Research - (2022) Volume 10, Issue 9
The Effect of Magnetized Water as a Mouthrinse on Chromium Ion Release and Surface Topography of Stainless Steel Orthodontic Archwires
Zinah N Al Zubaidy1* and Afrah K Al Hamdany2
*Correspondence: Zinah N Al Zubaidy, Ninavah Health Directorate, Iraq, Email:
Abstract
Aims: The current study aimed to discover if the use of magnetized water (MW) as a mouthrinse will affect the release of Chromum (Cr) ion from stainless steel (SS) orthodontic archwires. In addition to study surface topography of SS orthodontic archwires after and before immersion in MW by atomic force microscope (AFM). Materials and Methods: 80 (0.016” X0.022”) as received orthodontic SS archwires from the manufacturer company (Dentarum, Germany). The archwires were divided into four main groups according to the mouthrinse used as follows: magnetized water prepared at high power magnetic field group (MWH), magnetized water prepared at low power magnetic field group (MWL), Ortho Kin group (control positive) (OK) and distilled water group (control negative) (DW). Each group contain four times point intervals (24hr (hour), 1w (week), 3w and 4w). For each time interval, five archwires were used. The Cr ion release was measured at different time intervals (24hr, 1w, 3w and 4w) by using atomic absorption spectrometer (AAS) (BUCK Scientific, USA). And the surface topography of SS orthodontic archwires was measured by atomic force microscope (AFM) (NaioAFM Nanosurf, Switzerland). Statistical analysis was done using V26. SPSS Statistics, Data were analyzed with one-way ANOVA and Duncan’s multiple range tests. Results: MWH and MWL groups had significantly less amounts of Cr ions release from SS orthodontic archwires when compared with OK mouthrinse group. Conclusions: MW can be used as a safer alternative to other commercially current mouthrinses (as OK) for orthodontic patients.
Keywords
Archwire, Chromium ion, Corrosion, Magnetized water
Introduction
Orthodontic archwires and brackets are retained for prolong period intraorally throughout orthodontic treatment; therefore, they are exposed to continuous mechanical stress of wearing and chewing. Likewise, the presence of saliva and consumed liquids, changing pH and temperature produced by diet, oral microflora and their byproducts, enzymatic activity, proteins and topical fluoride modalities, all these factors affect the archwires surface [1]. In addition, several mouthrinses can affect the metallic appliances corrosion and lead to the release of metal ions into saliva of the patients [2], especially, toothpastes and mouthrinse solutions having fluoride ions [3,4]. For all these reasons, long-term use of any orthodontic appliances installed inside the mouth should ensure that no toxic ions will be significantly released [5]. Corrosion can lead to release of metal or alloy ions, which can cause local pains, discoloration of soft tissues and enamel and allergic reactions in susceptible patients [6].
Fixed orthodontic appliances allow accumulation of dental plaque, which promotes demineralization of enamel and lead to dental caries formation [7]. Furthermore, in the course of orthodontic treatment some patients may develop periodontal problems and need to use mouthrinses to diminish plaque accumulation. Thus, it is recommended to use different mouthrinses during orthodontic treatment [8]. The usage of mouthrinses and prophylactic agents can alter the oral environment. So, orthodontic archwires can be constantly subjected to these agents which may increase the corrosion rate. A prior study conducted by Schiff et al. stated that orthodontists should be cautious when they prescript mouthrinses for their orthodontic patients [9]. This fact developed the necessity to look for safer substitutes that are more acceptable and suitable for patients and have little influence on corrosion of metallic appliance, which can be provided by natural products [10]. Among different natural substitutes (to the chemical adjuncts) which have been studied, magnetized water (MW) has gained abundant concern in dentistry [11]. MW, which is water that has been treated with magnets or magnetically treated water, is defined as water that has been subjected to certain strength of magnetic field [12]. A study conducted by Goyal et al. showed that MW has better action in plaque as compared with saliva; therefore, MW is recently used as effective as a mouthrinse against S. mutans [13]. So MW can be used as an adjunct to the mouthrinses that are available commercially.
There is no prior study about the effect of MW as mouthrinse on release of metal ions from orthodontic archwires. So the present study was conducted to examine whether the use of MW as a mouthrinse will affect the release of Cr ions from SS orthodontic archwires and to study surface topography of SS orthodontic archwires immersed in MW by AFM.
The null hypotheses tested were that:
The concentration of Cr ions releases from SS archwires in the presence of MW does not differ from those immersed in commercially available Ortho Kin mouthrinse (OK).
The surface characteristics of SS archwires immersed in MW by AFM does not differ from those immersed in commercially available OK mouthrinse.
Materials and Methods
Materials
The study samples were consisted of eighty (0.016″ x 0.022″) brand new SS orthodontic archwires from the (Dentarum, Germany) manufacturer. The archwires were divided according to the mouthrinse used into four groups as follows: magnetized water prepared at high power (3000 Gauss) magnetic field group (MWH), magnetized water prepared at low power (1000 Gauss) magnetic field group (MWL), distilled water (control) group (DW) and Ortho Kin group (OK). There are four times point intervals for each group (24hr, 1w, 3w and 4w). The samples used for each time interval were five archwires so each group contains 20 archwires.
Preparation of magnetized water
The DW had been magnetized by a device that is locally made; magnets with two magnetic strengths (1000 and 3000 Gauss) of neodymium type were used. The magnetic strength was determined by Gauss Meter; also the Gauss Meter was used to determine the north poles of the magnets. Two magnets were fixed around the container in a north-north repulsion manner; DW was poured in the container and remained for 72 hr. After that, the pH value and electrical conductivity (EC) for the MW and DW were checked by pH Meter and EC Meter to ensure that DW was magnetized, the obtained values were shown in Table 1.
| pH | EC | |
|---|---|---|
| DW | 6.9 | 13.8 |
| MWL | 7 | 470 |
| MWH | 7.2 | 470 |
Table 1: EC and pH values for DW and MW.
Preparation of artificial saliva
Components of artificial saliva are 0.79g of CaCL2 .2H2O, 0.78g of NaH2 PO4.2H2O, 0.40g of KCl, 0.40g of NaCl, 0.1g of CO(NH2)2 Urea and 0.005g of Na2S.9H2O dissolved in 1 liter of DW (concentration g \l) (its pH was 7) [14].
Methods
SS archwires were firstly cleaned with acetone and washed with distilled water and then dried. Secondly, each archwire was divided into four pieces equal in length. The wires were placed in a glass container of 30 ml volume. After that, in each container 25 ml of solutions of the artificial saliva were poured. The samples were placed inside an incubator (nüve EN 400, Turkey) at 37°C for 24hr, 1w, 3w and 4w. At 12 hours’ time intervals, twice in a day, corresponding to the mouthrinse usage, the samples containing wires removed from the incubator and by using a plastic tweezers, the wire pieces were removed from their container and rinsed in DW, then the wires were dipped into another separate 30 ml glass containers that contain 25 ml of solution sample (MWH, MWL, OK and DW). The period of immersion inside mouthrinse solution was for one minute twice in a day. After the end of the period of immersion, the wires were washed with DW and returned to their artificial saliva container and the containers were putted in the incubator at 37°C until the next mouthrinse immersion time. This process was repeated at similar rhythm up to the forth week completion. At the end of each four time-points (24hr, 1w, 3w and 4w), a drop of 65% nitric acid was added after the removal of the wires from the artificial saliva solution for released ions stabilization [2]. The effect of MWH, MWL, OK and DW on Cr ion release from SS archwires at different time intervals was studied. The release of Cr ions was analyzed by using an atomic absorption spectrometer. And the surface topography of SS archwires was evaluated by AFM before and after four weeks of the experiment. Statistical analysis of the results was accomplished by using the computerized statistical program (IBM SPSS Statistics, V26). Normality of distribution was assessed by Shapiro–Wilk test and parametric statistical analysis was done which includes the descriptive Statistics (number of the samples in each group, minimum, maximum values of metal ions release, range, mean, standard error and standard deviation). One Way ANOVA Test and Duncan's Multiple Range Test were performed to assess the statistical significant difference at P≤0.05.
Results
Cr ion release from SS archwires
The descriptive data for Cr ion release from SS archwires are demonstrated in Table 2. These data include the number of the samples in each group, minimum and maximum values of the Cr ions release, range, mean (measured in part per billion (ppb)), standard error and standard deviation for the study groups. The descriptive analysis revealed that at 24hr, 1w, 3w and 4w intervals, OK showed the highest Cr ions release, while DW showed the lowest Cr ions release value.
| Groups | N | Min. | Max. | Range | Mean | S. E | S. D |
|---|---|---|---|---|---|---|---|
| DW 24hr | 5 | 0.3 | 0.5 | 0.2 | 0.38 | 0.03 | 0.08 |
| OK 24hr | 5 | 0.4 | 0.6 | 0.2 | 0.48 | 0.03 | 0.08 |
| MWL 24hr | 5 | 0.3 | 0.5 | 0.2 | 0.38 | 0.03 | 0.08 |
| MWH 24hr | 5 | 0.3 | 0.5 | 0.2 | 0.4 | 0.04 | 0.1 |
| DW 1w | 5 | 0.3 | 0.5 | 0.2 | 0.42 | 0.03 | 0.08 |
| OK 1w | 5 | 0.5 | 0.7 | 0.2 | 0.62 | 0.03 | 0.08 |
| MWL 1w | 5 | 0.4 | 0.6 | 0.2 | 0.48 | 0.03 | 0.08 |
| MWH 1w | 5 | 0.4 | 0.6 | 0.2 | 0.48 | 0.03 | 0.08 |
| DW SS 3w | 5 | 0.5 | 0.8 | 0.3 | 0.66 | 0.05 | 0.11 |
| OK SS 3w | 5 | 1.1 | 1.5 | 0.4 | 1.32 | 0.08 | 0.17 |
| MWL 3w | 5 | 0.7 | 0.9 | 0.2 | 0.8 | 0.03 | 0.07 |
| MWH 3w | 5 | 0.6 | 0.8 | 0.2 | 0.72 | 0.03 | 0.08 |
| DW 4w | 5 | 0.6 | 0.8 | 0.2 | 0.7 | 0.03 | 0.07 |
| OK 4w | 5 | 1.8 | 2.2 | 0.4 | 2 | 0.07 | 0.15 |
| MWL 4w | 5 | 0.7 | 0.9 | 0.2 | 0.82 | 0.03 | 0.08 |
| MWH 4w | 5 | 0.7 | 0.9 | 0.2 | 0.78 | 0.03 | 0.08 |
Table 2: Descriptive statistics for the Cr ion release from SS archwires.
The one way (ANOVA) statistical test results were shown in Table 3 presenting no significant differences at (P ≤ 0.05) among the groups at 24hr interval. While at 1w, 3w and 4w intervals, there are significant differences at (P ≤ 0.05) among the groups. A more specific Duncan’s multiple range test was performed among all the study groups as demonstrated in Table 4, and detected that at 24hr interval the DW, OK, MWL and MWH groups were not significantly differing in Cr ion release value. But at 1w, 3w and 4w intervals, the OK group had significantly the highest difference in Cr ion release value, while DW, MWL and MWH groups were not significantly differing in Cr ion release value.
| Sum of Squares | df | Mean Square | F | Sig. | ||
|---|---|---|---|---|---|---|
| 24hr | Between Groups | 0.034 | 3 | 0.011 | 1.462 | 0.262 |
| Within Groups | 0.124 | 16 | 0.008 | |||
| Total | 0.158 | 19 | ||||
| 1w | Between Groups | 0.108 | 3 | 0.036 | 5.048 | 0.011 |
| Within Groups | 0.112 | 16 | 0.007 | |||
| Total | 0.22 | 19 | ||||
| 3w | Between Groups | 1.369 | 3 | 0.456 | 32.035 | 0 |
| Within Groups | 0.228 | 16 | 0.014 | |||
| Total | 1.597 | 19 | ||||
| 4w | Between Groups | 5.742 | 3 | 1.914 | 173.985 | 0 |
| Within Groups | 0.176 | 16 | 0.011 | |||
| Total | 5.918 | 19 | ||||
Table 3: One way (ANOVA) for the mean values of Cr ion release from SS archwires after immersion in different mouthrinses at same interval.
| Interval | Mouthrinse group | N | Mean ± SE | Duncan Groups* |
|---|---|---|---|---|
| 24hr | DW | 5 | 0.38 ± 0.037 | A |
| OK | 5 | 0.48 ± 0.037 | A | |
| MWL | 5 | 0.38 ± 0.037 | A | |
| MWH | 5 | 0.40 ± 0.044 | A | |
| 1w | DW | 5 | 0.42 ± 0.037 | A |
| OK | 5 | 0.62 ± 0.037 | B | |
| MWL | 5 | 0.48 ± 0.037 | A | |
| MWH | 5 | 0.48 ± 0.037 | A | |
| 3w | DW | 5 | 0.66 ± 0.050 | A |
| OK | 5 | 1.32 ± 0.080 | B | |
| MWL | 5 | 0.80 ± 0.031 | A | |
| MWH | 5 | 0.72 ± 0.037 | A | |
| 4w | DW | 5 | 0.70 ± 0.031 | A |
| OK | 5 | 2.00 ± 0.070 | B | |
| MWL | 5 | 0.82 ± 0.037 | A | |
| MWH | 5 | 0.78 ± 0.037 | A |
Table 4: Duncan’s Multiple Range Test for multiple comparisons of the Cr ion release from SS archwires after immersion in different mouthrinses at same interval.
AFM Results
Table 5 represents the arithmetic mean height values (Sa) of the surface for new SS archwire and for SS archwires after immersion in different mouthrinses. It shows that the highest Sa value was for archwire immersed in OK, followed by archwire immersed in MWL, then followed by archwire immersed in MWH, and then for archwire immersed in DW, while new archwire shows the least Sa value.
| Sa value (nm) | |
|---|---|
| New | 2.821 |
| DW | 3.944 |
| OK | 6.481 |
| MWL | 5.746 |
| MWH | 4.107 |
Table 5: Surface roughness of SS archwires.
Figure 1 a, b, c, d and e represent the AFM results for new, DW, OK, MWL and MWH groups respectively, the general appearance of all these AFM images were corresponding to the Sa values. As shown in Figure 1a the surface of new archwire has generalized very shallow grooves, after 4wk immersion of the archwire in the DW, the depth of the grooves increased slightly as seen in Figure 1b. The surface of archwire immersed in OK has large and deep grooves as appear in Figure 1c. In MWL the archwire surface shows generalized pits and craters which is intermediate between OK and MWH as in Figure 1d. While in MWH the archwire surface shows generalized shallow grooves that are deeper than new and archwire that immersed in DW as seen in Figure 1e.
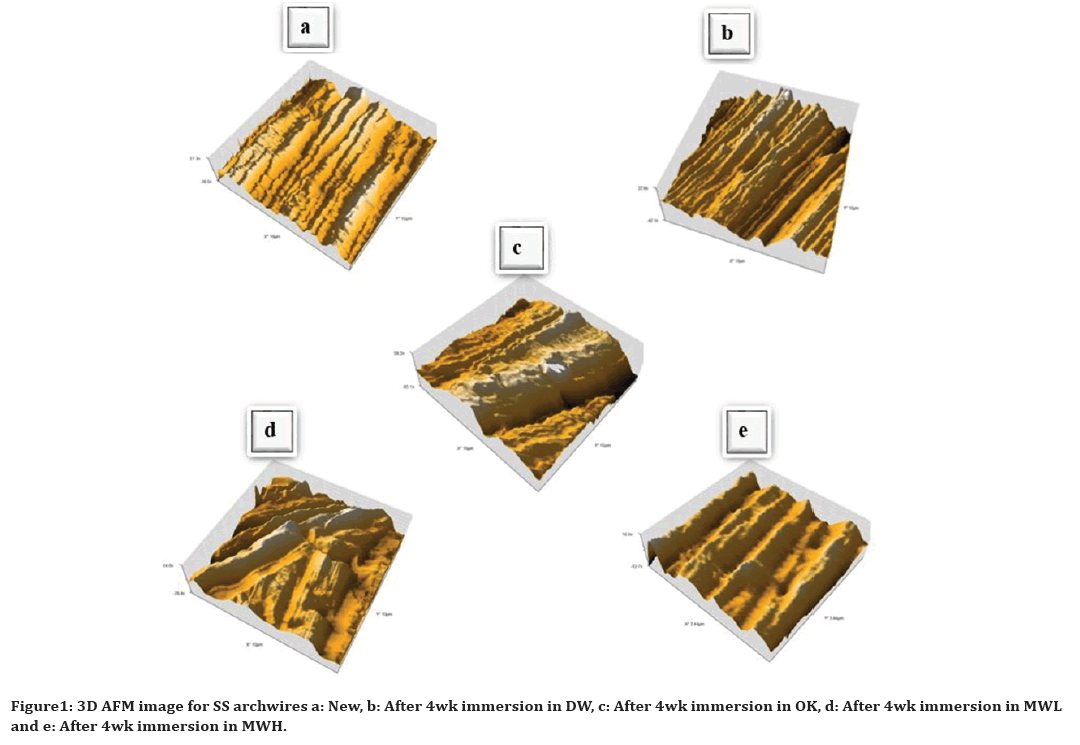
Figure 1. 3D AFM image for SS archwires a: New, b: After 4wk immersion in DW, c: After 4wk immersion in OK, d: After 4wk immersion in MWL and e: After 4wk immersion in MWH.
Discussion
In the present study, the general corrosion mechanism and consequent metal ions release from SS involves the loss of the passive layer consisting of Cr hydroxide and Cr oxide that forms on contact with oxygen on the surface of SS [15].
In this study, the release of Cr ion from SS archwires was significantly highest in OK mouthrinse at all-time points except for 24hr interval in which Cr ion release in OK mouthrinse was non significantly higher than other groups, this occurred as a result of corrosion process which increased due to the presence of corrosive ingredients in OK mouthrinse such as fluoride ion. Our results concerning OK which is a fluoridated mouthrinse, were in agreement with the results of a study which found that fluoride containing products corroded SS archwires and brackets, as demonstrated by the evaluation of surface roughness and measurement of metal ion release [16]. Another study stated that when fluoride ion concentration is increased, orthodontic archwires corrosion resistance is decreased [17]. Also reduction of pH caused by fluoridated mouthrinse may dissolve the surface oxide films and prevent its reformation which results in greater corrosion [18].
In the present study, Cr ion release amount from SS archwires that immersed in MWH and MWL were non significantly higher than that when immersed in DW due to variation in water characteristics when it was being magnetized, therefore the process of corrosion was affected. While in DW group, the lowest amount of Cr ions release from SS archwires may be due to pH of DW which is neutralized and the archwires immersed in DW group were only under the effect of chemical contents of artificial saliva in addition to influence of the temperature of incubation.
The AFM results in current study showed that the highest value of the surface roughness for SS was for archwire immersed in OK due to its known effect on the corrosion of the archwires as a result of its low pH value and aggressive fluoride ions in its component. However, as a fact, when corrosion increase the surface roughness increase. Fluoride containing mouthrinses have been seen to cause surface changes in orthodontic archwires and brackets [19]. Our study concerning the effect of fluoride on the surfaces of SS archwires was in agreement with the study conducted by Pulikkottil et al. to assess the corrosion resistance of SS, NiTi, TMA and ion-implanted TMA orthodontic archwires and to determine the effect of 0.5% Sodium fluoride (NaF) on corrosion resistance of these archwires, they concluded that resistance to corrosion of all archwires was significantly reduced in 0.5% acidic NaF containing artificial saliva because of the formation of fluoride complex compound [20].
Certainly new SS archwires showed the least roughness value because it did not suffer from the conditions that lead to corrosion and any major surface defect in it may be the result of the manufacturing process. The surface of SS archwires immersed in DW shows an increased surface roughness than the new ones because water is corrosive in its nature, in addition to the effect of incubator temperature (37°C) and the chemicals of artificial saliva during period of incubation, while SS archwires surfaces which immersed in MW at both high and low magnetic powers have a slightly larger values of surface roughness than that of the archwires immersed in DW due to changing of water properties after being magnetized. The surface roughness value of archwire immersed in MWH shows less roughness value than that which immersed in MWL due to different magnetizing power used which may effect on water properties.
The explanation of the effect of MW on the corrosion process may be due to the change in properties of water when subjected to the magnetic field as changing in pH, dissolved oxygen, total dissolved solids, conductivity, salinity, evaporating temperature, organic matter, minerals and total count of bacteria [21]. Al-Bayar et al. stated that water after being magnetized have more solubility for minerals and acids in addition to increasing in soluble oxygen percentage and becomes electrical conductor therefore it could be accelerating biochemical reactions [22]. Goyal et al. stated that the MW has a lots of oxygen that can be immediately dissolved in that water [13]. Mghaiouini et al. concluded that the magnetic field reduced the dielectric resistance of treated water and increased its EC [23]. This results about EC of MW were agreed with the results obtained in this study when measured by EC Meter which also displayed an increase of EC of DW after its exposure to magnetic field. However, many of these water characteristic that altered after its magnetization may effect on metal corrosion in a water environment, including water temperature, pH level and oxygen content [24].
In the current study, the pH of MWL and MWH was respectively 0.1 and 0.2 higher than DW pH when measured by pH-Meter. This slight amount of pH elevation was non-significant and has no weight on the rate of corrosion.
It has also been stated that the SS rates of corrosion increase with pure water aeration and dissolved oxygen that found in pure water is about 5 to 10 times more destructive than carbonic acid [15]. Also corrosion in MW may be produced by the fact that the rates of corrosion subjected to increase when EC of water increases. It can be assumed that the lower the electrolyte electric resistance is, the lower the corrosion rate is [25].
So that, according to all the above mentioned results, the proposed hypotheses were rejected as the concentration of Cr ions release from SS archwires in the presence of MW significantly lower than that from OK mouthrinse. And the surface roughness of SS archwires immersed in MW differ obviously from those immersed in OK when viewed by AFM.
Conclusion
MW can be used as a safer alternative to other commercially existing mouthrinses (such as OK) for orthodontic patients. Because MW has a minor effect on SS orthodontic archwire corrosion as well as on Cr ion release from SS archwire.
References
- Ahmed RA, Farghali RA, Alshahrani WA. Influence of fluoride and/or bovine albumin on electrochemical properties of bare and ionic liquid-coated Ni47Ti49Co4 orthodontic archwires in artificial saliva solution. Int J Electrochem Sci 2021; 16:2.
- Mirhashemi A, Jahangiri S, Kharrazifard M. Release of nickel and chromium ions from orthodontic wires following the use of teeth whitening mouthwashes. Prog Orthod 2018; 19:1-5.
- Chantarawaratit PO. Yanisarapan T. Exposure to the oral environment enhances the corrosion of metal orthodontic appliances caused by fluoride-containing products: Cytotoxicity, metal ion release, and surface roughness. Am J Orthod Dentofacial Orthop 2021; 160:101-112.
- Gopalakrishnan U, Felicita AS, Qureshi T, et al. Effect of fluoridated mouthwashes on corrosion property of orthodontic appliances: A narrative review. J Contemp Dent Pract 2022; 23:460-466.
- Olszewska A, Hanć A, Barałkiewicz D, et al. Metals and metalloids release from orthodontic elastomeric and stainless steel ligatures: In vitro risk assessment of human exposure. Biol Trace Elem Res 2020; 196:646-653.
- Tahmasbi S, Sheikh T, Hemmati YB. Ion release and galvanic corrosion of different orthodontic brackets and wires in Artificial saliva. J Contemp Dent Pract. 2017; 18:222-227.
- Choi YY. Relationship between orthodontic treatment and dental caries: Results from a national survey. Int Dent J 2020; 70:38-44.
- Aghili H, Yassaei S, Eslami F. Evaluation of the effect of three mouthwashes on the mechanical properties and surface morphology of several orthodontic wires: An in vitro study. J Dent Res 2017; 14:252-259.
- Schiff N, Dalard F, Lissac M, et al. Corrosion resistance of three orthodontic brackets: a comparative study of three fluoride mouthwashes. Eur J Orthod 2005; 27:541-549.
- Lone N, Sidiq M, Khan M, et al. Short term effects of magnetised water and chlorhexidine on plaque accumulation and gingival inflammation: A randomised clinical study. Ann Int Med Dent Res 2016; 2:91-94.
- Nagpal DI, Mankar SS, Lamba G, et al. Effectiveness of magnetized water and 0.2% chlorhexidine as a mouth rinse in children aged 12–15 years for plaque and gingivitis inhibition during 3 weeks of supervised use: A randomized control study. J Indian Soc Pedod Prev Dent 2020; 38:419-424.
- Alattar EM, Elwasife KY, Radwan ES, et al. Influence of magnetized water on the growth of corn (Zea mays) seedlings. Rom J Biophys 2019; 29:39-50.
- Goyal AK, Rathore AS, Garg M, et al. Effect of magnetized water mouthrinse on Streptococcus mutans in plaque and saliva in Children: An in vivo study. Int J Clin Pediatr Dent 2017; 10:335-339.
- Taqa A, Sulieman R, Al-Sarraf HA. Artificial saliva sorption for three different types of dental composite resin (An in vitro study). EC Dent Sci 2019; 18:2339-2344.
- Danaei SM, Safavi A, Roeinpeikar SM, et al. Ion release from orthodontic brackets in 3 mouthwashes: An in-vitro study. Am J Orthod Dentofac Orthop 2011; 139:730-734.
- Yanisarapan T, Thunyakitpisal P, Chantarawaratit PO. Corrosion of metal orthodontic brackets and archwires caused by fluoride-containing products: Cytotoxicity, metal ion release and surface roughness. Orthod Waves 2018; 77:79-89.
- Heravi F, Moayed MH, Mokhber N. Effect of fluoride on nickel-titanium and stainless steel orthodontic archwires: An in-vitro study. J Dent 2015; 12:49-59.
- Pataijindachote J, Juntavee N, Viwattanatipa N. Corrosion analysis of orthodontic wires: an interaction study of wire type, pH and immersion time. ADOH 2018; 10:1-7.
- Husain S, Kumar A. Influence of commercially available herbal mouthwash on the surface tomography of two different types of nickel titanium orthodontic arch wires: An in vitro study. Biosc Biotech Res Comm 2020; 13:1331-1335.
- Pulikkottil VJ, Chidambaram S, Bejoy PU, et al. Corrosion resistance of stainless steel, nickel-titanium, titanium molybdenum alloy, and ion-implanted titanium molybdenum alloy archwires in acidic fluoride-containing artificial saliva: An in vitro study. J Pharm Bioallied Sci 2016; 8:96-99.
- Ebrahim SA, Azab AE. Biological effects of magnetic water on human and animals. Biomed Sci 2017; 3:78-85.
- Al-Bayar MA, Mahmood RM, Saieed AY. Magnetic treated water, reality and applications: A review. Plant Arch 2020; 20:732-737.
- Mghaiouini R, Elmlouky A, El Moznine R, et al. The influence of the electromagnetic field on the electric properties of water. Mediterr J Chem 2020; 10:507-515.
- Riyaz K, Shamsuddin S, Kenneth FH. Comparitive evaluation of ion release from orthodontic brackets in two mouthwashes and two gels: an in vitro study. Int J Appl Dent Sci 2021; 7:36-44.
- Zakowski K, Narozny M, Szocinski M, et al. Influence of water salinity on corrosion risk: The case of the southern Baltic Sea coast. Environ Monit Assess 2014; 186:4871-4879.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Zinah N Al Zubaidy1* and Afrah K Al Hamdany2
1Ninavah Health Directorate, Mosul, Iraq2Department of Pedodontics, Orthodontics and Preventive Dentistry, College of Dentistry, University of Mosul, Mosul, Iraq
Received: 19-Aug-2022, Manuscript No. jrmds-22-75046; , Pre QC No. jrmds-22-75046(PQ); Editor assigned: 22-Aug-2022, Pre QC No. jrmds-22-75046(PQ); Reviewed: 06-Sep-2022, QC No. jrmds-22-75046(Q); Revised: 09-Sep-2022, Manuscript No. jrmds-22-75046(R); Published: 16-Sep-2022
