Research - (2019) Volume 7, Issue 5
The Effect of Sleep Deprivation on Cortical Oscillatory Waves of the EEG in Shift and Non-shift Health Workers
Mariam Salako1, Menizibeya O Welcome1, Cevat Unal2 and Senol Dane1*
*Correspondence: Senol Dane, Department of Physiology, Faculty of Basic Medical Sciences, College of Health Sciences, Nile University of Nigeria, Nigeria, Email:
Abstract
Background: The harmful effects of shift work on heart function shave been reported in previous studies. However, the impact of shift work on cortical functions as recorded on EEG has not been widely reported. We aimed to investigate the possible harmful effects of sleep deprivation secondary to shift working on brain function by recording the EEG in work shift health workers.
Method: Sixteen healthy health workers participated in this study. The study was conducted in Abuja, Nigeria, from January to May 2019. Night shift (sleep deprivation) group (n=9) remained awake for 26 hours. Non-shift group (n=7) slept in their homes. EEG was applied two times in the morning at 09.00 am and in the evening at 07.00 pm for both shift and non-shift groups.
Results: In the shift health workers (sleep deprivation group), EEG tracing of both right and left brain hemispheres revealed a decrease in EEG beta power and gamma powers in the evening compared to morning recording. But in the non-shift health workers, there was no statistically significant difference between morning and evening recordings.
Conclusion: Sleep deprivation due to work shift may cause disruption in the brain EEG recordings by affecting the biological rhythm.
Keywords
Sleep deprivation, Heart rate variability, Shift workers, Non-shift workers, Health workers
Introduction
Sleep is a normal physiological desire and is essential to maintain a healthy life and homeostasis in humans [1]. The health workers are involved in a round-the-clock activity that is indispensable for continuity of care in clinics and hospitals [1]. To this end, they are scheduled to work day and night in a rotating manner throughout the 24-hour period. This type of work is referred to as shift work. Therefore, illnesses that affect health workers due to pattern of work schedule or rotation will affect the quality of health care delivery [1,2].
Estimations indicate that about one-fifth of the world’s workforce is engaged in shift work [3]. However, the proportion of shift workers can reach 39% depending on the geographical region [4]. Research data indicate a steady increase of shift workers over the past decades worldwide [1-4]. Accumulating data suggest that shift work, and in particular, night shift, is one of the major causes of circadian rhythm disruption, resulting to disorders in sleep-wake cycle with fatigue, and altered alertness, which in turn increases the human factor “errors”, thereby increasing the likelihood of drug administration errors and substantially decreasing the quality of patient care [1,2,5]. The condition can predispose the worker to work-related injuries and also, reduces job satisfaction [1,2] and increase job-related stress [6]. Compared with day shift workers, night shifts workers come up with insomnia [7], mental health problems (somatization, interpersonal sensitivity, depression, anxiety, obsessive-compulsive and paranoid disorders) [2], cancer(breast cancer, prostate cancer, and colorectal cancer), cardiovascular disease and hypertension as well as associated complication such as ischemic stroke, gastrointestinal disorders (gastritis, peptic ulcers, dyspepsia, colitis, indigestion, appetite disorders, irregular bowel movements, constipation, heartburn etc.), poor reproductive outcomes (preterm delivery, and natural miscarriage) [1,2,8], back pain, menstrual disorders [1], and accidents, and significantly decrease productivity [9].
Shift work disorder has been recently identified a separate clinical syndrome that is characterized by alteration of circadian rhythm of sleep-wake, leading to insomnia, excessive day sleepiness, fatigue, and decreased alertness [2]. Based on available data, the disorders mentioned above can occur between 18 and 60% of the workforce [1]. On the basis of Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) [2], shift work disorder has been shown to be high among the workforce ranging from 10 to 44% depending on the assessment method [1,7,10] and from 5 to 44% depending on the work schedule [10].
Despite the growing number of literatures on the health problems associated with shift work, little is known about the changes that accompany the central nervous system after the night shift. However, the pathophysiological mechanisms associated with night shift work disorders have not been completely unravelled. Knowledge about these mechanisms may be important in shift work scheduling and treatment of related disorders. Furthermore, knowledge garnered from the mechanisms behind shift work disorders can help to effectively plan intervention programs to improve sleep quantity and quality among night shift workers, and also, develop guidelines on how to protect workers from night shift related abnormalities.
EEG is an important electrophysiological recording that is used to investigate electrocerebral activity (i.e. cortical activities) in both health and disease [11]. The cortical activities comprise action potentials and multiple excitatory and inhibitory postsynaptic potentials mostly of the neurons of the subcortical and cortical regions of the brain [12]. The cortical activities are usually reported in different frequency bands of EEG trace: Delta or δ, theta or θ, alpha or α, beta or β and gamma or γ. These frequency bands are the major waves of the EEG that represent brain information processing in sensorimotor, cognitive, emotional, and attentional domains [13,14].
Changes associated with EEG in health workers who worked both day and night shifts have not been reported. Therefore, the aim of this study was to investigate the effects of night shift on EEG in health workers.
Methods
Participants
Sixteen healthy health workers (nurses and physicians) aged 25–35 years (mean age ± standard deviation=29.61 ± 7.39) participated in this study. The study was conducted in Abuja, Nigeria, from January to May 2019. Of 16 subjects, 9 were work shift (sleep deprivation) group and 7 workers were non work shift group. Work shift (sleep deprivation) group (n=9) remained awake for 26 hours. Non-work shift group slept in their homes. EEG was applied at 09.00 am and 07.00 pm for both shift and non-shift groups. All participants were right-handed according to selfreport and confirmed by Edinburgh Handedness Inventory [15]. They had comparable education level (15–17 years).
Inclusion criteria
1. Absence of any health problem based on recent medical examination.
2. Willingness to participate.
3. Total abstinence from drugs.
Exclusion criteria
1. Unwillingness to participate in the study.
2. Presence of health problems such as psychiatric, respiratory, metabolic, cardiac or central and autonomic nervous system disease, which may affect EEG tracing. The Mini-Mental State Examination was initially used to screen participants of cognitive deficit. No participant had cognitive deficit on this test.
3. Individuals using medications or drugs were not considered for participation. Those who fail the drug abuse test were not involved in the study.
Procedure
The experimental protocol was in line with the declaration of Helsinki and approved by the local ethics committee. The aims and objectives of the study were explicitly explained to the participants before commencing the experiment. All participants gave written informed consent to participate in the study a day before the commencement of the study. To avoid artifacts in the EEG tracing, the participants were requested to relax comfortably in an arm chair. The study lasted for one week.
EEG recording
The EEG signal was recorded according to the standard international 10/20 system, with a sampling rate of 1 kHz. EEG data was recorded by two channel bipolar montage; F2–F4 (right brain hemisphere) and F3–F7 (left brain hemisphere) (Figure 1) [16,17].
Figure 1. Left and right brain before and after shift work, time domain EEG.
The digital EEG was recorded by using Power Lab 26T (AD Instruments, Bella Vista, Australia), a device used for multimodal monitoring of biosignals. In this study, the EEG was extracted morning at 09.00 am and evening at 07.00 pm for all participants. EEG frequency bands considered for analysis were delta waves (1–3 Hz), theta waves (4–7 Hz), alpha waves (8–12 Hz), beta waves (13–30 Hz), gamma1 waves (31– 40 Hz), gamma2 waves (41–50 Hz) and gamma3 waves (60–80 Hz).
The electroencephalographic signal processing analyses were performed in MATLAB. The changes in frequency and amplitude of the EEG were analyzed by means of power spectrum, measured as total power in microvolts-squared divided by frequency (μv2/Hz) [6]. We used Discrete Fourier Transform (DFT) to calculate the power spectrum of time domain discrete EEG signal. Power spectral density (PSD) is frequency response of a periodic or random signal. PSD shows distribution of signal strength depending on the frequency. The EEG data we were recorded is a time domain discrete signal.
Power spectral density can be expressed with Equation 1.
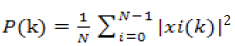 (1)
(1)
Where N is the number of samples and xi(k) is the Discrete Fourier Transform (DFT) of the time domain discrete xi(n) signal. xi(k) is calculated as shown in Equation 2.
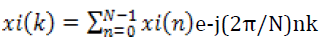 (2)
(2)
Statistical analysis
The SPSS software (version 18.0 for Windows) was used for statistical data analysis. Results are expressed as mean ± standard deviation (SD) as well as in percentages (%). The pattern of data distribution was evaluated by Kolmogorov Smirnov test. Unpaired Student T-test was used for comparison of results of EEG waves in shift and non-shift health workers. Differences were considered statistically significant at p˂0.05.
The EEG tracing of F2–F4 (right brain hemisphere) of shift health workers revealed a decrease in beta power (μV2/Hz), gamma1 power (μv2/Hz), gamma2 power(μv2/Hz)and total gamma power (μv2/Hz) in the evening compared to morning recording (beta: t=2.21, p=0.04; gamma1: t=2.32, p=0.04; gamma2: t=2.13, p=0.04; total gamma power: t=2.24, p=0.04) (Table 1). In non-shift health workers, there was no difference between morning and evening EEG tracings for the all parameters in F2-F4 (right brain hemisphere) (Table 2).
| Power (μv2/Hz) | Morning | Evening | t-test | p |
|---|---|---|---|---|
| Total power (μv2/Hz) | 85.58 ± 42.85 | 87.73 ± 36.76 | 0.25 | NS |
| Delta power (μv2/Hz) | 78.36 ± 42.65 | 80.99 ± 37.02 | 0.32 | NS |
| Theta power (μv2/Hz) | 3.47 ± 1.68 | 3.65 ± 1.22 | 0.42 | NS |
| Alpha power (μv2/Hz) | 1.43 ± 0.89 | 1.38 ± 0.56 | 0.33 | NS |
| Beta power (μv2/Hz) | 1.48 ± 0.13 | 1.18 ± 0.39 | 2.21 | 0.04 |
| Gamma1 power (μv2/Hz) | 0.48 ± 0.07 | 0.32 ± 0.06 | 2.32 | 0.04 |
| Gamma2 power (μv2/Hz) | 0.29 ± 0.04 | 0.18 ± 0.07 | 2.13 | 0.04 |
| Gamma3 power (μv2/Hz) | 0.32 ± 0.03 | 0.22 ± 0.05 | 1.89 | NS |
| Total Gamma power (μv2/Hz) | 1.09 ± 0.14 | 0.72 ± 0.09 | 2.24 | 0.04 |
Table 1: Powers of the different EEG waves in the right brain of shift health workers.
| Power (μv2/Hz) | Morning | Evening | t-test | p |
|---|---|---|---|---|
| Total power (μv2/Hz) | 69.01 ± 40.47 | 81.65 ± 35.02 | 0.61 | NS |
| Delta power (μv2/Hz) | 61.57 ± 35.29 | 74.58 ± 35.47 | 0.66 | NS |
| Theta power (μv2/Hz) | 3.76 ± 3.91 | 3.32 ± 2.73 | 0.19 | NS |
| Alpha power (μv2/Hz) | 1.41 ± 1.25 | 1.24 ± 0.46 | 0.34 | NS |
| Beta power (μv2/Hz) | 1.48 ± 1.19 | 1.68 ± 2.12 | 0.34 | NS |
| Gamma1 power (μv2/Hz) | 0.48 ± 0.61 | 0.51 ± 0.63 | 0.54 | NS |
| Gamma2 power (μv2/Hz) | 0.27 ± 0.39 | 0.28 ± 0.45 | 0.35 | NS |
| Gamma3 power (μv2/Hz) | 0.32 ± 0.44 | 0.35 ± 0.49 | 1.05 | NS |
| Total Gamma power (μv2/Hz) | 1.06 ± 1.44 | 1.14 ± 1.58 | 0.66 | NS |
Table 2: Powers of the different EEG waves in the right brain in non-shift health workers.
Also, in the shift health workers, EEG tracing of F3–F7 (left brain hemisphere) revealed a decrease in beta power (μV2/Hz), gamma1 power Also, in the shift health workers, EEG tracing of F3–F7 (left brain hemisphere) revealed a decrease in beta power (μV2/Hz), gamma1 power (μv2/Hz), gamma2 power (μv2/Hz), gamma3 power (μv2/Hz) and total gamma power (μv2/Hz) in the evening EEG compared to morning recording EEG (beta: t=2.21, p=0.04; gamma1: t=2.37, p=0.04; gamma2: t=2.17, p=0.04; gamma3: t=2.29, p=0.04; total gamma power: t=2.37, p=0.04) (Table 3). But in the non-shift health workers, there was no difference between morning and evening for the all EEG parameters in EEG tracing of F3-F7 (left brain hemisphere) (Table 4).
| Power (μv2/Hz) | Morning | Evening | t-test | p |
|---|---|---|---|---|
| Total power (μv2/Hz) | 90.03 ± 44.29 | 84.01 ± 31.95 | 0.81 | NS |
| Delta power (μv2/Hz) | 82.79 ± 44.22 | 77.33 ± 32.31 | 0.74 | NS |
| Theta power (μv2/Hz) | 3.46 ± 1.67 | 3.59 ± 1.12 | 0.31 | NS |
| Alpha power (μv2/Hz) | 1.45 ± 0.88 | 1.36 ± 0.56 | 0.54 | NS |
| Beta power (μv2/Hz) | 1.49 ± 0.13 | 1.20 ± 0.19 | 2.21 | 0.04 |
| Gamma1 power (μv2/Hz) | 0.48 ± 0.09 | 0.32 ± 0.06 | 2.37 | 0.04 |
| Gamma2 power (μv2/Hz) | 0.29 ± 0.07 | 0.19 ± 0.05 | 2.17 | 0.04 |
| Gamma3 power (μv2/Hz) | 0.32 ± 0.03 | 0.22 ± 0.08 | 2.29 | 0.04 |
| Total Gamma power (μv2/Hz) | 1.08 ± 0.14 | 0.72 ± 0.11 | 2.37 | 0.04 |
Table 3: Powers of the different EEG waves in the left brain of shift health workers.
| Power (μv2/Hz) | Morning | Evening | t-test | p |
|---|---|---|---|---|
| Total power (μv2/Hz) | 75.59 ± 54.51 | 75.41 ± 47.86 | 0.16 | NS |
| Delta power (μv2/Hz) | 72.21 ± 51.59 | 66.35 ± 44.98 | 0.24 | NS |
| Theta power (μv2/Hz) | 2.89 ± 1.91 | 3.85 ± 3.97 | 0.54 | NS |
| Alpha power (μv2/Hz) | 1.42 ± 0.93 | 1.83 ± 1.13 | 0.87 | NS |
| Beta power (μv2/Hz) | 1.99 ± 2.37 | 2.17 ± 1.93 | 0.64 | NS |
| Gamma1 power (μv2/Hz) | 0.66 ± 0.84 | 0.75 ± 0.67 | 0.66 | NS |
| Gamma2 power (μv2/Hz) | 0.38 ± 0.61 | 0.41 ± 0.45 | 0.21 | NS |
| Gamma3 power (μv2/Hz) | 0.44 ± 0.66 | 0.43 ± 0.47 | 0.08 | NS |
| Total Gamma power (μv2/Hz) | 1.49 ± 2.12 | 1.59 ± 1.58 | 0.29 | NS |
Table 4: Powers of the different EEG waves in the left brain in non-shift health workers.
Discussion
The physiological importance of sleep has been reported in many studies. Indeed majority of humans spend approximately one-third of their lives asleep. It is therefore not a surprise that any disruption of the processes that regulate sleep can result to substantial consequences [18]. The results of a previous recent study made by recording HRV (heart rate variability) showed that sleep deprivation was associated with sympatho-vagal imbalance with sympathetic dominance [19]. This suggests that sleep deprivation impairs processes associated with regulation of cardiac rhythm. Available data suggest that sleep deprivation or shift work causes a multi-system hazard, probably due to impairment in circadian rhythm [7,20,21]. This is based on the hypothesis that circadian rhythm is essential for homeostasis of several systems of the body [7,22]. Unfortunately, however, data on the effects of sleep deprivation or shift work on cortical functions as recorded with the EEG are scanty. The results of our study have added substantial data to the literature on EEG markers of shift work.
Sleep deprivation disrupts synchronization of the supra chiasmatic nucleus, affecting the secretion of hormones and neurotransmitters such as acetylcholine, noradrenaline, serotonin, corticosteroids, and melatonin, which participate in cortical rhythm-genesis and modulation of EEG waves. The imbalances in hormone or neurotransmitter secretion can result to different disorders in cortical rhythms and other physiological processes [7,20-22].
Sustained wakefulness or sleep deprivation has been reported to increase neuronal firing. Indeed staying asleep counterbalances the increased neuronal firing during prolonged wakefulness or sleep deprivation [23]. However, emerging evidences indicate that various brain regions respond differently to sleep deprivation. For instance, Greer et al. reported decreased neuronal activity in appetitive evaluation regions within the human frontal cortex and insula cortex following sleep deprivation. In contrast, neuronal activity in the amygdala was amplified [24]. Previous studies have showed high variability in EEG rhythms in wakefulness/sleep deprivation or sleep [25]. Bersagliere et al. showed that sleep deprivation results to an increase in mid-delta activity (1.25–2 Hz), particularly in parietal and frontal brain. Interestingly, independent on the duration of sleep, occipital and temporal brain had increased low-delta (0.5–1 Hz) activity [26]. A couple of authors have shown conflicting results regarding changes associated with EEG δ waves after sleep deprivation. It appears that δ power is regulated independently of sleep duration [27]. However, Pressman [28] has indicated a role of delta waves in evaluating sleep processes and disorders. Comparable findings about the role of delta waves as potential indicator of sleep restriction were reported by Stephenson et al. [29]. Indeed predominance of low-frequency EEG such as delta waves indicates decreased alertness, which reflects a decrease in activation of the cerebral cortex, indicating fatigue and decreased cognition [30]. The role of delta EEG in sleep processes has been discussed elsewhere [31]. Although further investigations are necessary, findings from our study suggest that there were no significant differences between morning and evening recordings of delta EEG for the shift workers or non-shift workers. Therefore, there is need to revise EEG δ power as an indicator of sleep deprivation [27] and also, investigate how this EEG wave changes with shift-work adaptation in different categories of workers.
In line with literature data, our study revealed that alpha, theta, delta powers are not affected by sleep deprivation. The shift health workers (sleep deprivation group) had a decrease in beta power and gamma powers in the evening EEG compared to morning recording for both right and left brain hemisphere. However, in the nonshift health workers, there was no difference between morning and evening EEG tracing for the all parameters in EEG tracing of both right and left brain hemispheres. It can be suggested that the differences observed in shift workers may indicate a possible adaptation to their work schedule. But there is severe lack of data regarding adaptation of circadian clock (master controller of sleep-wake cycle and homeostasis) in chronic shift work or sleep deprivation. Evidences from human studies indicate a possible role of circadian clock adaptation in shift-work, sleep deprivation, lifestyle habits [31], sleep disorders [32,33]. Adaption of the circadian rhythm to day-night cycle has also been reported in drosophila [34], suggesting this property of the master circadian clock may be evolutionarily determined.
Conclusion
Though scheduling and diversification of working time is important in work organization, contributing to the improvement of human life, there are potentially serious health consequences that are associated with such work arrangement on the workers. Thus the need to develop measures and interventions that will be directed towards mitigating the negative effects of shift work on workers’ health, in addition to providing solutions for a better work scheduling that will have substantially lower negative health impact on workers’ health.
References
- Anbazhagan S, Ramesh N, Nisha C, et al. Shift work disorder and related health problems among nurses working in a tertiary care hospital, Bangalore, South India. Indian J Occup Environ Med 2016; 20:35–38.
- Ferri P, Guadi M, Marcheselli L, et al. The impact of shift work on the psychological and physical health of nurses in a general hospital: a comparison between rotating night shifts and day shifts. Risk Manag Health Policy 2016; 9:203–211.
- Thirion A, Biletta I, Cabrita J, et al. Sixth European working conditions survey: Overview report. Luxembourg: Publications Office of the European Union 2016.
- Bae MJ, Song YM, Shin JY, et al. The association between shift work and health behavior: Findings from the Korean national health and nutrition examination survey. Korean J Fam Med 2017; 38:86–92.
- Muecke S. Effects of rotating night shifts: Literature review. J Adv Nurs 2005; 50:433–439.
- McVicar A. Scoping the common antecedents of job stress and job satisfaction for nurses (2000–2013) using the job demands-resources model of stress. J NursManag 2016; 24:112–136.
- Drake CL, Roehrs T, Richardson G, et al. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep 2004; 27:1453–1462.
- Nojkov B, Rubenstein JH, Chey WD, et al. The impact of rotating shift work on the prevalence of irritable bowel syndrome in nurses. Am J Gastroenterol 2010; 105:842–847.
- Roth T. Shift work disorder: Overview and diagnosis. J Clin Psychiatry 2012; 73:9.
- Flo E, Pallesen S, Magerøy N, et al. Shift work disorder in nurses: Assessment, prevalence and related health problems. PLoS One 2012; 7:e33981.
- Badrakalimuthu RV, Swamiraju R, Waal H. EEG in psychiatric practice: To do or not to do? Adv Psychiatr Treat 2011; 17:114–121.
- Binienda ZK, Beaudoin MA, Thorn BT, et al. Analysis of electrical brain waves in neurotoxicology: Gamma-hydroxybutyrate. Curr Neuropharmacol 2011; 9:236–239.
- Vyazovskiy VV, Cui N, Rodriguez AV, et al. The dynamics of cortical neuronal activity in the first minutes after spontaneous awakening in rats and mice. Sleep 2014; 37:1337–1347.
- Janssen TWP, Bink M, Weeda WD, et al. Learning curves of theta/beta neuro feedback in children with ADHD. Eur Child Adolesc Psychiatry 2017; 26:573–582.
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971; 9:97–113.
- Olanipekun A, Alhassan AK, Musa FH, et al. The effect of foot bath therapy on the dynamics of cortical oscillatory waves in healthy humans: An EEG study. J Res Med Dent Sci 2019; 7: 57-61.
- Unal C, Welcome MO, Salako M, et al. The effect of foot reflexotherapy on the dynamics of cortical oscillatory waves in healthy humans: An EEG study. Complement Ther Med 2018; 38: 42-47.
- Moszczynski A, Murray B. Neurobiological aspects of sleep physiology. Sleep 2012; 30:963–85.
- Cebeci S, Canbal M, Yuksel R, et al. The effect of sleep deprivation on heart rate variability in shift and non-shift physicians. Clin Invest Med 2015; 38(4):E233-E236.
- Tobaldini E, Nobili L, Strada S, et al. Heart rate variability in normal and pathological sleep. Front Physiol 2013; 16(4): 294.
- Buccelatti E, Gilardi E, Scaini E, et al. Heart rate variability and myocardial infarction: systematic literature review and meta-analysis. Eur Rev Med PharmacolSci 2009; 13:299-307.
- Redwine L, Hauger RL, Gillin JC, et al. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab 2000; 85: 3597-3603.
- Vyazovskiy VV, Olcese U, Lazimy YM, et al. Cortical firing and sleep homeostasis. Neuron 2009; 63:865–878.
- Greer SM, Goldstein AN, Walker MP. The impact of sleep deprivation on food desire in the human brain. Nat Commun 2013; 4:2259.
- Manganotti P, Formaggio E, Del Felice A, et al. Time-frequency analysis of short-lasting modulation of EEG induced by TMS during wake, sleep deprivation and sleep. Front Hum Neurosci 2013; 7:767.
- Bersagliere A, Pascual-Marqui RD, TarokhL, et al. Mapping slow waves by EEG topography and source localization: Effects of sleep deprivation. Brain Topogr 2018; 31:257–269.
- Davis CJ, Clinton JM, Jewett KA, et al. EEG delta wave power: An independent sleep phenotype or epiphenomenon? J Clin Sleep Med 2011; 7:16–18.
- Pressman MR. Hypersynchronous delta sleep EEG activity and sudden arousals from slow-wave sleep in adults without a history of parasomnias: Clinical and forensic implications. Sleep 2004; 27:706–710.
- Stephenson R, Caron AM, Famina S. Behavioral sleep-wake homeostasis and EEG delta power are decoupled by chronic sleep restriction in the rat. Sleep 2015; 38:685–697.
- Cheng SY, Hsu HT. Mental fatigue measurement using EEG. In: Nota G, Risk management trends. 2011.
- Forni D, Pozzoli U, Cagliani R, et al. Genetic adaptation of the human circadian clock to day-length latitudinal variations and relevance for affective disorders. Genome Biol 2014; 15:499.
- Dall'Ara I, Ghirotto S, Ingusci S, et al. Demographic history and adaptation account for clock gene diversity in humans. Heredity (Edinb) 2016; 117:165-172.
- Landgraf D, McCarthy MJ, Welsh DK. The role of the circadian clock in animal models of mood disorders. Behav Neurosci 2014; 128:344-359.
- Menegazzi P, Benetta ED, Beauchamp M, et al. Adaptation of circadian neuronal network to photoperiod in high-latitude European drosophilids. Curr Biol 2017; 27:833-839.
Author Info
Mariam Salako1, Menizibeya O Welcome1, Cevat Unal2 and Senol Dane1*
1Department of Physiology, Faculty of Basic Medical Sciences, College of Health Sciences, Nile University of Nigeria, Abuja, Nigeria2Department of Electrical and Electronics Engineering, Faculty of Engineering, Nile University of Nigeria, Abuja, Nigeria
Citation: Mariam Salako, Menizibeya O Welcome, Cevat Unal, Senol Dane, The Effect of Sleep Deprivation on Cortical Oscillatory Waves of the EEG in Shift and Non-shift Health Workers, J Res Med Dent Sci, 2019, 7(5):103-109.
Received: 24-Jul-2019 Accepted: 14-Oct-2019
