Research - (2020) Advances in Dental Surgery
The Effect of Chicken Eggshell Extract on Microhardness of Artificially Induced Dental Erosion in Permanent Teeth (In Vitro Study)
Shatha A Abbas* and Alhan A Qasim
*Correspondence: Shatha A Abbas, Department of Pedodontic and Preventive Dentistry, College of Dentistry, University of Baghdad, Iraq, Email:
Abstract
Background: Chicken eggshell mainly comprises of calcium and phosphorous along with other minerals as trace elements, so it has been considered as a natural source of calcium for enamel remineralization.
Aim of the study: Test and compare the efficacy of two various application intervals of chicken eggshell powder (CESP) solution on the microhardness of artificial erosion like lesion of enamel, compared with sodium fluoride and de-ionized.
Materials and Methods: twenty sound permanent upper first premolars were used. Samples were distributed randomly as three study and one control groups, each one comprised of five teeth. Then all samples were subjected to demineralization by soft cola drink. The study groups are: Group A: untreated (demineralization followed by immersion in deionized water ,as control), Group B : demineralization followed by immersion in CESP solution for 7 consecutive day , Group C: demineralization followed by immersion in CESP solution 30 minutes twice daily for 7 days, Group D : demineralization followed by immersion in 0.05% sodium fluoride (NaF) solution 4 minutes daily for 7 days. Vickers microhardness test were performed before and after demineralization with coca cola drink and following treatment with the selected agent for pre-determined time intervals.
Results: There was a significant reduction in microhardness of all samples after demineralization. For groups B, C and D, a significant increase in microhardness (P < 0.05) following treatment with therapeutic solutions. Group B was statistically higher than other groups (54.13%, P= 0.001), followed by group C (44.52%, P=0.001) which was significantly higher than group D and A.
Conclusion: According to the results of the current study, eggshell has a remineralizing potential against initial enamel erosion.
Keywords
Chicken eggshell powder (CESP) solution, Erosion, Surface microhardness, Remineralization
Introduction
At recent years dental practice shifts towards focusing on minimally invasive dentistry which follow a more conservative techniques that mainly emphasize on caries detection at initial stage, remineralization of dental surfaces and maintenance of tooth structure surrounding the lesion [1]. Beside dental caries, dental erosion become threatening problem affecting nearly all ages at many developing countries [2].
Dental Erosion is a chemical process affecting the tooth surfaces owing to the acids from either extrinsic or intrinsic origins that result in dissolution of the tooth minerals. Most frequent extrinsic agents are carbonated drinks, acidic food, and repeated exposure to environmental acids. Intrinsic source of erosion is primarily associated with gastro esophageal reflux and vomiting in which gastric acid reaches the oral cavity [3]. Even though, teeth are subjected continuously to demineralization and remineralization cycles, this equilibrium can certainly be disturbed because of frequent consumption of low pH beverage; for instance, acidic drinks, juices and wines which produced tooth minerals dissolution followed by damage of tooth structure. This critical process leads to irreversible loss of dental substance, so teeth susceptibility to abrasion increases and may as well causes wear of dental tissues, tooth sensitivity and in advance cases pulpal exposure [4].
Topical fluoride application is one of preventive techniques in the management of teeth erosion, its effectiveness as anti-erosive agent has been proved by previous studies [5,6].
sodium fluoride is one of fluoride compound that has anti-caries and anti-erosion effects. Fluoride application results in calcium fluoride (CaF2) layer formation on the enamel surface, which has the function of being a fluoride reservoir [7]. When pH in the oral cavity decreases, CaF2- deposits at first release fluoride ions which later integrated with tooth minerals forming fluoroapatite or fluorohydroxyapatite, leading to reduction in tooth vulnerability to additional dissolution [8]. Additional mechanism for anti-erosion is the CaF2-layer may act as a mechanical barrier hindering acids contact with the underlying enamel [9].
CESP has considered to be an important natural source of calcium that can be used in different fields. It comprises of calcium carbonate, calcium phosphate, magnesium carbonate, and organic matter in percentage of 94%, 1%, 1% and 4% respectively [10]. CESP has tremendous antirachitic properties as demonstrated in animals and humans’ studies. Furthermore, it decreases pain, bone resorption and rises bone density in female after menopause and in osteoporosis, thus it is appropriate in the management of osteoporosis [11,12]. Researchers had used eggshell for synthesis of hydroxyapatite with good properties [13,14].
CESP was used for production of bio-preparation known as Biomin H which is considered as a natural, rich calcium source. Studies demonstrated that Biomin H had positively affected the health of bone and cartilage and is appropriate for prevention and management of osteoporosis [12].
Eggshell was also used in treatment of maxillofacial defect as a substitute to bone graft and the results of these studied had shown that it is a biocompatible and effective in bone regeneration [14].
CESP was as well evaluated for remineralization of initial enamel caries lesion and erosive enamel lesion and the results demonstrated its remineralizing potential [15-19].
No previous study has evaluated different application intervals of CESP solution against dental erosion, so this study was aimed to compare the effectiveness of two various application intervals of CESP to that of sodium fluoride for remineralizing an intial enamel erosion in permanent teeth.
Materials and Methods
CESP solution preparation
CESP had prepared according to the calcination protocol (WO/2004/105912: Method of Producing eggshell powder). Calcium carbonate presented in 95% is converted into calcium oxide owing to calcination, this in turn be responsible for increasing alkalinity [20]. Twenty chicken eggs were used. After washing with de-ionized water, these were left in boiled water for ten minutes to assist in membranes removal. The eggshells were then grinded into small particles with sterile mortar and pestle. The small particles gained were kept in an ofen (Combilabor CL-V, Heraus Hanau, Japan) at 1000°C for 90 minutes to be sure that the obtained powder is free of pathogen. The CESP solution was prepared by dissolving a gram of CESP in 20ml of de-ionized water containing acetic acid in 4%. The fluid at the top of the graduated cylinder was taken and used in this study. The solution pH was measured by a digital pH meter Consort, Belgium) and found to be 11.8.
Sample preparation
Twenty maxillary first premolars extracted for orthodontic purpose were cleaned, polished with conventional low speed hand piece and non-fluoridated pumice, then washed with deionized water and wiped with acetone to insure a thorough removal of pumice remnants. Any tooth with crack or enamel defect were excluded, after that they were kept in a plastic screwed container filled with de-ionized water, 0.1% thymol crystals were added for the purpose of inhibiting bacterial growth. Samples stored at room temperature until use.
Enamel surface preparation
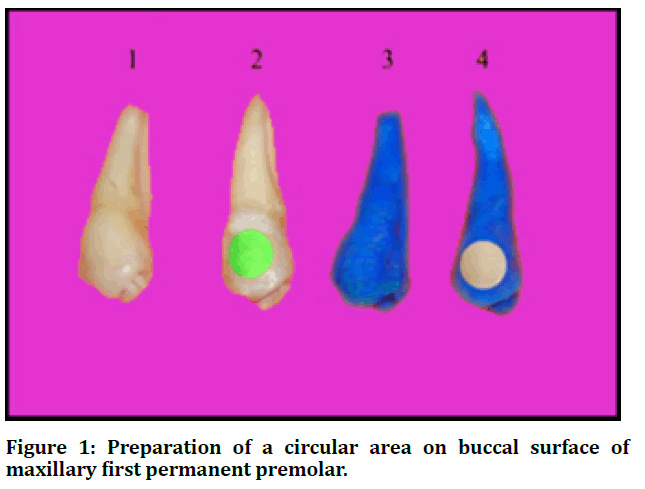
A circular area (6 mm in diameter) was drawn in the middle of the buccal surface of each tooth. An adhesive tape circle of the same diameter was then placed on the circular area, subsequently each tooth was coated with a nail varnish, and the tape was removed exposing a circular area on the buccal surface; (Figure 1). Teeth were adjusted in an acrylic mold by means of red wax. Finally, the circular area of each tooth was polished 10 times in single direction with grit paper (number 400) that was positioned in manual device shown in figure 2. This technique performed to produce a flat tooth surface designed for microhardness measurement [21].
Figure 1: Preparation of a circular area on buccal surface of maxillary first permanent premolar.
Figure 2: Special manual device.
Demineralization procedure
A carbonated beverage (Pepsi Cola, Baghdad) was selected for induction of initial enamel erosion. The pH of the beverage was 2.1 measured at 20°C. The teeth were immersed individually in six milliliters of Pepsi cola for two minutes at room temperature. Four sequential cycles were performed at 6 hours interval, in between the cycles the samples were rinsed with de-ionized water and stored in containers filled with de-ionized water [22].
Sample grouping
Teeth samples were distributed randomly to four groups:
Group A: untreated (demineralization followed by immersion in deionized water, as control)
Group B: demineralization followed by immersion in CESP solution for 7 consecutive days
Group C: demineralization followed by immersion in CESP solution 30 minutes twice daily for 7 days
Group D: demineralization followed by immersion in 0.05% NaF solution 4 minutes daily for 7 days
Vickers microhardness device was used for enamel microhardness measurement. The measurement was done primarily for normal enamel and following initiation of dental erosion. Subsequently, teeth were treated with a certain agent solution by immersing each sample individually in 20 ml of the chosen solution prepared daily for pre-determined period, then; teeth were washed individually with de-ionized water for 2 minutes and kept in de-ionized water at room temperature for the next day. This process was daily repeated for 7 days, and finally teeth were subjected to a third microhardness measurement.
Surface microhardness assessment
The measurement was accomplished with digital Vickers micro hardness tester in the Metal testing laboratory, Department of Production Engineering and Metallurgy, University of Technology. The used load was 500 grams for a period of 30 seconds. For each sample, three records were taken, and the mean of records was calculated. Vickers microhardness test was done using optical microscope and the used magnification was X50. The device has a square tipped diamond indenter. The included angle between the opposed faces was 136°.
Statistical analysis
The data was analyzed using SPSS version 25. Analysis of Variance (ANOVA) was used to compare the mean of enamel microhardness between the four groups. To confirm the differences occurred between groups, post hoc analysis (LSD) was used. Paired t-test had been used for comparing the mean of enamel microhardness at baseline, following demineralization and after remineralization. A level of P – value less than 0.05 had been considered significant.
Results
Table 1 demonstrated the comparison between study groups by baseline enamel microhardness of teeth, no statistically significant difference (P=0.23) in enamel microhardness was found between study groups. The mean and SD for all samples at this stage was (382.55 ± 29.2). After demineralization there is a significant reduction in enamel microhardness for all groups (P=0.001) and the mean was measured to be (222.51 ± 13.6). Following treatment, a significant increase in the microhardness for groups B, C and D (P<0.05) while for group A, no statistically significant difference (P=0.306) in enamel microhardness compared to that following demineralization (Table2). Table 3 shows the comparison between study groups in percentage of change in enamel microhardness after remineralization compared to demineralization. It was obvious that this percentage of change in enamel microhardness was significantly higher in group B than in other groups (54.13%, P= 0.001). Post hoc analysis (LSD) was done to confirm the variances occurred between groups and showed that the percentage of change in enamel microhardness was significantly higher in group B than that in all other groups. Also, it was significantly higher in group C than that in groups (A and D), significantly higher in group D than that in group A as shown in table 4.
Study group |
Baseline enamel microhardness | P–Value | ||
|---|---|---|---|---|
| Mean ± SD | Lower Bound | Upper Bound | ||
| A | 375.26 ± 29.0 | 338.15 | 409.1 | 0.23 |
| B | 407.82 ± 13.3 | 392.45 | 428.55 | |
| C | 378.48 ± 15.1 | 397.18 | 359.85 | |
| D | 368.67 ± 48.9 | 297 | 428.55 | |
Table 1: Comparison between study groups by baseline enamel microhardness.
Study group |
Enamel microhardness | P – Value | |
|---|---|---|---|
| After demineralization | After remineralization | ||
| Mean ± SD | Mean ± SD | ||
| Group A | 223.68 ± 11.6 | 224.5 ± 12.9 | 0.306 |
| Group B | 227.35 ± 12.5 | 349.77 ± 6.7 | 0.001 |
| Group C | 209.38 ± 8.4 | 302.14 ± 4.3 | 0.001 |
| Group D | 229.63 ± 9.2 | 280.55 ± 8.0 | 0.001 |
Table 2: Comparison in enamel microhardness after remineralization with that following demineralization in each study group.
Study Group |
Percentage of change in enamel microhardness after remineralization compared to demineralization (%) | P – Value | |
|---|---|---|---|
| Mean ± SD | Range | ||
| A | 0.34 ± 0.7 | - 0.5 – 1.29 | 0.001 |
| B | 54.13 ± 6.9 | 46.41 – 60.66 | |
| C | 44.52 ± 7.2 | 35.81 – 51.79 | |
| D | 22.3 ± 5.3 | 17.37 – 31.17 | |
Table 3: Comparison between study groups in percentage of change in enamel microhardness after remineralization with that following demineralization.
Study group |
||||
|---|---|---|---|---|
| A | B | C | D | P-Value |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| 0.34 ± 0.7 | 54.13 ± 6.9 | - | - | 0.001 |
| 0.34 ± 0.7 | - | 44.52 ± 7.2 | - | 0.001 |
| 0.34 ± 0.7 | - | - | 22.3 ± 5.3 | 0.001 |
| - | 54.13 ± 6.9 | 44.52 ± 7.2 | - | 0.043 |
| - | 54.13 ± 6.9 | - | 22.3 ± 5.3 | 0.001 |
| - | - | 44.52 ± 7.2 | 22.3 ± 5.3 | 0.001 |
Table 4: Post hoc tests (LSD) to confirm the differences occurred between groups after remineralization compared to demineralization stage.
Discussion
Although dental erosion is a preventable disease, it remains common and is still a public health problem. Hence, there is a continuous need to prevent the occurrence of dental erosion and search for additional techniques intended for disease prevention, or new means for enhancing the existing preventive programs.
Eggs shell is a non-palatable material, commonly discarded as a waste product. Recently, its usage in different fields has been extensively studied [11,12]. Owing to its higher calcium constituent, it has been used as human dietary calcium supplement particularly for aged poupulations and women after menopause [11]. The bioavailablility of calcium in higher concentration ensures CESP role in enamel remineralization when its applied topically [16]. The purpose of calcination process of CESP is to increase its alkalinity and eliminate pathogens. Additionally, the acetic acid (4%) confirmed that the prepared solution is nearly pathogens free [20]. PH of CESP solution was measured to be 11.8, this higher pH is encouraging for the improved ionic activity of hydroxyl and phosphate ions. Accordingly, enamel remineralization is enhanced by the availability of these ions. If the therapeutic solution has low PH, the H+ ions will combine with the presented anions, hence the availability of these ions for remineralization are reduced [16].
Sodium fluoride can reinforce and strengthen enamel. The effectiveness of different fluoride formulations in prevention of erosive lesion has been revealed in some studies [5,6].
In this in vitro study, the anti-erosive effects of CESP solution on permanent teeth were studied. Moreover, the effect of CESP was compared to a commonly used sodium fluoride solution by using micro hardness tester. Additionally, for the reason of being most frequently consumed, Pepsi cola was selected as a demineralizing solution to stimulate surface erosion. Besides, to lessen the buffering action created by ionic dis¬solution of tooth surfaces the carbonated beverage was replaced for every 2 minutes immersion [23].
Enamel subsurface has less minerals than enamel surface. So, polished tooth samples were used to reduce the natural difference of enamel surface among the samples that may react in a different way to acidic dissolution [24]. A primary manifestation of dental erosion is enamel softening that can be measured with either Knoop or Vickers indenter. In the current study Vickers micro hardness tester was used. The results of the current study suggested a significant difference in enamel microhardness values between the four different groups after treatment with the selected solutions.
There was a significant reduction in enamel microhardness following demineralization with Pepsi cola in all groups as an indication of enamel demineralization and initiation of erosive lesion. The results of this study revealed that the two treatments provided to Group B, C, and D could increase enamel hardness as compare to specimens in Group A receiving no treatment after demineral¬ization in the permanent teeth.
After treatment of enamel samples with CESP in group B and C, a statistically significant increase in microhardness values was found. This revealed the incorporation of calcium and phosphorus ions which repaired surface defect and increased microhardness of enamel surface. The calcium ions are the major component of apatite crystals so upon this element incorporation in the outer enamel surface the values of the microhardness elevated. This result agrees with (Feroz et al.) which revealed that CESP can significantly increases the microhardness as well as calcium and phosphorous level of all treated tooth samples. In addition to erosive demineralization prevention, CESP likewise can remineralize the initial enamel carious lesion [18].
Group B showed a statistically significant difference and the highest enamel microhardness (54.13%) followed by group C (44.52%), so this revealed a continuous increase in microhardness value with increasing time intervals. However, group C showed an efficacy for remineralization of induced erosive enamel lesion as compared with group A(control group) and group D, so it appeared that lesser time interval could also increase the microhardness value and had a remineralizing effect. No previous study tested different application intervals is available to compare with.
Group D showed an increase in enamel microhardness which is statistically significant in comparison to group A but lesser than that provided by CESP in other groups. This result is in agreement with (Wiegand et al.) [9] and (Feroz et al.) [18] according to which fluoride was found to have a protective effect on tooth enamel, but it cannot absolutely inhibit the demineralization process. Even though remineralization occurred, none of the used solutions was capable of increasing enamel microhardness to the primary values. This may possibly be owing to the period of application used in this study. Samples were treated for different intervals in one-week period. Using more treatment time may increase the microhardness values; but this may require to be investigated by other studies.
Conclusion
The effectiveness of CESP in remineralizing erosive enamel lesions was showed by statistically significant increase in the microhardness of groups treated with it. Although microhardness value increased with increasing the period of immersion in CESP solution, the lesser immersion time used could also produce a significant increase in microhardness as compared to control group and group treated with NaF. CESP with its natural and economic availability has a promising future in prevention and treatment of dental erosion. However, there is a need for more clinical studies to prove its remineralizing potential in vivo and to recommend its addition to commercial dental products.
References
- Murdoch_Kinch CA, McLean M. Minimally invasive dentistry. J Am Dent Assoc. 2003; 134:87-95.
- Jaeggi T, Lussi A. Prevalence, Incidence and distribution of erosion. Monogr Oral Sci 2006; 20:44-65.
- Poggio C, Lombardini M, Dagna A, et al. Protective effect on enamel demineralization of a CPP-ACP paste: An AFM invitro study. J Dent 2009; 37:949-54.
- Lussi A, Jaeggi T, Zero D. The role of diet in the aetiology of dental erosion. Caries Res 2004; 38:34-44.
- Ren Y, Zhao Q, Malmstrom H, et al. Assessing fluoride treatment and resistance of dental enamel to soft drink erosion in vitro: Applcations of focus variation 3D scanning microscopy and stylus profilometry. J Dent 2009; 37:167-176.
- Murakami C, Bonecker M, Correa MS, et al. Effect of fluoride varnish and gel on dental erosion in primary and permanent teeth. Arch Oral Biol 2009; 54:997-1001.
- Navarro M, Alto LAM, Cruz RA, et al. Calcium fluoride uptake by human enamel after use of fluoridated mouthrinses. Braz Dent J 2001; 12:178-182.
- Ten Cate J. Review on fluoride, with special emphasis on calcium fluoride mechanisms in caries prevention. Europ J Oral Sci 1997; 105:461-465.
- Wiegand A, Attin T. Influence of fluoride on the prevention of erosive lesions-A review. Oral Health Preventive Dent 2003; 1:245-253.
- Stadelman WJ. Eggs and egg products. In: Francis FJ. Encyclopedia of food science and technology. 2nd Edn NewYork: John,Wiley and Sons 2000; 593-599.
- Schaafsma A, Doormaal JJ, Muskiet FA, et al. Positive effects of a chicken eggshell powder-enriched vitamin-mineral supplement on femoral neck bone mineral density in healthy late post-menopausal Dutch women. Br J Nutr 2002; 87:267-275.
- Rovenský J, Stancíková M, Masaryk P, et al. Eggshell calcium in the prevention and treatment of osteoporosis. Int J Clin Pharmacol Res 2003; 23:83-92.
- Krishna DS, Siddharthan A, Seshadri SK, et al. A novel route for synthesis of nanocrystalline hydroxyapatite from eggshell waste. J Mater Sci Mater Med 2007; 18:1735-1743.
- Kattimani VS, Chakravarthi PS, Kanumuru NR, et al. Eggshell derived hydroxyapatite as bone graft substitute in the healing of maxillary cystic bone defects: A preliminary report. J Int Oral Health 2014; 6:15-19.
- Haghgoo R, Mehran M., Ahmadvand M, et al. Remineralization effect of eggshell versus nano-hydroxyapatite on caries-like lesions in permanent teeth (in vitro). J Int Oral Health 2016; 8:435-439.
- Mony B, Ebenezar A, Ghani MF, et al. Effect of chicken egg shell powder solution on early enamel carious lesions: An Invitro preliminary study. J Clin Diagn Res 2015; 9:30-32.
- Yaberi M, Haghgoo R. A comparative study of the effect of nanohydroxyapatite and eggshell on erosive lesions of the enamel of permanent teeth following soft drink exposure: A randomized clinical trial. J Int Oral Health 2018; 10:176-179.
- Feroz S, Moeen F. Protective effect of two different remineralizing agents on artificially induced dental erosion in primary and permanent teeth: An in-vitro analysis. Pakistan Oral Dent J 2017; 37:657-666.
- Feroz S, Moeen F, Haq SN. Protective effect of chicken egg shell powder solution (CESP) on artificially induced dental erosion: An in Vitro atomic force microscope study. Int J Dent Sci Res 2017; 5:49-55.
- Shen P, Manton D, Cochrane NJ, et al. Effect of added calcium phosphate on enamel remineralization by fluoride in a randomized controlled in situ trial. J Dent 2011; 39:518-525.
- Al-Sayyab M. The potential effect of combined CO2 laser and fluoride on acid resistance of human dental enamel and root surface in vitro. PhD thesis, Preventive Dentistry, University of Baghdad. 2000.
- Tantbirojin D, Hung A, Ericson MD, et al. Change in surface hardness of enamel by a cola drink and a CPP-ACP paste. J Dent 2008; 36: 74-79.
- Johansson A, Sorvari R, Birkhed D, et al. Dental erosion in decid¬uous teeth-an in vivo and in vitro study. J Dent 2001; 29:333-340.
- Adebayo OA, Burrow M, Tyas MJ. An SEM evaluation of conditioned and bonded enamel following carbamide peroxide bleaching and casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) treatment. J Dent 2009; 37:297-306.
Author Info
Shatha A Abbas* and Alhan A Qasim
Department of Pedodontic and Preventive Dentistry, College of Dentistry, University of Baghdad, IraqCitation: Shatha A Abbas, Alhan A Qasim, The Effect of Chicken Eggshell Extract on Microhardness of Artificially Induced Dental Erosion in Permanent Teeth (In Vitro Study), J Res Med Dent Sci, 2020, 8 (7): 42-47.
Received: 27-Sep-2020 Accepted: 13-Oct-2020 Published: 20-Oct-2020