of Myrrh mouthwash in reducing gingival inflammation and plaque accumulation in comparison with Chlorhexidine.
Methods and Methods: Seventy-five patients diagnosed with gingivitis were randomly assigned to two groups (Commiphora Myrrh mouthwash) and (0. 2% Chlorhexidine). Patients were instructed on the use of the mouthwash including quantity, frequency, and duration of use. Gingival index (GI) and plaque control record (PCR) were obtained at pre-treatment and two weeks follow up visit. A feedback questionnaire was used to assess compliance and report side effects.
Results: Both groups showed improvement in oral hygiene after the mouthwash use. The GI of the Myrrh group was reduced from 1.3 ± 0.5 at initial exam to 0.3 ± 0.38 (p=0.0001). PCR index was also significantly lower than the initial examination (81.6 % ± 23.5 vs 24.2% ± 22 p=0.0001). Intergroup comparison at two weeks, Chlorhexidine showed significantly lower PCR (13% ±21.2 vs. 24.2 ± 22.1, p=0.03) and GI (0.1 ± 0.2 Vs. 0.3±0.38270, p=0.01). The feedback questionnaire showed more compliance and reported minimal side effects with Myrrh mouthwash use. Chlorhexidine group reported taste alteration, staining and uncomfortable sensation more frequently.
Conclusion: Myrrh-based mouthwash is an effective method to improve oral hygiene. it demonstrated clinical effectiveness in reducing dental plaque and gingival inflammation on the short term with minimal side effects. Key messages: Myrrh mouthwash is an effective antiplaque and anti-gingivitis agent. It can be used as an adjunct to mechanical plaque control in the treatment of periodontitis patients with a fewer reported side effect and a high compliance rate. However, safety over a longer duration of use needs to be evaluated.
Introduction
Dental plaque accumulation initiates inflammatory changes in the gingival tissue leading to gingivitis and subsequently periodontal breakdown. Therefore, elimination of dental plaque and prevention of its formation is essential for the treatment of periodontal disease [1]. Self-performed mechanical plaque control alone is insufficient to eliminate dental plaque completely [2]. Studies showed the addition of antimicrobial agents is beneficial to augment biofilm control [3]. Mouthwashes or mouth rinses can reach areas that are hard to reach with a toothbrush or a floss. They are used for antiinflammatory or anti-septic purposes, or simply for mouth refreshing. In recent years, herbal mouthwash use is on the rise due to increased interest in alternative medicine. However, their effectiveness in controlling plaque, gingivitis, and mouth odour is controversial [4].
Chlorhexidine gluconate (CHX) is a well-studied antiplaque and anti-gingivitis agent. It has a strong antibacterial action through its ability to disturb bacterial cell wall causing cell death [5]. CHX is recognized for its substantivity to dental structures resulting in a slow release and prolonged chemotherapeutic effect [6]. CHX mouthwash is available in several concentrations with a comparable therapeutic effect. However, its use has been associated with multiple side effects such as taste alteration, burning sensation, gingival irritation and tongue and teeth discoloration [7]. The numerous side effects limit the use of CHX and negatively affects patient compliance to the recommend oral hygiene practices [3]. CHX typically prescribed when short term plaque control is needed following surgical procedures or in patients with advanced periodontal disease but not as a long term, daily home care agent [8].
The Myrrh derived its name from the Arabic word “mur”, which means bitter. Fundamentally Myrrh is an oleo-gum resin extracted from the tree Commiphora molmol consists of volatile oil (Myrrhol), resin (Myrrhin), gum and impurities [9,10]. Myrrh contains many active ingredients with strong anti-inflammatory effects such as 1(10), 4-furanodien-6-one (78) which significantly reduces the levels of pro-inflammatory cytokines IL-6, IL- 23, IL-17, TGF-B, and INF-gamma induced by lipopolysaccharide [11]. In addition, Myrrh has an antimicrobial effect against streptococcus mutans, [12], Staphylococcus aureus and Candida albicans which are common oral pathogens. It was as effective as CHX in decreasing microbial load after one week of use as a mouthwash [3,13,14]. Myrrh also promoted oral wound healing [9] and was an effective over the counter remedy for the treatment of aphthous ulcers [15]. Studies that compared the clinical effect of Myrrh on plaque accumulation or gingival inflammation when used to augment oral hygiene practices are scarce [16]. This study aimed to evaluate the effectiveness of Myrrh mouthwash in reducing gingival inflammation and plaque accumulation in dental patients in comparison with Chlorhexidine mouthwash.
Subjects and Methods
Overview
This study is a randomized clinical trial to evaluate the effect of Myrrh containing mouthwash on gingival inflammation and plaque accumulation in patients with gingivitis and compare it to CHX mouthwash. All subjects provided an informed written consent before enrolment in the study. Study design has been approved by Institutional Review Board at Umm Al-Qura University, l-Abdia campus, 21955, Makkah, Saudi Arabia, at 1st of January 2019 with approval number as (119-19).
Study sample
Subjects were recruited from patients seeking treatment at Umm Alqura University Dental Hospital. Selection criteria included: Medically healthy adults, above the age of 18 years who are diagnosed with gingivitis or mild periodontitis and have a minimum of 20 teeth present. Smokers, pregnant women, or those who require antibiotic prophylaxis were excluded from the study. As well as patients who were diagnosed with moderate or severe periodontitis.
All subjects received a comprehensive periodontal examination. Plaque accumulation was evaluated following the use of a disclosing agent [16]. Each tooth surface is examined by the tip of the dental explorer for soft accumulations at the gingival margins. Plaque control record (PCR) is calculated by dividing the number of plaques containing surfaces by the total number of available surfaces.
Gingival inflammation was evaluated using the gingival index (GI) [17]. In brief, the GI is assessed at four sites around each tooth (Buccal, Lingual, Distal, and Mesial).The assessment is as follows: 0=Healthy or normal gingiva, 1=Mild inflammation -mild color change, slight edema and without bleeding on probing (BOP), 2=Moderate inflammation, redness, edema and BOP, 3=Sever inflammation, redness, edema, ulceration and spontaneous bleeding.
All subjects received non-surgical periodontal treatment, oral hygiene education, prophylaxis and scaling and root planning as determined by the need of their periodontal status. Subjects were randomly assigned to experimental or control group. Experimental group received Myrrh mouthwash (Weleda, Nature Certified Natural, Switzerland); while the control group received CHX Mouthwash (0.2% Chlorhexidine Gluconate (National pharmaceuticals factory co., Riyadh. Saudi Arabia).
Following the completion of the periodontal debridement, all subjects were given the following oral hygiene instructions:
Tooth brushing with soft toothbrush and fluoridated toothpaste twice a day for two minutes.
Interdental tooth flossing once daily.
Instructions for mouthwash use: use the mouthwash after toothbrushing twice a day. Switch 10 ml of the mouthwash in a half cup of water for at 30 seconds and spit it out without swallowing.
Follow up examination was done two weeks after the completion of the periodontal debridement and GI and PCR were obtained. Also, all subjects were given a feedback questionnaire to document their experience using the mouthwash and record any side effects. The questionnaire consisted of 10 questions. The first four questions evaluated participants compliance with the mouthwash use and the instructions given at the initial visit. Questions 5-10 addressed different possible side effects of the mouthwash use.
Statistical analysis
The collected data were analyzed using SPSS program. Descriptive analysis, student T test were used for intra-group comparison and inter groups analysis. Additionally, Chi-square test used to analyze data from the given questionnaire. p value<0.05 was considered significant.
Results
Seventy-five subjects participated in the study. The average age of the participants was 34 years (range 18-50 years). Thirty-nine (52%) patients were females and 36 (48%) were males. Participants were divided in to 2 groups: Experimental group received Myrrh-based mouthwash (n=45, 60%) and control group received Chlorhexidine mouthwash (n=30, 40%). Demographic data of the groups are presented in Table 1.
| Characteristic | Total participants | Myrrh group | CHX group |
|---|---|---|---|
| Number (%) | 75 | 45 (60%) | 30 (40%) |
| Age (range) yrs | 34 (18-50) | 34 (18-50) | 34.5 (21- 48) |
| Gender: Female (n, %) | 39 (52%) | 23 (51%) | 14 (46%) |
| Gender: Male (n, %) | 36 (48%) | 22 (49%) | 16 (54%) |
Table 1: Demographic data of all participants.
Initial examination
At the initial examination, all subjects presented with poor oral hygiene and gingivitis. Plaque covered most of the teeth surfaces, the average PCR was 75% ± 27.7. Most teeth have signs of inflammation and the mean GI was 1.3 ± 0.53. The experimental group had significantly more plaque accumulation (PCR 81.6 ± 23.5% as compared with 65 ± 30.7 % in the control group (p=0.01). However, both groups had similar gingivitis level (1.3 ± 0.5 and 1.2 ± 0.5 for experimental and control group respectively, (p=0.67).
Final examination
Both groups showed a significant improvement of oral hygiene at the two weeks followup examination. This was reflected as a reduction in both GI and PCR indices. The GI of the experimental group 0.3 ± 0.38 which was significantly lower than the initial exam (p=0.0001). PCR index was also significantly lower than the initial examination (24.2% ± 22.1 p=0.0001). The control group had similar findings with GI in the final examination is 0.11 ± 0.27 and PCR index is 13 % ± 21.2. Both were significantly reduced (p=0.0001).
Comparing both groups, subjects in the control group had on average less teeth surfaces with plaque accumulation compared with experimental group (PCR 13 % ± 21.2 Vs 24.2% ± 22.1 p=0.03). Clinically, both groups showed minimal gingival inflammation. The GI in the control group was significantly lower than the experimental group (0.11 ± 0.27 Vs 0.3 ± 0.38 respectively p=0.01). However, the reduction of GI mean score was similar in the two groups (1.0 ± 0.2 vs. 1.09 ± 0.2 p=0.08) (Figures 1 and 2).
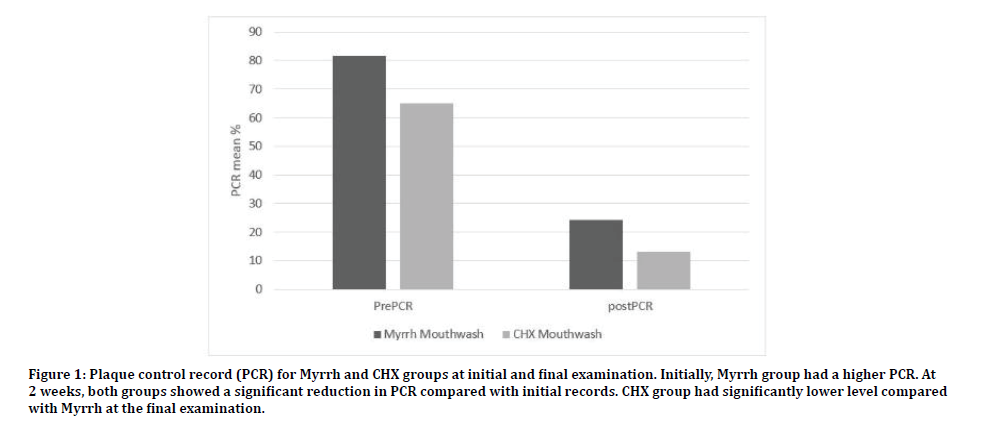
Figure 1: Plaque control record (PCR) for Myrrh and CHX groups at initial and final examination. Initially, Myrrh group had a higher PCR. At 2 weeks, both groups showed a significant reduction in PCR compared with initial records. CHX group had significantly lower level compared with Myrrh at the final examination.
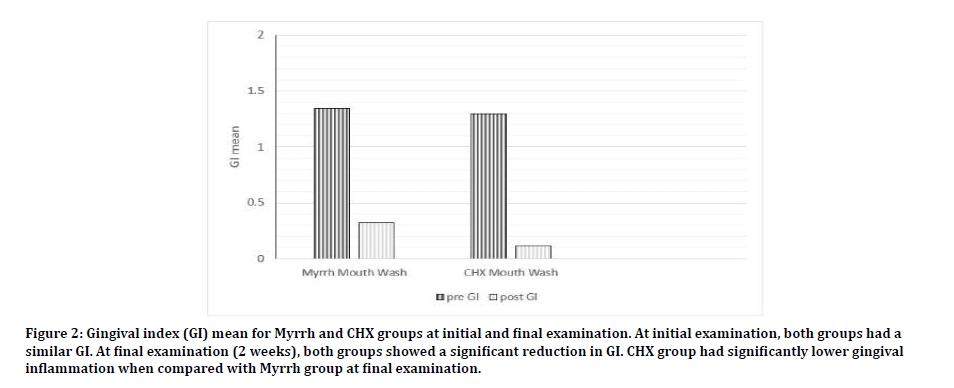
Figure 2: Gingival index (GI) mean for Myrrh and CHX groups at initial and final examination. At initial examination, both groups had a similar GI. At final examination (2 weeks), both groups showed a significant reduction in GI. CHX group had significantly lower gingival inflammation when compared with Myrrh group at final examination.
Patient response questionnaires
A total of 66 questionnaires were completed. Thirty-nine participants in Myrrh mouthwash (86%) and 27 of CHX (90%) were evaluated. Questions and responses are presented in Table 2. Most participants followed the instructions of mouthwash use carefully. However, Myrrh mouthwash users were more compliant (p=0.02). More participants in the Chlorhexidine group did not use the mouthwash daily for the entire study period and one third of them did not use it twice daily as instructed (p=0.04). Side effects were reported more frequently with the Chlorhexidine group. All (100%) of the participants complained of altered taste sensation compared to only 7 % in Myrrh group (p=0.0001)). A significant number of Chlorhexidine users also complained of burning sensation and staining of teeth. On the other hand, less than 10% of Myrrh users reported such side effects. (p= 0.0006 and p=0.002 respectively).
| Question | Myrrh group | CHX group |
|---|---|---|
| Did you used the mouthwash twice or more daily? | 87 | 66* |
| Did you used given mouthwash as instructed? | 82 | 87 |
| Did you used the mouthwash for more than 30 seconds each time? | 77 | 87.5 |
| Did you used the mouthwash daily for 14 days? | 87 | 57* |
| Did you notice any changes in the teeth or gums colour? | 5 | 30† |
| Did you notice taste alteration during mouthwash usage? | 7 | 100 † |
| Did you suffer any uncomfortable sensations (burning, itching, tingling) | 0 | 40† |
| Did you notice plaque accumulation around teeth? | 13 | 16 |
| Did you notice gum bleeding during or after teeth brushing? | 13 | 5 |
| Did the mouthwash cause you nausea or loss of appetite? | 5 | 53† |
*p< 0.05, †p< 0.005
Table 2: Patient’s feedback questionnaire. The data indicate the percentage of positive responses to each item.
Discussion
This study confirmed the added benefits of using an antimicrobial agent for the management of periodontal disease. Myrrh mouthwash improved oral hygiene by reducing plaque accumulation and decreasing gingival inflammation over a short period of use. Statistically significant differences were found between the initial and final examination in both groups. However, intergroup comparison indicates CHX remains the gold stander of chemical biofilm control in periodontal therapy.
Myrrh mouthwash users had plaque scores lowered from initial 84% to 24 % at 2 weeks follow-up. Similar finding was reported by Bassiony et al. In which Myrrh has the greatest reduction in plaque and gingival scores after 3 weeks of use in comparison to Chlorhexidine mouthwash and Miswak based mouthwash [18]. Interestingly, Myrrh significantly reduced plaque while the reduction in gingival score did not reach a significant level. In an experimental gingivitis model, Myrrh mouthwash was effective in reducing plaque score over a period of 14 days; however, this did not reflect on the gingival inflammation [19]. To be considered, subjects in Zahid et al. study refrained from mechanical plaque control and only mouthwash was used as means of oral hygiene [19]. This in contrary to our findings were gingival inflammation was significantly reduced when Myrrh mouthwash was used as an adjunct to mechanical plaque control.
Although Myrrh demonstrated effective outcomes, the Chlorhexidine group had a significantly higher reduction in plaque and gingival index. This is opposite to what Zahid et al. reported in which CHX mouthwash was less effective than Myrrh mouthwash in reducing plaque and gingival index [19]. The small sample size could explain the lack of significant differences in their study. Bassioni et al. also showed a superior result of Myrrh over CHX mouthwash although the difference was not significant [18]. Differences on Myrrh mouthwash concentration could explain the variability in the outcomes as we tested a commercially available product while both Zahid et al. and Bassiony et al. used a customized preparation [18,19].
A few side effects were reported by Myrrh mouthwash users. On the other hand, CHX mouthwash users reported all the characteristic side effects associated with CHX use. Almost all subjects reported altered taste sensation, and one third of them noticed staining of teeth and tongue despite of the short duration of the mouthwash use. Burning sensation was also a common finding. Other studies reported altered taste incidence of 57 % of with the use of 0.2% CHX and an incidence of burning sensation of 52% [3]. The severity of the side effects of CHX is usually proportional to the duration of use [8,20]. Comparing with Myrrh mouthwash, only 7%of the users reported altered taste and only 5 % of Myrrh group noticed dental staining. Overall, there were significantly fewer side effects reported with Myrrh mouthwash use, which is an advantage of Myrrh over CHX mouthwash. To our knowledge, this study is the first to documents the side effects of Myrrh mouthwash. In general, documenting side effects associated with herbal mouthwash use is lacking in the literature [4]. Fewer CHX users used the mouthwash for the entire 2 weeks of the study. The higher incidence of side effects may play a role in patient compliance with oral hygiene instructions [6].
Myrrh is widely used in Saudi Arabia as at home remedy for the treatment of infection [21]. In addition to its anti-inflammatory [22], antiulcer [9] and astringent effect, Myrrh exhibits antibacterial effects on different species [23,24] including oral flora [13]. The marked antibacterial and anti-inflammatory properties of Myrrh could explain the reduction of dental plaque and gingival inflammation observed in this study. Although CXH showed a greater statistical reduction in gingivitis score, GI mean scores were similar between the two groups indicating clinically comparable effects. Since gingivitis is a reversible disease and could be treated effectively by supragingival plaque control [25], Myrrh extract has the potential to be an alternative remedy for daily oral hygiene routine as an adjunct to mechanical plaque control. However, potential toxic effect with the prolonged application of Myrrh must be tested [9].
The present study used aged matched participants with initial comparable level of gingivitis. Smokers and those with severe periodontal disease were excluded from the study and all participants had at least 20 teeth present to control cofounders. All participants were given clear and standard instructions of the oral hygiene and the use of mouthwash, which included the control of the quantity, frequency, and duration of use. This explains the high compliance rate of the participants.
Limitations
The study has many limitations, it evaluated the effectiveness of the mouthwash over a short duration of use and only evaluated clinical signs of inflammation. It did not evaluate other periodontal parameters like reduction in probing pocket depth or clinical attachment level changes. The absence of negative control is another limitation.
Conclusion
Myrrh-based mouthwash is an effective method to improve oral hygiene by controlling plaque accumulation and decreasing gingival inflammation on the short term with minimal side effects expected.
References
- Trombelli L, Farina R, Silva CO, et al. Plaque-induced gingivitis: Case definition and diagnostic considerations. J Periodontol 2018; 89:S46-S73.
- Chapple IL, Van der Weijden F, Doerfer C, et al. Primary prevention of periodontitis: Managing gingivitis. J Clin Periodontol 2015; 42 :71-76.
- Guerra F, Pasqualotto D, Rinaldo F, et al. Therapeutic efficacy of chlorhexidine-based mouthwashes and its adverse events: Performance-related evaluation of mouthwashes added with anti-discoloration system and cetylpyridinium chloride. Int J Dent Hyg 2019; 17:229-36.
- Manipal S, Hussain S, Wadgave U, et al. The mouthwash war chlorhexidine vs. herbal mouth rinses: A meta-analysis. J Clin Diagn Res 2016; 10:81-83.
- Van Strydonck DA, Slot DE, Van der Velden U, et al. Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: a systematic review. J Clin Periodontol 2012; 39:1042-1055.
- Cortellini P, Labriola A, Zambelli R, et al. Chlorhexidine with an anti-discoloration system after periodontal flap surgery: A cross-over, randomized, triple-blind clinical trial. 2008; 35:614-620.
- Gürgan CA, Zaim E, Bakirsoy I, et al. Short-term side effects of 0.2% alcohol-free chlorhexidine mouthrinse used as an adjunct to non-surgical periodontal treatment: A double-blind clinical study. J Periodontol 2006; 77:370-384.
- James P, Worthington HV, Parnell C, et al. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst Rev. 2017; 3:CD008676.
- Al-Mobeeriek A. Effects of myrrh on intra-oral mucosal wounds compared with tetracycline- and chlorhexidine-based mouthwashes. Clin Cosmet Investig Dent 2011; 30:53-58.
- El Ashry ES, Rashed N, Salama OM, et al. Components, therapeutic value and uses of myrrh. Pharmazie 2003; 58:163-168.
- Cao B, Wei XC, Xu XR, et al. Seeing the unseen of the combination of two natural resins, frankincense and myrrh: Changes in chemical constituents and pharmacological activities. Molecules 2019; 24:3076.
- Abu-Obaid E, Salama F, Abu-Obaid A, et al. Comparative Evaluation of the antimicrobial effects of different mouthrinses against streptococcus mutans: An in Vitro study. J Clin Pediatr Dent 2019; 43:398-407.
- Sambawa ZM, Alkahtani FH, Aleanizy FS, et al. Comparison of antibacterial efficacy chlorohexidine gluconate and Saudi myrrh mouthwashes in the oral cavity. Orient J Chem 2016; 32:2605-2610.
- Almekhlafi S, Thabit A, Alwossabi A, et al. Antimicrobial activity of Yemeni myrrh mouthwash. J Chem Pharm Res 2014; 6:1006-1013.
- Burgess JA, Van der Ven PF, Martin M, et al. Review of over-the-counter treatments for aphthous ulceration and results from use of a dissolving oral patch containing glycyrrhiza complex herbal extract. J Contemp Dent Pract 2008; 9:88-98.
- O'Leary TJ, Drake RB, Naylor JE. The plaque control record. J Periodontol 1972; 43:38.
- Loe H, Silness J. Periodontal disease in pregnancy. Acta Odont Scand 1963; 21:533-551.
- Bassiouny G, Al Barrak H. The anti-plaque effect of miswak and myrrh mouthwashes versus chlorhexidine in the treatment of chronic gingivitis: A comparative clinical trial. Med Sci 2014; 9:32-37.
- Zahid TM, Alblowi JA. Anti-inflammatory and anti-plaque effects of commiphora myrrh mouthwash: A preliminary pilot clinical study. Open Dent J 2019; 13:1-5.
- Tartaglia GM, Tadakamadla SK, Connelly ST, et al. Adverse events associated with home use of mouthrinses: A systematic review. Ther Adv Drug Saf 2019; 10:1–16.
- Alsanad S, Aboushanab T, Khalil M, et al. A descriptive review of the prevalence and usage of traditional and complementary medicine among saudi diabetic patients. Scientifica 2018; 2018:6303190.
- Kim MS, Bae GS, Park KC, et al. Myrrh inhibits LPS-induced inflammatory response and protects from cecal ligation and puncture-induced sepsis. Evid Based Complement Alternat Med 2012; 2012:278718.
- Khalil N, Fikry S, Salama O. Bactericidal activity of Myrrh extracts and two dosage forms against standard bacterial strains and multidrug-resistant clinical isolates with GC/MS profiling. AMB Express 2020; 10:21.
- Mahboubi M, Kashani LM. The anti-dermatophyte activity of commiphora molmol. Pharm Biol 2016; 54:720-725.
- Figuero E, Nóbrega DF, García-Gargallo M, et al. Mechanical and chemical plaque control in the simultaneous management of gingivitis and caries: A systematic review. J Clin Periodontol 2017; 44:S116-S134.
Author Info
Reem A Alotaibi, Salwa Aldahlawi and Fatimah M Alyami*
1Department of Basic and Clinical Oral Sciences, Faculty of Dentistry, l-Abdia campus, 21955, Makkah, Saudi ArabiaCitation: Reem A Alotaibi, Salwa Aldahlawi, Fatimah M Alyami, The Effects of Commiphora Myrrh Mouthwash Verses Chlorhexidine on Dental Plaque and Gingivitis: A Comparative Study, J Res Med Dent Sci, 2020, 8 (4):65-70.
Received: 15-Jun-2020 Accepted: 01-Jul-2020 Published: 08-Jul-2020
