Research - (2021) Volume 9, Issue 6
The Effects of Carbamylation on HBA1C in Chronic Renal Failure Patient
*Correspondence: B Shanthi, Department of Biochemistry, Sree Balaji Medical College & Hospital Affiliated to Bharath Institute of Higher Education and Research, India, Email:
Abstract
Chronic renal failure occurs, when disease or disorders damages the kidneys so that they are no longer capable of adequately removing fluids and wastes from the body or of maintaining the proper level of certain kidney - regulated chemicals in the blood stream. It is caused by several diseases and inherited disorders, but the progression of chronic kidney failure is always the same. The kidneys, which serve as the body's natural filtration system, gradually lose their ability to remove fluids and waste products (urea) from the bloodstream. They also fail to regulate certain chemicals in the bloodstream, and deposit protein into the urine. Chronic kidney failure is irreversible, and will eventually lead to total kidney failure, also known as end stage renal disease (ESRD). Without proper treatment intervention to remove wastes and fluids from the bloodstream, ESRD is fatal. Glycated hemoglobin (hemoglobin A1c, HbA1c, A1C, or Hb1c) is a form of hemoglobin that is measured primarily to identify the average plasma glucose concentration over prolonged periods of time. This serves as a marker for average blood glucose levels over the previous 3 months prior to the measurement as this is the half-life of red blood cells. Our present study based on the estimation of HbA1c level in diabetic patients with and without chronic kidney diseases (CKD) and HbA1C on serial monitoring can be marker for assessing renal disease.
Keywords
Chronic renal failure, Inherited disorders HbA1C, Renal disease
Introduction
Chronic kidney failure describes the gradual loss of kidney function or it is a progressive loss in renal function over a period of months or years. Kidneys filter wastes and excess fluids from your blood, which are then excreted in the urine. When chronic kidney disease reaches an advanced stage, dangerous levels of fluid, electrolytes and wastes can build up in our body. In the early stages of chronic kidney disease, you may have few signs or symptoms. Chronic kidney disease may not become apparent until kidney function is significantly impaired. Chronic kidney disease is identified by a blood test for creatinine, which is a breakdown product of muscle metabolism. Higher levels of creatinine indicate a lower glomerular filtration rate and as a result a decreased capability of the kidneys to excrete waste products. Creatinine levels may be normal in the early stages of CKD, and the condition is discovered if urinalysis (testing of a urine sample) shows the kidney is allowing the loss of protein or red blood cells into the urine. To fully investigate the underlying cause of kidney damage, various forms of medical imaging, blood tests, and sometimes a renal biopsy (removing a small sample of kidney tissue) are employed to find out if a reversible cause for the kidney malfunction is present [1].
Screening of at -risk people is important because treatments exist that delay the progression of CKD. In more advanced stages, treatments may be required for anemia and renal bone disease (also called renal osteodystrophy, secondary hyperparathyroidism, or chronic kidney disease - mineral bone disorder (CKD -MBD). Chronic kidney disease resulted in 956,000 deaths in 2013 up from 409,000 deaths in 1990 [2-4]. Treatment for chronic kidney disease focuses on slowing the progression of the kidney damage, usually by controlling the underlying cause. Chronic kidney disease can progress to end -stage kidney failure, which is fatal without artificial filtering (dialysis) or a kidney transplant. The leading cause of death in patients with chronic kidney disease is cardiovascular disease, regardless of whether [5 -7] there is progression to stage 5. While renal replacement therapies can maintain patients indefinitely and prolong life, the quality of life is severely affected [8].
Transplantation aside, high -intensity home hemodialysis appears to be associated with improved survival and a greater quality of life, when compared to the conventional three-timesa- week hemodialysis and peritoneal dialysis. [9] Uremia is a clinical syndrome associated with fluid, electrolyte, and hormone imbalances and metabolic abnormalities, which develop in parallel with deterioration of renal function. Uremia more commonly develops with chronic kidney disease (CKD), especially the later stages, of CKD, but it also may occur with acute kidney injury (AKI) if loss of renal function is rapid. Glycated hemoglobin (hemoglobin A1c, HbA1c, A1C, or Hb1c) is a form of hemoglobin that is measured primarily to identify the average plasma glucose concentration over prolonged periods of time. It is formed in a non -enzymatic glycation pathway by hemoglobin's exposure to plasma glucose. HbA 1 c is a measure of the beta -N-1-deoxy [9-11] fructosyl component of hemoglobin. Normal levels of glucose produce a normal amount of glycated hemoglobin. As the average amount of plasma glucose increases, the fraction of glycated hemoglobin increases in a predictable way. This serves as a marker for average blood glucose levels over the previous 3 months prior to the measurement as this is the half-life of red blood cells.
Materials and Methods
This study was conducted in the Department of Biochemistry, Sree Balaji Medical College and Hospital, Chromepet, Chennai during the period of January 2014 – June 2015 among 50 Diabetic patients with chronic renal failure visiting the nephrology outpatient services of the Department of nephrology and 50 diabetic individuals without chronic renal failure, who came for routine check - up in diabetic outpatient service, department of medicine. The groundwork for the study was started after getting clearance from the research committee and the Institutional human ethical committee (reference number for approval: 002/SBMC/ IHEC/2014 -2) of Sree Balaji Medical College and Hospital, Chromepet, Chennai.
Demographic data, age, gender, height, weight, BMI, duration of type 2 Diabetes Mellitus, general history and medications and blood pressure were recorded. Routine clinical examination was done. The laboratory parameters that were measured include serum urea, serum creatinine, glycated hemoglobin (HbA1C), Fasting plasma glucose (FBS). The inclusion criteria are diabetes patients with chronic renal failure of both sexes (20 – 70 years of age) and diabetic patient without chronic renal failure of both sexes (20– 70 years of age) and exclusion criteria are those with advanced malignancy and those who not regular follow up.
Sample collection
The study was explained to the participants and informed consent obtained from them before taking the blood sample. The blood samples were collected from subjects by venipuncture fasting (8 hours overnight fasting) samples were collected under aseptic precautions. Estimation of Fasting plasma glucose by GOD/ POD enzymatic photometric Method, HbA1 c by Ion Exchange Resin method, Serum Urea By Using Berthelot, Serum Creatinine By Alkaline Picrate Method.
Results and Discussion
Results are described in the form of Tables (Tables 1-3) and Figures (Figures 1-4).
| HBA1C | Urea | Creatinine | FBS | |
|---|---|---|---|---|
| Valid | 50 | 50 | 50 | 50 |
| N Missing | 0 | 0 | 0 | 0 |
| Mean | 7.779 | 25.32 | 0.85 | 166.04 |
| Std. Error of Mean | 0.1461 | 1.4349 | 0.0374 | 2.5948 |
| Std. Deviation | 1.0332 | 10.1467 | 0.265 | 18.348 |
Table 1: Descriptive statistics cases (Group 1).
| HBA1C | Urea | Creatinine | FBS | |
|---|---|---|---|---|
| Valid | 50 | 50 | 50 | 50 |
| N Missing | 0 | 0 | 0 | 0 |
| Mean | 5.444 | 98.26 | 4.039 | 151.4 |
| Std. Error of Mean | 0.0925 | 5.5393 | 0.4164 | 1.8883 |
| Std. Deviation | 0.654 | 39.1693 | 2.9446 | 13.3523 |
Table 2: Descriptive statistics: Cases (Group 2).
| Group | N | Mean | Std. Deviation | Std. Error Mean | |
|---|---|---|---|---|---|
| HBA1C | 1 | 50 | 7.779 | 1.0332 | 0.1461 |
| 2 | 50 | 5.444 | 0.654 | 0.654 | |
| Urea | 1 | 50 | 25.32 | 10.1467 | 1.4349 |
| 2 | 50 | 98.26 | 39.1693 | 5.5393 | |
| Creatinine | 1 | 50 | 0.85 | 0.265 | 0.0374 |
| 2 | 50 | 4.039 | 2.9446 | 0.4164 | |
| FBS | 1 | 50 | 166.04 | 18.348 | 2.5948 |
| 2 | 50 | 151.4 | 13.3523 | 1.8883 |
Table 3: Descriptive statistics-Comparison group statistics.
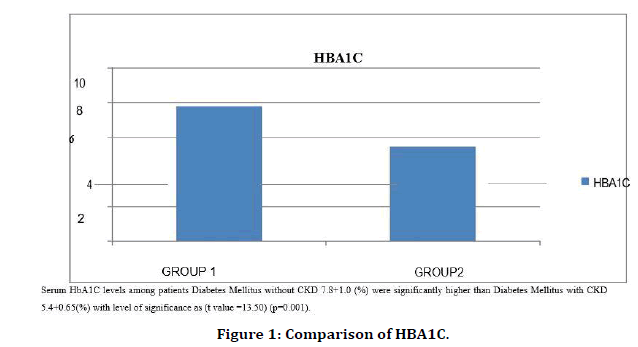
Figure 1. Comparison of HBA1C.
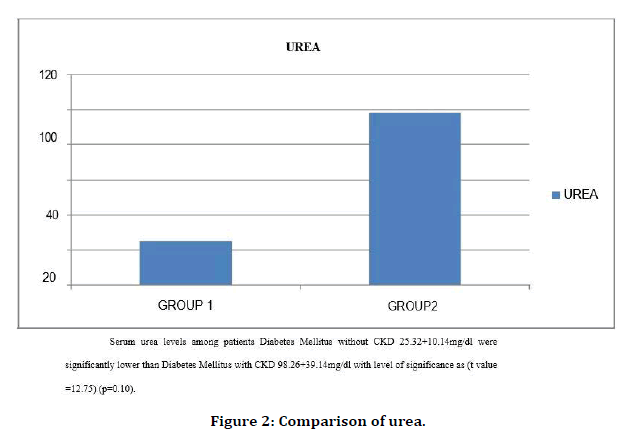
Figure 2. Comparison of urea.
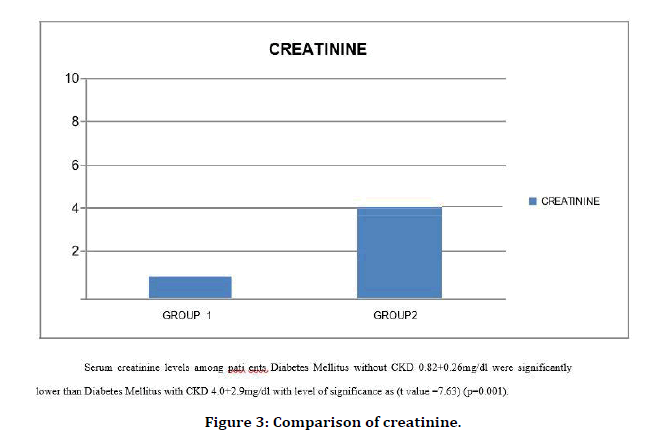
Figure 3. Comparison of creatinine.
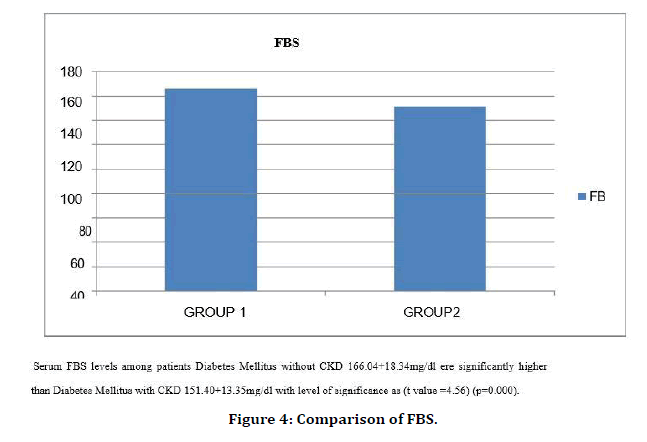
Figure 4. Comparison of FBS.
This study was done on Diabetic with Chronic Renal Failure. Age, Sex matched diabetic individuals were taken as controls. In diabetic patients with end -stage renal disease, erythrocyte lifespan tends to be decreased. This may result in part from iron deficiency anemia, recent transfusions, or other effects of kidney disease on erythrocyte survival. Uremic patients with blood urea nitrogen levels greater than about 85 mg/dL also develop significantly high levels of carbamylated hemoglobin, which interferes with some. HbA1c assays in spite of these complications, HbA1c (measured with an assay that does not cross -react with carbamylated hemoglobin) has been shown to correlate well with average blood glucose levels in diabetic patients on hemodialysis, with questionably significant overestimation of average blood glucose for values of HbA1c greater than 7.5% [12,13]
Other factors, such as chronic alcohol, opioid, and salicylate abuse and ingestion of large amounts of vitamin C and E have been reported to skew A1c results [11,12,14]. Given the lack of large studies, the sometimes-contradictory conclusions of these reports, and the unclear mechanisms of these effects, it is impractical to dismiss A1c as invalid in all these cases. However, knowing that these effects may exist can help guide decision -making in situations in which the index of suspicion for an inaccurate A1c is already high [15-17]. Chronic renal failure develops in many diabetic patients. The role of glycemic control and the value of HbA1c in diabetic subjects with renal disease are controversial. While interference from carbamylated Hb can be evaluated, the role of renal anemia, erythropoietin intake, and other factors in chronic renal failure is more difficult to evaluate. Recent reports suggest HbA1c underestimates glycemic control in diabetic patients on dialysis and that glycated albumin is a more forceful indicator of glycemic control [18-20] . Further studies are needed to clarify the role of HbA1c in diabetic patients with chronic renal failure.
Re-measuring carbamylated hemoglobin after in vitro glycation showed lower levels compared to those obtained from RBCs samples incubated only with the same urea concentrations. This indicated that in presence of a fixed level of urea, carbamylated hemoglobin decreases with increase glucose concentration which can be explained by that glycation of some hemoglobin free amino groups prevents their participation in carbamylation reaction. This result agreed with a previous report6 which demonstrated that carbamylated hemoglobin was significantly lower in diabetic patients and provided further evidence of decrease carbamylation in samples with abnormally high glucose concentrations through in vitro carbamylation experiment using a fixed urea concentration of 70 mg/dl. It is not surprising that the measured HbA1c is elevated in presence of high urea concentration when the former is measured by a method depending on the positive charges on non -altered hemoglobin. We were expecting an attenuation of this elevation at high glucose and urea concentrations due to interference of the two reactions: glycation and carbamylation involving the same amino groups. However, the carbamylation of hemoglobin appeared to add to the measured value of HbA1c almost equally at all concentrations of glucose, depending on the urea concentration. This means that carbamylated hemoglobin was already measured by th is method and expressed as hemoglobin A1c. Therefore, this study represents another evidence on the mutual interference of glycation and carbamylation of hemoglobin witnessed by the significant decrease of valine hydantoin production with increase glucose concentration and the additive effect of urea that produced carbamylated hemoglobin leading to elevation of glycated hemoglobin levels estimated by this cation-exchange based method. The impact of carbamylation on glycation was evaluated using two concentrations of urea (40 and 80 mg/dl) to confirm our results and to help establishment of the equation which can correct the results of cation - exchange based assay methods of glycated hemoglobin in blood samples with abnormally high levels of glucose and urea so that the corrected result reflects the actual glycation of hemoglobin and helps in estimation of carbamylated hemoglobin through an easy widely used method. The measuring of HbA1c in uremic patients either nondiabetic or a diabetic showed falsely high values which confirmed the results of our in vitro experiments.
Conclusion
Our study shows a moderate significant relationship between change in HbA1c and fasting blood glucose concentration values. However, a significant difference in HbA1c is seen with fall in eGFR. In summary, this study showed significantly lower values of Hb A1c among diabetes with CKD. This decrease HbA1c among diabetes with CKD patients is because of carbamylation. carbamylated hemoglobin decreases with increase glucose concentration which can be explained by that glycation of some hemoglobin free amino groups prevents their participation in carbamylation reaction. Therefore, HbA1c should not be used alone for diagnosis of severity of diabetes in patients with ESRD.
Funding
No funding sources.
Ethical Approval
The study was approved by the Institutional Ethics Committee.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledegements
The encouragement and support from Bharath University, Chennai is gratefully acknowledged. For provided the laboratory facilities to carry out the research work.
References
- National Kidney Foundation. "K/DOQI clinical practice guidelines for chronic kidney disease" 2002.
- National Institute for Health and Clinical Excellence. Clinical guideline 73: Chronic kidney disease. London 2008.
- Plantinga LC, Tuot DS, Powe NR. Awareness of chronic kidney disease among patients and providers. Adv Chronic Kidney Dis 2010; 17:225-236.
- GBD 2013 Mortality and causes of death, collaborators (17 December 2014). "Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990- 2013: A systematic analysis for the Global Burden of Disease Study 2013." Lancet 385:117–171.
- Perazella MA, Khan S. Increased mortality in chronic kidney disease: A call to action. Am J Med Sci 2006; 331:150–153.
- Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American heart association councils on kidney in cardiovascular disease, high blood pressure research 2003.
- Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation 2003; 108:2154–2169.
- Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol 2006; 17:2034–2047.
- Heidenheim AP, Kooistra MP, Lindsay RM. "Quality of life". Contrib Nephrol 2004; 145:99–105.
- Pierratos A, McFarlane P, Chan CT . "Quotidian dialysis –update 2005". Curr Opin Nephrol Hypertens 2005; 14:119–24.
- Miedema K. Standardization of HbA1c and optimal range of monitoring. Scandinavian J Clin Laboratory Investigation 2005; 240:61–72.
- Peterson KP, Pavlovich JG, Goldstein D, et al. What is hemoglobin A1c? An analysis of glycated hemoglobins by electrospray ionization mass spectrometry. Clin Chem 1998; 44:1951–1958.
- Sacks DB, Bruns DE, Goldstein DE, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 2002; 48:436-472.
- Bunn FH, Forget BG. Hemoglobin: Molecular, genetic and clinical aspects. Philadelphia, PA: WB Saunders Co 1986; 425-427.
- Ansari A, Thomas S, Goldsmith D. Assessing glycemic control in patients with diabetes and end stage renal failure. Am J Kidney Dis 2003; 41:523 -531.
- Joy MS, Cefalu WT, Hogan SL, et al. Long -term glycemic control measurements in diabetic patients receiving hemodialysis. Am J Kidney Dis 2002; 39:297-307.
- Davie SJ, Gould BJ, Yudkin JS. Effects of vitamin C on glycosylation of proteins. Diabetes. 1992; 41:167-173.
- Inaba M. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: Effect of anemia and erythropoietin injection. J Am Soc Nephrol 2007; 18:896-903.
- Freedman BI, Shenoy RN, Planer JA, et al. Comparison of glycated albumin and hemoglobin A1c concentrations in diabetic subjects on peritoneal and hemodialysis. Peritoneal Dialysis Int 2008; 30:72-79.
- Freedman BI, Shihabi ZK, Andries L, et al. Relationship between assays of glycemia diabetic subjects with advanced chronic kidney disease. Am J Nephrol 2010; 31:375-379.
Author Info
Department of Biochemistry, Sree Balaji Medical College & Hospital Affiliated to Bharath Institute of Higher Education and Research, Chennai, Tamil Nadu, IndiaCitation: P Ravisekar, B Shanthi, The Effects of Carbamylation on HBA1C in Chronic Renal Failure Patients, J Res Med Dent Sci, 2021, 9 (6):539-544.
Received: 03-Apr-2021 Accepted: 23-Apr-2021
