Research Article - (2025) Volume 13, Issue 4
The cytotoxic Effect of Iraqi Hyacinthus orientalis against breast Cancer Cells
Noor Mohammed Shareef* and Thukaa Z Abdul-jalil
*Correspondence: Noor Mohammed Shareef, Department of Pharmacognosy and Medicinal Plants, Collage of Pharmacy, University of Baghdad, Baghdad, Iraq, Email:
Abstract
Plants are significant stocks for cytotoxic agents especially compounds having role to deal with multidrug resistant cells. With the aim of finding promising chemotherapy players, Hyacinthus orientalis L. is one of the ornamental plant cultivated in Iraq being in the circle of interest coming up with our best knowledge, there are no published reports comparing between the three different Iraqi’s plant parts (flowers, leaves and bulbs) contents with cytotoxic activities. Extraction were done, for each plant parts, by Soxhlet apparatus follow by fractionation with petroleum ether and ethyl acetate to obtain the target of this study, evaluation of the ethyl acetate fraction of Hyacinthus orientalis L. different parts was done in vitro as cytotoxic agent against human breast cancer (MCF-7) cell line, using the MTT assay. The most effective fraction in the MCF-7 cell line was the flower ethyl acetate fraction (IC50 2.014). The bulbs ethyl acetate fraction (IC50 5.507) and the leaves ethyl acetate fraction (IC50 11.38) were also found to be effective. These results demonstrated that phytoconstituents of Iraqi Hyacinthus orientalis L. should be digged deep for further possible promising chemotherapy players in cancer treatment.
Keywords
Hyacinthus orientalis L., Michigan cancer foundation-7, Cytotoxic activities, Flavonoids and phenolic compounds
Introduction
Cancer is a leading cause of death worldwide accounting for nearly 10 million deaths in 2020, accounting to recent World Health Organization (WHO) there were 2.3 million women diagnosed with breast cancer [1]. Traditional cancer therapies including surgery, radiation and chemotherapy have their own disadvantages. The use of chemotherapy is associated with cancer recurrence, emergence of resistance and toxicity to the immune system and normal cells. Therefore, finding new medications with low toxicity and higher activity is a priority demand in cancer researches [2].
Plants exhibit an unlimited source of biologically active natural products having high potential to target cancer cells with relatively limited toxicity. However, the use of many plant derived anticancer agents is associated with known toxic effects and development of treatment resistance. Estimations worldwide showed that around 70,000 plant species are used for medical purposes [3]. Natural products are gaining a significant attention as effective anticancer agents recently. Exploring the ability of plants to treat cancers revealed more than 3000 species with anticancer properties [4]. Currently, more than 60% of anticancer agents originate from plants like vincristine and vinblastine (from Catharanthus roseus), Taxol (from Taxus brevifolia), Camptothecin (from Camptotheca acuminate) and Harringtonine and Homoharringtonine (from Cephalotaxus harringtonia) [5].
The anticancer effect of these natural products is mediated by different mechanisms, including apoptosis induction, immune system modulation and angiogenesis inhibition [6]. The use of herbal medicine by cancer patients is a common practice, as in the USA, more than 35% of cancer patients are using herbal medicine with chemotherapy [7]. Additionally; in the UK herbal medication is effectively used for multiple purposes, such as preventing or relieving some of the direct symptoms of the cancer itself or dampening down the direct side effects of chemotherapy and radiotherapy used in the initial treatment process [8]. Despite this, there have been a limited number of studies to evaluate the biological activity of a broad spectrum of herbs traditionally used to treat cancer.
Hyacinthus orientalis L. is an ornamental plant belonging to Hyacinth genus of the Asparagaceae (formerly, Hyacinthaceae) family, as shown in Figure 1. Hyacinth is a small genus of bulbous herbs native to the Mediterranean region and tropical Africa, widely grown in Turkey, France and Netherlands [9,10].

Figure 1: Hyacinthus orientalis L. plant.
In traditional medicine, the whole plant flowers, leaves and bulbs of H. orientalis L. were used as hemostatic for the treatment of prostate disease and hemorrhoids and wound healing. Although limited studies have tested, the biological activities of this plant, chemical analysis revealed the presence of nine acylated anthocyanins in the flowers of H. orientalis L. [11-15]. Anthocyanins are well-known for their anti-oxidant, anti-inflammatory and anti-mutagenesis effects [16]. Also chemical studies show the presence of phenolic and flavonoid compounds with antioxidant and anticancer effects, compounds isolated from this plant showed potent inhibitory activity toward α-glucosidase and therefore have the potential to be used in the treatment of diabetes [17,18]. The present study investigates the in vitro cytotoxicity of the ethyl acetate fractions of three different parts of Iraqi Hyacinthus orientalis L. against human breast cancer (MCF-7) cell line. The selection of L. plants was based on different literature sources, folklore and traditional medicine to discover the new source of bioactive compounds with anticancer effects.
Materials and Methods
Chemicals and reagents
The main chemicals and reagents used in this study will be illustrated in Table 1 as shown below:
| No. | Chemicals and reagents | Company | Country | Uses |
|---|---|---|---|---|
| 1 | DMSO | Santacruz biotechnology | USA | Amphipathic solvent. |
| 2 | Ethyl acetate 99.5% | Scharlab S.L | Spain | Extraction solvent. |
| 3 | Ethanol absolute | Alpha chemika | India | Extraction solvent. |
| 4 | Ferric Chloride (FeCl3) powder | Fluka | Switzerland | Phenolic acid test. |
| 5 | Fetal bovine serum | Capricorn | Germany | Supplement to the basal growth medium in cell culture applications. |
| 6 | Hydrochloric acid (36%-38%) | Thomas baker | India | Flavonoid test. |
| 7 | MTT stain | Bio-world | USA | Indicator of cell viability, proliferation and cytotoxicity. |
| 8 | NaOH (Sodium Hydroxide) | Xilong chemical Co | China | Flavonoid test. |
| 9 | Petroleum ether (40-60) | Siuopharm chemical reagent Co. Ltd | China | Extraction solvent. |
| 10 | RPMI 1640 | Capricorn | Germany | Growth medium. |
| 11 | Trypsin/EDTA | Capricorn | Germany | Metal chelator. |
| 12 | (MCF-7) | Michigan cancer foundation-7 is a breast cancer cell line isolated from a 69-year-old White woman. | ||
Table 1: Chemicals and reagents used.
Instruments
The main instruments used in this study will be illustrated in Table 2 as shown below:
| No. | Item | Company | Country |
|---|---|---|---|
| 1 | Cell culture plates | Santa cruz biotechnology | USA |
| 2 | Incubator | Cypress diagnostics | Belgium |
| 3 | Laminar flow hood | K and K scientific supplier | Korea |
| 4 | Microtiter reader | Gennex lab | USA |
| 5 | Micropipette | Cypress diagnostics | Belgium |
| 6 | Oven: Memmert 854 | Buchi | Germany |
| 7 | Rotary evaporator | BUCHI\Germany, rotatory evaporator attached to vacuum pump | Germany |
Table 2: Instruments used.
Collection and extraction
The different parts (flowers, leaves and bulbs) of Hyacinthus orientalis L. were collected from Baghdad nursery plantation in February 2021. The plant was identified and authenticated by Prof. Dr. Zainab Abd Aon/department of biology/college of sciences/university of Baghdad. The plant parts were washed thoroughly, dried under shade and ground in a mechanical grinder to a fine powder. The air-dried powder of the plant is weighted then extracted in Soxhlet with (1:10) aqueous ethanol 85% for 18 hr. then the extract was dried by rotary evaporator the dry extract is weighted and the yield of extraction is calculated. The dry extract is dissolved in water and then partitioned 3 times with various solvent of increasing polarity, started with petroleum ether to get rid of chlorophyll and waxy material follow by ethyl acetate. Ethyl acetate fraction is dried, weighted and prepared for phytochemical screening and cytotoxic study [19].
Preliminary phytochemical examination of plant extracts
Phytochemical analysis for the screening and identification of bioactive chemical constituents in the medicinal plants under study was carried out on ethyl acetate fractions of different parts using the standard procedures as described by Harborne [20-23].
Flavonoid detection (alkaline reagent test): One ml of ethyl acetate fraction for each plant parts (flowers, leaves and bulbs)+two mL of 2% NaOH solution+few drops diluted HCl, an intense yellow color, becomes colorless on the addition of dilute acid indicates the presence of flavonoids.
Ferric chloride test for phenolic compounds: One mL of ethyl acetate fraction for each plant parts (flowers, leaves and bulbs)+3 drops 10% Ferric chloride solution, a blue-green color indicates the presence of phenolic compounds.
Cytotoxic evaluation
Maintenance of cell cultures: MCF-7 cell lines, was maintained in MEM supplemented with 10% fetal bovine, 100 unit/mL penicillin and 100 μg/mL streptomycin. Cells were passaged using trypsin-EDTA reseeded at 50% confluence twice a week and incubated at 37°C [24,25].
Cytotoxicity assays: The cytotoxicity effect of Hyacinthus orientalis was determined by the MTT assay using 96 well plates [26]. Cell lines were seeded at 1 × 104 cells/well and after 24 hrs (a confluent monolayer was achieved); cells were treated with tested compounds at different concentrations. Cells viability was measured after 72 hrs of treatment by removing the medium, adding 28 μL of 2 mg/mL solution of MTT, and incubating the cells for 2.5 h at 37°C. After removing the MTT solution, the crystals remaining in the wells were solubilized by the addition of 130 μL of DMSO (Dimethyl Sulphoxide) followed by 37°C incubation for 15 min with shaking [27]. The absorbency was determined on a microplate reader at 492 nm the assay was performed in triplicate. The inhibition rate of cell growth (the percentage of cytotoxicity) was calculated by the following equation [28].
% Cell viability=(Absorbance of treated cell/Absorbance of non-treated cell) × 100
% Cytotoxicity=100-cell viability
Statistical analysis
The obtained data were statically analyzed using an unpaired T-test with graph pad prism 6 [29]. The values were presented as the mean ± SD of triplicate measurements and P value<0.05 which was considered as significant [30].
Results and Discussion
Soxhlet apparatus is used as it represents a closed system, plant material is not in direct contact with the source of heat and minimum volume of solvent is required. The use of a hot extraction method is preferable as heat will facilitate the penetration of plant material by the solvent by breaking the plant tissue fibers. The percentage of yield of crude ethanolic extracts and weight of ethyl acetate fraction of each plant parts (flowers, leaves and bulbs) are illustrated in Table 3.
| Parts of plant | % yield of crude ethanolic extracts | Weight of ethyl acetate fraction |
|---|---|---|
| Flowers | 40% | 0.92 gm |
| Leaves | 45% | 0.93 gm |
| Bulbs | 13.60% | 0.34 gm |
Table 3: Percentage of yield of crude ethanolic extracts and weight of ethyl acetate fraction of each plant parts.
The phytochemical screening in the present study, indicated the presence of, flavonoids and phenolic acids in the ethyl acetate fraction of flowers, bulbs and leaves extracts as shown in Table 4 below:
| Phytochemicals | Flowers | leaves | Bulbs |
|---|---|---|---|
| Flavonoids | + | + | + |
| Phenols | + | + | + |
Table 4: Preliminary phytochemical examination of different plant parts extracts.
The cytotoxic effect of Hyacinthus orientalis against breast cancer cells was evaluated for its antitumor activity assessed by studying their ability to inhibit the proliferation of cancer cells through breast cancer cells line, and considered as first study that deals with effect of each part (flowers, leaves and bulbs) of the Iraqi Hyacinthus orientalis plant on breast cancer cell line. The results of this research were highly significant for the cytotoxic activity of flowers having the lowest IC50 in relation for bulbs and leaves against the human breast cancer cell lines as shown in Figures 2-8, that means that; the flowers of Hyacinthus orientalis have higher anticancer activity compared by others parts of plant (leaves and bulbs). The growth suppression of cancer cell lines is concentration dependent. The proposed mechanisms for the cytotoxicity against cancer cells may be related to apoptosis induction.
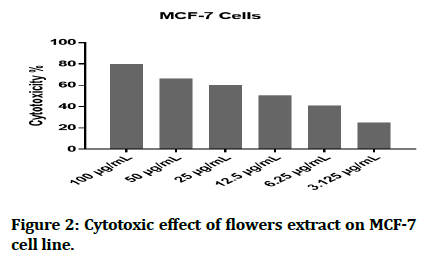
Figure 2: Cytotoxic effect of flowers extract on MCF-7 cell line.
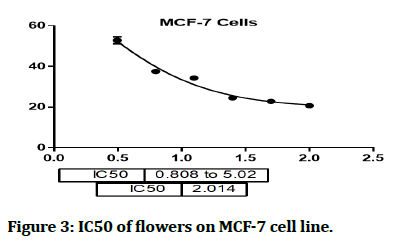
Figure 3: IC50 of flowers on MCF-7 cell line.
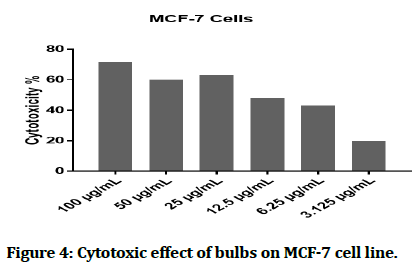
Figure 4: Cytotoxic effect of bulbs on MCF-7 cell line.
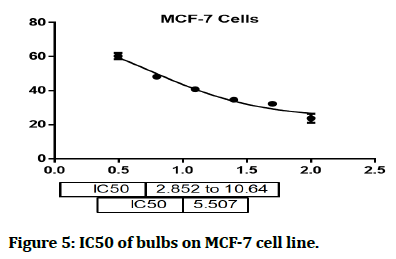
Figure 5: IC50 of bulbs on MCF-7 cell line.
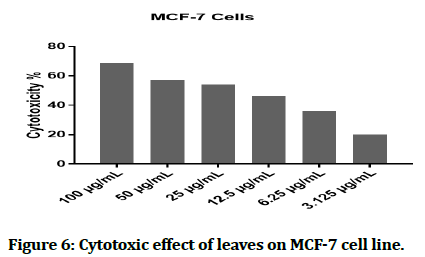
Figure 6: Cytotoxic effect of leaves on MCF-7 cell line.
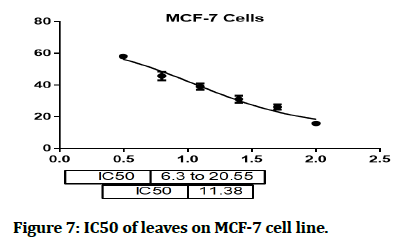
Figure 7: IC50 of leaves on MCF-7 cell line.
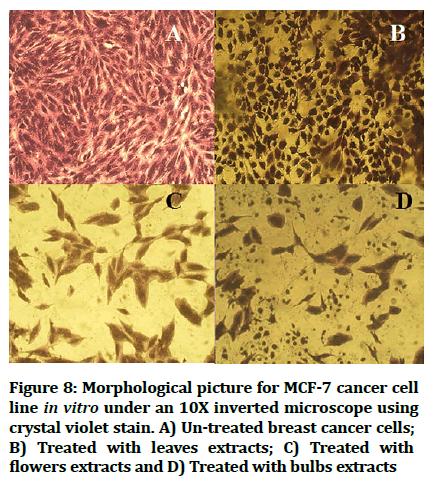
Figure 8: Morphological picture for MCF-7 cancer cell line in vitro under an 10X inverted microscope using crystal violet stain. A) Un-treated breast cancer cells; B) Treated with leaves extracts; C) Treated with flowers extracts and D) Treated with bulbs extracts.
The workout phytochemical screening indicates the presence of flavonoids and phenolic acids in the ethyl acetate fraction of flowers, bulbs and leaves extracts. Flavonoids are known for their antioxidant and free radicals scavenging properties, also phenolic compounds exhibited anticancer activity affecting multiple key elements related to cell proliferation, apoptosis, angiogenesis and metastasis [31].
Conclusion
In this study, the cytotoxic activity of ethyl acetate fraction from three different parts of Iraqi Hyacinthus orientalis was examined. These fractions could represent potential cytotoxic agents against the examined human breast cancer (MCF-7) cell line in a concentration dependent manner. The most effective fraction in the MCF-7 cell line was the flower ethyl acetate fraction (IC50 2.014). The bulbs ethyl acetate fraction (IC50 5.507) and the leaves ethyl acetate fraction (IC50 11.38) were also found to be effective. These fractions represent a new step option for inhibition of cancer cell metastasis. Further studies are needed to clarify the phytoconstituents which are responsible for the anticancer effects and reassessment of the dose response relationship in vitro and in vivo.
References
- Ferlay J, Ervik M, Lam F, et al. Global cancer observatory: Cancer today. International Agency for Research on Cancer. Lyon, France. 2020.
- Housman G, Byler S, Heerboth S, et al. Drug resistance in cancer: An overview. Cancers 2014; 6:1769-1792.
[Crossref] [Google Scholar] [PubMed]
- Kuruppu AI, Paranagama P, Goonasekara CL. Medicinal plants commonly used against cancer in traditional medicine formulae in Sri Lanka. Saudi Pharm J 2019; 27: 565-573.
[Crossref] [Google Scholar] [PubMed]
- Graham JG, Quinn ML, Fabricant DS, et al. Plants used against cancer-An extension of the work of Jonathan Hartwell. J Ethnopharmacol 2000; 73:347-377.
[Crossref] [Google Scholar] [PubMed]
- Khan T, Ali M, Khan A,et al. Anticancer plants: A review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules 2019; 10:47.
[Crossref] [Google Scholar] [PubMed]
- Rayan A, Raiyn J, Falah M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS One 2017; 12: e0187925.
[Crossref] [Google Scholar] [PubMed]
- Leng JC, Gany F. Traditional Chinese medicine use among Chinese immigrant cancer patients. J Cancer Educ 2014; 29: 56-61.
[Crossref] [Google Scholar] [PubMed]
- Damery S, Gratus C, Grieve R, et al. The use of herbal medicines by people with cancer: A cross sectional survey. Br J Cancer 2011; 104: 927-933.
[Crossref] [Google Scholar] [PubMed]
- Christopher B. The royal horticultural society az encyclopedia of garden plants. Dorling Kindersley Publisher. London, UK. 1996:884-885.
- Hu F, Liu H, Wang F, et al. Root tip chromosome karyotype analysis of hyacinth cultivars. Genet Mol Res 2015; 14:10863-10876.
[Crossref] [Google Scholar] [PubMed]
- Altundag E, Ozturk M. Ethnomedicinal studies on the plant resources of east Anatolia, Turkey. Procedia-Soc Behav Sci 2011; 19: 756–777.
- Ghafari S, Tavakoli Z, Shirooyeh P, et al. The herbal medicine proposed by Iranian traditional medicine (Persian Medicine) for treatment of primary Dysmenorrhea: A Review. Tradit Integr Med 2018; 3:30-42.
- Hosokawa K, Fukunaga Y, Fukushi E, et al. Acylated anthocyanins from red Hyacinthus orientalis. Phytochemistry 1995; 39:1437-1441.
- Karaman S, Kocabas YZ. Traditional medicinal plants of K. Maras (Turkey). Science 2001; 1:125-128.
[Crossref]
- Hosokawa K, Fukunaga Y, Fukushi E, et al. Acylated anthocyanins in red flowers of Hyacinthus orientalis regenerated in vitro. Phytochemistry 1996; 42:671-672.
- Lin BW, Gong CC, Song HF, et al. Effects of anthocyanins on the prevention and treatment of cancer. Br J Pharmacol 2017; 174:1226-1243.
[Crossref] [Google Scholar] [PubMed]
- Yin Z, Zhang W, Feng F, et al. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci Hum Wellness 2014; 3:136-174.
- Kury LTA, Taha Z, Talib WH. Immunomodulatory and anticancer activities of Hyacinthus orientalis L. an in vitro and in vivo study. Plants 2021; 10:617.
[Crossref] [Google Scholar] [PubMed]
- Jasim WH, Hamad MN. Extraction and identification of phenolic compounds from Iraqi Heliotropium europaeum L plant separation and identification of phenolic compounds from the iraqi plant scorpion hashish walaa hamid jasim and maha nouri hamad. Iraqi J Pharm Sci 2021; 30:2021.
- Harborne JB. Phytochemical methods; A guide to modern techniques of plant analysis. J Chem Inf Model. 1998; 3:317.
- Kokate CK, Gokhale SB, Purohit AP. A textbook of pharmacognosy. 29th Edition. Nirali Prakashan Publication. Mumbai, India. 2009:1-635.
- Sarker SD, Latif Z, Gray AI. Natural products isolation. 2nd Edition. Humana Press. New Jersey, USA. 2005:515.
- Satheesh KB, Suchetha KN, Vadisha SBhat. Preliminary phytochemical screening of various extracts of Punica granatum peel, whole fruit and seeds. J Health Allied Sci NU. 2012; 2:34-38.
- Soule HD, Vazguez J, Long A, et al. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst 1973; 51:1409-1416.
[Crossref] [Google Scholar] [PubMed]
- Al Shammari AM, Alshami MA, Umran MA, et al. Establishment and characterization of a receptor negative, hormone nonresponsive breast cancer cell line from an Iraqi patient. Breast Cancer: Targets Ther 2015; 7:223-230.
[Crossref] [Google Scholar] [PubMed]
- Adil BH, Al Shammari AM, Murbat HH. Breast cancer treatment using cold atmospheric plasma generated by the FE-DBD scheme. Clin Plasma Med 2020; 19-20.
- Abdullah SA, Al Shammari AM, Lateef SA. Attenuated measles vaccine strain have potent oncolytic activity against Iraqi patient derived breast cancer cell line. Saudi J Biol Sc 2020; 27:865-872.
[Crossref] [Google Scholar] [PubMed]
- Al Shammari AM, Jalill RDA, Hussein MF. Combined therapy of oncolytic Newcastle disease virus and rhizomes extract of Rheum ribes enhances cancer virotherapy in vitro and in vivo. Mol Biol Rep 2020; 47:1691-1702.
[Crossref] [Google Scholar] [PubMed]
- Mohammed MS, Al Taee MF, Al Shammari AM. Caspase dependent and independent anti-hematological malignancy activity of AMHA1 attenuated newcastle disease virus. Int J Mol Cell Med 2019; 8:211-222.
[Crossref] [Google Scholar] [PubMed]
- Al Ziaydi AG, Al Shammari AM, Hamzah MI, et al. Newcastle disease virus suppress glycolysis pathway and induce breast cancer cells death. VirusDisease 2020; 31:341-348.
[Crossref] [Google Scholar] [PubMed]
- Basli A, Belkacem N, Amrani I. Health benefits of phenolic compounds against cancers. In phenolic compounds-biological activity. Intechopen Publisher. London, UK. 2017:193-210.
Author Info
Noor Mohammed Shareef* and Thukaa Z Abdul-jalil
Department of Pharmacognosy and Medicinal Plants, Collage of Pharmacy, University of Baghdad, Baghdad, IraqCitation: Noor Mohammed Shareef, Thukaa Z Abdul-jalil, The Cytotoxic Effect of Iraqi Hyacinthus orientalis against Breast Cancer Cells, J Res Med Dent Sci, 2023, 11 (08): 063-068.
Received: 09-Jul-2022, Manuscript No. JRMDS-22-69008; , Pre QC No. JRMDS-22-69008 (PQ); Editor assigned: 11-Jul-2022, Pre QC No. JRMDS-22-69008 (PQ); Reviewed: 25-Jul-2022, QC No. JRMDS-22-69008; Revised: 14-Aug-2023, Manuscript No. JRMDS-22-69008 (R); Published: 21-Aug-2023
