Research Article - (2022) Volume 10, Issue 9
The Alterations in Some Salivary Biomarkers and Oral Microbiome in COVID-19 Patients and Healthy Individuals
Mustafa Kareem Aziz* and Abbas S Al-Mizraqchi
*Correspondence: Mustafa Kareem Aziz, Department of Basic Sciences, College of Dentistry, University of Baghdad, Baghdad, Iraq, Email:
Abstract
A saliva samples have been collected from 42 COVID-19 patient and a 42 healthy individuals and tested for salivary biomarkers of, α-amylase, lysozyme in addition to and mutans streptococci. Results showed non-significant difference between mean concentration of α-amylase in COVID-19 patients (5.48 µ/ml) and healthy individuals (6.05 µg/ml), high significant difference in mean concentration of lysozyme in COVID-19 patient (170.42 µg/ml) and healthy individuals (8.47 µg/ml), and non-significant difference in mean concentration of mutans streptococci in COVID-19 patients (6.88) × 10⁶ CFU/ml and healthy individuals (6.58 × 10⁶ CFU/ml).
Keywords
COVID-19, SARS-CoV-2, Salivary lysozyme Salivary α-amylase, Salivary lysozyme Mutans streptococciIntroduction
Saliva is a bio-fluid constituted of various fluids of secretions of salivary glands, respiratory secretions, crevicular and exfoliated epithelial cells. Presence of SARS-CoV-2 in saliva is attributed to the viral replication and RNA secretion in any cells and tissues related to the production of salivary components like salivary glands, periodontal tissues and the upper respiratory tract cells [1].
The microbial constituents of a eubiotic Humam Oral Microbiome (HOM) can inhibit colonization of pathogens through competitive exclusion and/or via facilitation of immune response to exclude the pathogen [2,3]. It is reported that a vital collaborative interactions happen between viruses and the micro biome and that the micro biome could be regulate and it, in turn, could be regulated by viruses through various mechanisms [4]. The oral micro biota can produce anti-viral substances (defensins) against various viruses, including respiratory tract viruses like coronaviruses, herpes viruses, adenoviruses, orthomyxoviruses and papillomaviruses [5]. Otherwise, invading viruses could results in dysbiosis and progression of disease [6].
The pandemic of coronavirus SARS-CoV-2, the causative of COVID-19 is respiratory virus that invades the oropharynx as the primary site of replication but the possible impact on oral micro biome through development of infection remains un-clarified. In particular, there are no date available concerning the non-bacterial constituents of HOM (fungi and viruses), that have been shown vital for other diseases. Regarding Covid-19, it is reported that the presence of gingival inflammation/periodontitis was associated with a 3.5-fold increase in the risk for admission to Intensive Care Units (ICU), a 4.5-fold increase in the probability for assisted ventilation and a risk of 8.81-fold increase in the probability for death as a consequences of COVID-19, separately from any other concomitant risk factors [7].
The functions of salivary biomarker of α-amyalse extensively studied for their biological activities but their correlation to each other in COVID-19 patient and healthy individuals to COVID-19 are still unrevealed. The current study is designed to assess whether there is any association between salivary α-amylase, lysozyme, and total a count of mutans Streptococci. The study aimed to measure the salivary α-amylase level in both groups (COVID-19 patients and heathy individuals), to measure the salivary lysozyme level in both groups (COVID-19 patients and control group), to calculate the total viable count of mutans Streptococci and C. albicans among COVID-19 patients and to evaluate the relationship between salivary α-amylase, lysozyme, and total viable a count of mutans Streptococci among COVID-19 patients.
Materials and Methods
Saliva collection: After taking patients agreement for collecting saliva samples, checking that the patients didn’t take antibiotics or any medications for the latest two weeks, then giving the patients plain tubes that numbered before sample collection. Saliva sample collection was made in early morning at time between 8 am to 10 am.
The amount of the collected saliva was between 1-3 ml of un-stimulated saliva by allowing the saliva to accumulate in the mouth and then spitting into a tube. After collecting the salivary sample from each patient, the tubes were placed in a cool box with ice to transfer them to the laboratory to be cultured within less than an hour, then 0.1 ml would be taken from the salivary sample by micro pipette for the serial dilution tubes using PBS. The resting saliva was centrifuged for 3000 rpm for 10 min, and the clear supernatant was e and stored in freezer at -20°C until the determinations of salivary α-Amylase, lysozyme and melatonin were done by ELISA test
Determination of lysozyme level
This kit was based on Competitive-ELISA detection method (Cell Biolabs, USA). The microtiter plate provided in this kit has been pre-coated with antibody. During the reaction, target in the sample or standard competes with a fixed amount of Biotin-Antigen. Excess conjugate and unbound sample or standard are washed from the plate. HRP-Streptavidin was added and unbound conjugates were washed away with wash buffer. Then TMB substrate solution is added to each well. The enzyme substrate reaction is terminated by the addition of a sulfuric acid solution and the colour change is measured spectrophotometrically at a wavelength of 450 nm. The concentration of target in the samples is then determined by comparing the OD of the samples to the standard curve.
Determination of salivary α-Amylase
The ELISA test kit (LDN, Germany) provides a quantitative in vitro assay for free α-amylase in human saliva. The test kit contains microtiter strips each with 8 break off reagent wells coated with anti-rabbit antibodies. In the first reaction step, diluted patient samples are pipetted into the reagent wells together with peroxidase-labelled α-amylase and a specific rabbit anti-α-amylase antibody. α-amylase from the patient sample and the labelled α-amylase in the conjugate compete for the free binding sites of the specific antibody. In the third incubation step, the bound peroxidase catalyses a colour reaction with the peroxidase substrate Tetra Methyl Benzidine (TMB). The intensity of the colour formed is inversely proportional to the concentration of α-amylase in the sample. The results for the samples are determined using the standard curve.
Cultivation of mutans streptococci
Saliva samples were centrifuged at 3000 rpm for 10 min. Precipitate has been discarded and the supernatant forwarded for culture and identification of mutans Streptococci and The supernatant has been serially 10-folds diluted in PBS enumeration of mutans streptococci, the supernatant of saliva was serially 10-folds diluted in PBS and streaked on MSBA agar for calculation of CFU/ml of Mutans Streptococci.
Statistical analysis: Statistical analysis and processing of the data were carried out using SPSS version 21 (Statistical Package for Social Sciences) under Windows 10. Data were subjected to the following:
Descriptive statistics
- Arithmetic Means (M), Standard Deviation (SD) and Standard Error (SE).
- Minimum (mini) and maximum (maxi).
Inferential statistics
The statistical tests that were used in this study:
- Anova test.
- S.D. test.
- Student's t-test and.
- Pearson correlation coefficients.
The level of significance was accepted at P< 0.05, and highly significance when P<0.01.
Results
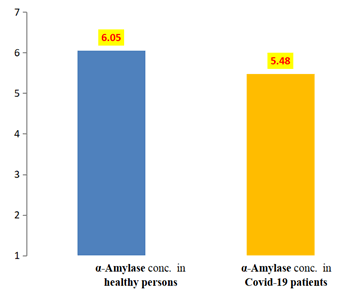
α-Amylase: Salivary α-amylase level in COVID-19 patients group and healthy individuals group is shown in Table 1 and Figure 1, which revealed the presence of mean value of salivary α-amylase among the COVID-19 patients group (5.48) u/ml less than healthy individuals group (6.05) u/ml, with a statistical non-significant difference between the two groups, the t-value was (1.602) and the p value was (0.117).
| Variable | No. of cases | Minimum | Maximum | Mean | SE | SD |
|---|---|---|---|---|---|---|
| Healthy individuals | 42 | 3.6 | 9.09 | 6.05 | 0.25 | 1.66 |
| COVID-19 patients | 42 | 1.79 | 11.2 | 5.48 | 0.33 | 2.18 |
Table 1: Descriptive of α-amylase.
Figure 1: Means of a-amylase among COVID-19 patients and healthy individuals groups.
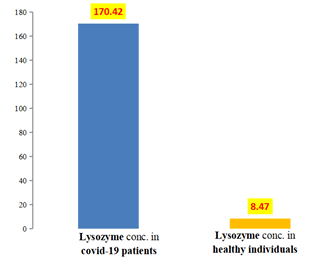
Lysozyme: Salivary lysozyme level in COVID-19 patients group and healthy individuals group is shown in Table 2 and Figure 2, which revealed the presence of higher mean value of salivary lysozyme among the COVID-19 patients group (170.42) g/ml than healthy individuals group (8.47) g/ml, with a statistical high significant difference between the two groups, the t-value was (7.668) and the p value was (0.001).
| Variables | No. of cases | Minimum | Maximum | Mean | SE | SD |
|---|---|---|---|---|---|---|
| COVID-19 patients | 42 | 24.38 | 513.3 | 170.42 | 21.11 | 136.86 |
| Healthy individuals | 42 | 4.76 | 9.29 | 8.47 | 0.16 | 1.07 |
Table 2: Descriptive of lysozyme.
Figure 2: Means of lysozyme among COVID-19 patients and healthy individuals group.
Total Viable Count of Mutans Streptococci (CFU/ml)
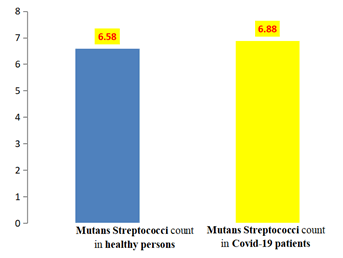
Result in Table 3 and Figure 3 shows the statistical analysis of viable count (CFU/ml) × 10ⶠof Total Viable Count of Salivary Mutans streptococcus, there was Non-significant difference between two groups, the t-value was (0.43), the p value (0.66), the mean value in healthy individuals group (6.58) × 10ⶠCFU/ml was less than COVID-19 patients group mean value (6.88) × 10ⶠCFU/ml
| Variables | No. of cases | Minimum | Maximum | Mean | SE | SD |
|---|---|---|---|---|---|---|
| Healthy individuals | 42 | 2.8 | 11.28 | 6.58 | 0.36 | 2.39 |
| COVID-19 patients | 42 | 3 | 17.44 | 6.88 | 0.55 | 3.61 |
Table 3: Descriptive of mutans streptococcus.
Figure 3: Means of Total viable count of salivary mutans streptococcus among COVID-19 patients and healthy individuals group.
Discussion
Salivary α-amylase level in COVID-19 patients group and healthy individuals group
α-amylase works to digest the large polysaccharides like glycogen and starch into smaller units of glucose and maltose through breakage of α-1,4 bond of such sugars [8]. α-amylase is synthesized in acinar cells and stored in secretory granules [9]. It is released from salivary cells upon response to mastication motion of the jaw or taste [10-12].
The SG could be a target for SARS-CoV-2 due to the intense expression of Angiotensin-Converting Enzyme 2 (ACE2) and Trans Membrane Protease Serine 2 (TMPRSS2) receptors by the ductal epithelium and serous acinar of SG [13]. The infection of SG by SARS-CoV-2 results in histopathological changes in the ductal lining cell cytoplasm, ductal lumen and acinar cells with the presence of viral particles in different parts of SG [14-16].
The slight difference in the mean and the non-significant difference of SAA values in both COVID-19 patients and healthy individuals groups in indicated that secretion of α-amylase by salivary gland does not been affected greatly by SARS-Cov-2 infection in COVID-19 patient group, and only slight increase of amylase was detected that could not be interpreted as hyperamylasia. Only the infection of pancreas by SARS-Cov-2 could results in pancreatic injury and thus hyperamylasemia [17,18].
Salivary lysozyme level in COVID-19 patients group and healthy individuals group
The presence of lysozyme in body fluids, including saliva, is an important factor in non-specific mechanisms towards microbial infections [19-21]. The high significant difference between the COVID-19 patients and healthy individuals groups is a strong marker that SARS-CoV-2 provokes the salivary glands to secrete a 20 fold of lysozyme concentration of healthy individuals to counteract the viral infection.
Lysozyme is bactericidal for gram-positive bacteria via hydrolyzing the β-1,4 glycosidic bond between N-acetylglucosamine andN-acetylmuramic acid of the bacterial cell wall [22]. Furthermore, the lysozyme exerts antimicrobial activity through binding to negatively charged surfaces of microbes owing to its cationic nature [23,24]. The immunomodulatory action of lysozyme has only been appreciated in last few years.
Within neutrophils and marcophages, lysozyme works to increase their pro-inflammatory response, but when it secreted outside the above mentioned cells as well as epithelial cells, it limits inflammation through decreasing the chemotaxis and oxidative burst in neutrophils [25], it suppresses the production of IL-6 and TNF-α in macrophages [26], it binds and decreases the circulating levels of Advanced Glycation End products (AGEs) (that are pro-oxidative) in addition to increasing their renal excretion [27] and it disrupts the capacity of peptidoglycan to bind the complement factors that works as anaphylotoxins [22]. Moreover, when subjected to artificial conditions of gastro-intestinal conditions, the hydolyzate of lysozyme of hen egg white showed remarkable Angiotensin Converting Enzyme (ACE) and anti-oxidant activity [28,29]. As mentioned above, the oxidative stress (including participation of AGEs), cytokines of IL-6 and TNF-α, inflammation caused by macrophages and neutrophils and the Renin–Angiotensin System (RAS) are characteristic in the Acute Respiratory Distress Syndrome (ARDS) and/or severe COVID-19. It is most interested that the activity of lysozyme beside lactoferrin in neuroprotection of Alzheimer’s patients through inhibition of amyloid-beta aggregation [30] could be an active mechanism in treatment of potential neurological manifestations in severe cases of COVD-19.
The increase in Salivary Antimicrobial Proteins (sAMPs) like lysozyme and lactoferrin in Lower Respiratory Tract Infection (LRTI) was reported in several previous studies [31,32]. The advantage of increasing level of lysozyme is to raise the immunomodulatory effects of lysozyme to counteract SARS-CoV-2 infection [25-28]
The viable total count of salivary mutans streptococci in COVID-19 patients group and healthy individuals group
The count concentration of mutans streptococci in COVID-19 patients and healthy individuals was attributed to the fact that the natural habitat of mutans streptococci is the oral cavity. The mutans streptococci have the ability to exert effective persistence in oral cavity due to formation of diverse human associated-biofilms [33-65] reported that the bacterial profile of oral microbiota in COVID-19 patients is characterized by dominance of Prevotella salivae and Veillonella infantium, whereas Neisseria perflava and Rothia mucilaginosa were dominant in healthy individuals along with N. perflava, K. gabonensis, G. elegans, Porphyromonas pasteri, Gemella taiwanensis, R. mucilaginosa, and Streptococcus oralis. Mutans streptococci concentration in oral cavity is unaffected by COVID-19 [66-95].
Conclusion
It is concluded that salivary lysozyme is highly responsive through COVID-19 to immunomodulate the infection. There is an inverse relationship between salivary melatonin level and COVID-19 that is resulted, probably, from fast utilization of melatonin as free radical scavenger to counteract the inflammation-generated high level of ROS. The secretory function of salivary glands for secretion of α-amylase is unaffected during COVID-19 due to the homeostatis of the enzyme in both COVID-19 patients and healthy individuals. Oral candidiasis is a sequeleae of COVID-19 as result of saliva low flow rate (Xerostomia) occurred during SARS-CoV-2 infection to salivary glands. The total count of Mutans Streptococci is stable during COVID-19.
Recommendations
It is recommended to study the effect of COVID-19 on oral microbiota of gram-positive and gram-negative bacteria and monitor the potential presence of bacteremia and/or septicemia in COVID-19 patients due to overgrowth of any member of oral bacteria, study level of other salivary biomarkers through course of COVID-19 pathology like C-Reactive Protein (CRP), myoglobin, creatine kinase isoform MB, α-2-macroglobulin, glycosylated hemoglobin (HbA1c) and various Interleukins (ILs), study the involvement of periodontal diseases in the COVID-19 due to depletion of salivary melatonin level in COVID-19 patients that is correlated to periodontal diseases, study the correlation between severity of COVID-19 and levels of salivary biomarkers and oral micro biome to uncover the contribution of salivary biomarkers and oral micro biome in determination of susceptibility of an individual to COVID-19.
Conflict of Interest
The author declare that there are no conflicts of interest
Acknowodgement
This works was sponsored by Department of basic sciences, College of Dentistry, University of Baghdad.
References
- Chen T, Hudnall SD. Anatomical mapping of human herpesvirus reservoirs of infection. Mod Pathol 2006; 19:726-737.
- Wilks J, Beilinson H, Golovkina TV. Dual role of commensal bacteria in viral infections. Immunol Rev 2013; 255:222–229.
- Wilks J, Golovkina T. Influence of microbiota on viral infections. PLoS Pathog 2012; 8:1002681.
- Li N, Ma WT, Pang M, et al. The Commensal Microbiota and Viral Infection: A Comprehensive Review. Front immunol 2019; 10:1551.
- Pfeiffer JK, Sonnenburg JL. The intestinal microbiota and viral susceptibility. Front Microbiol 2011; 2:92.
- Lynch SV. Viruses and microbiome alterations. Ann Am Thorac Soc 2014; 11:57–60.
- Marouf N, Cai W, Said KN, et al. Association between periodontitis and severity of COVID-19 infection: A case-control study. J clin periodontal 2021; 48:483-491.
- Granger DA, Kivlighan KT, El-Sheikh M, et al. Salivary alpha-amylase in biobehavioral research: Recent developments and applications. Ann N Y Acad Sci 2007; 1098:122-144.
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders: Overview of physical and behavioral homeostasis. Erratum in JAMA 1992; 267:244-1252.
- Kirschbaum C, Read GF, Hellhammer DH. Assessment of hormones and drugs in saliva in biobehavioral research. Gottingen: Hogrefe and Huber 1994.
- Scannapieco FA, Torres G, Levine MJ. Salivary α-amylase: Role in dental plaque and caries formation. Crit Rev Oral Biol Med 1993; 4:301-307.
- Rogers JD, Palmer RJ Jr, Kolenbrander PE. Role of Streptococcus gordonii amylase-binding protein A in adhesion to hydroxyapatite, starch metabolism, and biofilm formation. Infect Immun 2001; 69:7046-7056.
- Matuck BF, Dolhnikoff M, Duarte-Neto AN, et al. Salivary glands are a target for SARS-CoV-2: a source for saliva contamination. J Pathol 2021; 254:239-243.
- Chen M, Shen W, Rowan NR, et al. Elevated ACE2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. bioRxiv : the preprint server for biology 2020.
- Song G, Liang G, Liu W. Fungal Co-infections Associated with Global COVID-19 Pandemic: A Clinical and Diagnostic Perspective from China. Mycopathologia 2020; 185:599–606.
[Crossref][Google Scholar][Indexed]
- Song J, Li Y, Huang X, et al. Systematic analysis of ACE2 and TMPRSS2 expression in salivary glands reveals underlying transmission mechanism caused by SARS-CoV-2. J Med Virol 2020; 92:2556-2566.
- Farshidfar N, Hamedani S. Hyposalivation as a potential risk for SARS-CoV-2 infection: Inhibitory role of saliva. Oral diseases 2021; 3:750-751.
- Pribadi RR, Simadibrata M. Increased serum amylase and/or lipase in coronavirus disease 2019 (COVID-19) patients: Is it really pancreatic injury. JGH Open 2021; 5:190-192.
- Jenzano JW, Hogan SL, Lundblad RL. Factors influencing measurement of human salivary lysozyme in lysoplate and turbidimetric assays. J clin microbiol 1986; 24:963–967:
- Nasiri K. COVID-19 and the Antiviral Effect of Saliva. Eur J Dent 2020; 14:177–178.
- Pedrosa MS, Sipert CR, Nogueira FN. Salivary Glands, Saliva and Oral Findings in COVID-19 Infection. Odontopediatria Clin. Integr 2020; 20:0104.
- Ragland SA, Criss AK. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog 2017; 13:1006512.
- Sava G. Pharmacological aspects and therapeutic applications of lysozymes. Lysozymes: Model Enzymes in Biochemistry and Biology. Jolles P. (Lysozyme: Model Enzymes in Bichemistry and Biology). Birkhäuser Verlag, Basel, Switzerland, 1996; 75:433-449.
- Ibrahim HR, Imazato K, Ono H. Human lysozyme possesses novel antimicrobial peptides within its N-terminal domain that target bacterial respiration. J Agric Food Chem 2011; 59:10336–10345.
- Gordon LI, Douglas SD, Kay NE, et al. Modulation of neutrophil function by lysozyme. Potential negative feedback system of inflammation. J Clin Invest 1979; 64:226–232.
- Tagashira A, Nishi K, Matsumoto S, et al. Anti-inflammatory effect of lysozyme from hen egg white on mouse peritoneal macrophages. Cytotechnol 2018; 70: 929–938.
- Aldini G, Vistoli G, Stefek M, et al. Molecular strategies to prevent, inhibit, and degrade advanced glycoxidation and advanced lipoxidation end products. Free radical res 2013; 47:93-137.
- Rao S, Sun J, Liu Y, et al. ACE inhibitory peptides and antioxidant peptides derived from in vitro digestion hydrolysate of hen egg white lysozyme. Food Chem 2012; 135:1245-1252.
- Kaabi SAG. Prophylactic Phage Therapy in Infant Rabbits Model of cholera. Karbala Int J Mod Sci 2021; 7:2.
[Crossref]
- Helmfors L, Boman A, Civitelli L, et al. Protective properties of lysozyme on β-amyloid pathology: implications for Alzheimer disease. Neuro biol Dis 2015; 83:122–133.
- Akinbi HT, Epaud R, Bhatt H, et al. Bacterial killing is enhanced by expression of lysozyme in the lungs of transgenic mice. J immunol 2000; 165:5760–5766.
- Canto E, Roca E, Perea L, et al. Salivary immunity and lower respiratory tract infections in non-elite marathon runners. PloS one 2018; 13.
- Kreth J, Merritt J, Qi F. Bacterial and host interactions of oral streptococci. DNA and cell biology 2009: 28:397-403.
- Iebba V, Zanotta N, Campisciano G, et al. Profiling of Oral Microbiota and Cytokines in COVID-19 Patients. Front microbiol 2021; 12:1603.
- J Zeng L, Kajfasz J, Palmer SR, et al. Biology of Oral Streptococci. Microbiol spectrum 2018; 6.
- Acuna-Castroviejo D, Escames G, Figueira JC, et al. Clinical trial to test the efficacy of melatonin in COVID-19. J. Pineal Res 2020; 69.
- Aizawa S, Miyasawa-Hori H, Nakajo K, et al. Effects of alpha-amylase and its inhibitors on acid production from cooked starch by oral streptococci. Caries res 2009; 43:17–24.
- Almughrabi OM, Marzouk KM, Hasanato RM, et al. Melatonin levels in periodontal health and disease. Journal of periodontal research 2013; 48:315–321
- Anderson G, Reiter RJ. Melatonin: Roles in influenza, COVID-19, and other viral infections. Rev Med Virol 2020; 30.
- Arastehfar A, Carvalho A, Nguyen MH, et al. COVID-19-Associated Candidiasis (CAC): An Underestimated Complication in the Absence of Immunological Predispositions? J Fungi 2020; 6:211.
- Baghizadeh Fini M. Oral saliva and COVID-19. Oral oncol 2020; 108.
- Barbieri DSV, Vicente VA, Fraiz FC, et al. Analysis of the in vitro adherence of Streptococcus mutans and Candida albicans. Braz J Microbiol 2007; 38:624-632.
- Bencharit S, Altarawneh SK, Baxter SS,et al. Elucidating role of salivary proteins in denture stomatitis using a proteomic approach. Mol Biosyst 2020; 8:3216-3223.
- Buranarom N, Komin O, Matangkasombut O. Hyposalivation, oral health, and Candida colonization in independent dentate elders. PloS one 2020; 15.
- Caplan DJ, Hunt RJ. Salivary flow and risk of tooth loss in an elderly population. Com Dent Oral Epidemiol 1996; 24:68–71.
- Castillo RR, Quizon GRA, Juco MJM, et al. Melatonin as adjuvant treatment for coronavirus disease 2019 pneumonia patients requiring hospitalization (MAC-19 PRO): a case series. Melatonin Res 2020; 3:297–310.
- Cengiz MI, Cengiz S, Wang H. Melatonin and Oral Cavity. Int J Dent 2012.
- Chen L, Zhao J, Peng J, et al. Detection of SARS-CoV-2 in saliva and characterization of oral symptoms in COVID-19 patients. Cell Prolif 2020; 53.
- Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev 2005; 9:11–24.
- Culp DJ, Robinson B, Cash MN. Murine Salivary Amylase Protects Against Streptococcus mutans-Induced Caries. Front physiol 2021; 12.
- Cutando A, Galindo P, Gomez-Moreno G, et al. Relationship between salivary melatonin and severity of periodontal disease. J periodontal 2006; 77:1533–1538.
- Cutando A, Gomez-Moreno G, Arana C, et al. Melatonin: potential functions in the oral cavity. J Periodontol 2007; 78:1094-1102.
- Dodds M, Roland S, Edgar M, et al. Saliva A review of its role in maintaining oral health and preventing dental disease. BDJ Team 2015; 2.
- Farsi NM. Signs of oral dryness in relation to salivary flow rate, pH, buffering capacity and dry mouth complaints. BMC oral health 2007; 7:1-6:
- Bacaksiz F, Ebik B, Ekin N, et al. COVID-19 and Hyperamylasemia. Authorea 2021.
- Gomez-Moreno G, Guardia J, Ferrera MJ, et al. Melatonin in diseases of the oral cavity. Oral dis 2010; 16: 242–247.
- Herrera D, Serrano J, Roldan S, et al. Is the oral cavity relevant in SARS-CoV-2 pandemic? Clin Oral Invest 2020; 24:2925–2930.
- Kaabi SAG, Musafer HK. Novel Phage Cocktail for the Treatment of Bacteria Causing Chronic Suppurative Otitis Media. Trop J Nat Prod Res 2020; 4:680-686.
[Indexed]
- Kalsbeek A, Buijs RM. Output pathways of the mammalian suprachiasmatic nucleus: coding circadian time by transmitter selection and specific targeting. Cell Tissue Res 2002:309:109-118.
- Kitamura M, Kiyak HA, Mulligan K. Predictors of root caries in the elderly. Community Dent Oral Epidemiol 1986; 14:34-38.
- Lai CC, Wang CY, Hsueh PR. Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? JMicrobiol Immunol Infect Wei Mian Yu Gan Ran Za Zhi 2020; 53:505-512.
- Li X, Wang L, Yan S, et al. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical recordsin a single medical center, Wuhan, China. Int J Infect Dis 2020; 94:128–132.
- Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV-2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol 2020; 92:552–555.
- Llena-Puy C. The role of saliva in maintaining oral health and as an aid to diagnosis. Medicina oral, patologia oral y cirugia bucal 2006; 11:449–455.
[Indexed]
- Lynge Pedersen AM, Belstrom D. The role of natural salivary defences in maintaining a healthy oral microbiota. J dent 2019; 80:3–12.
- Marsh PD, Do T, Beighton D, et al. Influence of saliva on the oral microbiota. Periodontol 2000 2016; 70:80-92.
- Mastrangelo A, Germinario BN, Ferrante M, et al. COVID-Bio Study Group. 2020. Candidemia in COVID-19 patients: incidence and characteristics in a prospective cohort compared to historical non-COVID-19 controls. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America, ciaa 1594. Advance online publication.
- Mortazavi H, Baharvand M, Movahhedian A, et al. Xerostomia due to systemic disease: A review of 20 conditions and mechanisms. Annals Med Health Sci Res 2014; 4:503–510.
- Moser D, Biere K, Han B, et al. COVID-19 Impairs Immune Response to Candida albicans. Front immunol 2021; 12:640-644.
- Motahari P. Relationship of oral candidiasis with salivary lysozyme and lactoferrin in HIV-positive patients: a systematic review. HIV and AIDS Review. Int J HIV-Related Problems 2012; 20:17-20.
[Crossref][Google Scholar][Indexed]
- Murakami Y, Machino M, Fujisawa S. Porphyromonas gingivalis Fimbria-Induced Expression of Inflammatory Cytokines and Cyclooxygenase-2 in Mouse Macrophages and Its Inhibition by the Bioactive Compounds Fibronectin and Melatonin. ISRN dent 2012; 350-859.
- Nambiar M, Varma SR, Jaber M, et al. Mycotic infections-mucormycosis and oral candidiasis associated with COVID-19: a significant and challenging association. J oral microbiol 2021; 13:1967699.
- Noto Y, Sato T, Kudo M, et al. The relationship between salivary biomarkers and state-trait anxiety inventory score under mental arithmetic stress: a pilot study. Anesthesia and Analgesia 2005; 101:1873–1876.
- Nowak R, McMillen IC, Redman J, et al. The correlation between serum and salivary melatonin concentrations and urinary 6-hydroxymelatonin sulphate excretion rates: two non-invasive techniques for monitoring human circadian rhythmicity. Clin Endocrinol 1987; 27:445–452.
- Nowroozi N, Kawata T, Liu P, et al. High β-galactosidase and ganglioside GM1 levels in the human parotid gland. Archives of Otolaryngology-Head Neck Surgery 2001; 127:1381-1384.
- Reiter RJ, Paredes SD, Manchester LC, et al. Reducing oxidative/nitrosative stress newly discovered gene for melatonin. Criti Rev Biochemi Mol Biol 2009;44: 175-200.
- Romero AC, Ibuki FK, Nogueira FN. Sialic acid reduction in the saliva of streptozotocin induced diabetic rats. Archiv Oral Biol 2012; 57:1189-1193.
- Saeralaathan S, Rajkumar A, Balaji TM, et al. Salivary melatonin is depleted in patients with dental caries due to the elevated oxidative stress. J oral biol craniofac res 2021; 11:547-551.
- Salaric I, Karmelic I, Lovric J, et al. Salivary melatonin in oral squamous cell carcinoma patients. Sci Rep 2021; 11:13201.
- Saldiva PHN, Braz-Silva PH, Caldini EG, et al. Salivary glands are a target for SARS-CoV-2: a source for saliva contamination. J Pathol 2021; 254:239-243.
- Salehi M, Ahmadikia K, Mahmoudi S, et al. Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: Species identification and antifungal susceptibility pattern. Mycoses 2020; 63:771–778.
- Samaranayake YH, Samaranayake LP, Pow EH, et al. Antifungal effects of lysozyme and lactoferrin against genetically similar, sequential Candida albicans isolates from a human immunodeficiency virus-infected southern Chinese cohort. J clin microbiol 2001; 39:3296–3302.
- Sardi JCO, Scorzoni L, Bernardi T, et al. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J med microbiol 2013; 62:10-24.
- Sebaa S, Hizette N, Boucherit-Otmani Z, et al. Doseâ??dependent effect of lysozyme upon Candidaalbicans biofilm. Molecular medicine reports 2017; 15:1135–1142.
- Sehirli A, Aksoy U, Koca-Unsal RB, et al. Role of NLRP3 inflammasome in COVID-19 and periodontitis: Possible protective effect of melatonin. Med hypotheses 2021; 151:110588.
- Sharma A, Subramaniam P, Moiden S. Analysis of Salivary IgA, Amylase, Lactoferrin, and Lysozyme Before and After Comprehensive Dental Treatment in Children: A Prospective Study. Contemporary Clin Dent 2017; 8:526-530.
- Shneider A, Kudriavtsev A, Vakhrusheva AV, et al. Can melatonin reduce the severity of COVID-19 pandemic? Int Rev Immunol 2020; 19:153-162.
- Srinath R, Acharya AB, Thakur SL. Salivary and gingival crevicular fluid melatonin in periodontal health and disease. J periodontol 2010; 81:277–283.
- Vaarala MH, Porvari KS, Kellokumpu S, et al. Expression of transmembrane serine protease TMPRSS2 in mouse and human tissues. J Pathol 2001;193:134-140.
- Vakkuri O, Leppaluoto J, Kauppila A. Oral administration and distribution of melatonin in human serum, saliva and urine. Life Sciences 1985; 37:489-495.
- Villa A, Connell CL, Abati S. Diagnosis and management of xerostomia and hyposalivation. Ther Clin Risk Manag 2014; 11:45-51.
- Wang WK, Chen SY, Liu IJ, et al. SARS Research Group of the National Taiwan University/National Taiwan University Hospital. Detection of SARS-associated coronavirus in throat wash and saliva in early diagnosis. Emerg Infect Dis 2004; 10:1213-1219.
- Xu X, Wang G, Ai L, et al. Melatonin suppresses TLR9-triggered proinflammatory cytokine production in macrophages by inhibiting ERK1/2 and AKT activation. Sci Rep 2018; 8:15579.
- Xu J, Li Y, Gan F, et al. Salivary Glands: Potential Reservoirs for COVID-19 Asymptomatic Infection. J dent res 2020; 99:989.
- Zhang R, Wang X, Ni L, et al. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci 2020; 250:117583.
- Zhou Y, Hou Y, Shen J, et al. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov 2020; 6:1–18.
Author Info
Mustafa Kareem Aziz* and Abbas S Al-Mizraqchi
Department of Basic Sciences, College of Dentistry, University of Baghdad, Baghdad, IraqReceived: 04-Jul-2022, Manuscript No. JRMDS-22-55999; , Pre QC No. JRMDS-22-55999; Editor assigned: 07-Jul-2022, Pre QC No. JRMDS-22-55999; Reviewed: 21-Jul-2022, QC No. JRMDS-22-55999; Revised: 05-Sep-2022, Manuscript No. JRMDS-22-55999; Published: 12-Sep-2022