Review Article - (2023) Volume 11, Issue 1
Synthesis, characterization and evaluation of antioxidant activity of New pyrazolines derivatives
Zeinab G Younus* and Tagreed NA Omar
*Correspondence: Zeinab G Younus, Department of Pharmaceutical Chemistry, College of Pharmacy, University of Baghdad, Iraq, Email:
Abstract
A series of pyrazoline antioxidant derivatives were synthesized from chalcones and evaluated for their pharmacological activities. Chalcones (1-6) were prepared by claisen schimidt condensation methods by the reaction of furfural with different acetophenones. Various pyrazolines derivatives were prepared by reflux reaction of chalcones (1-6) with hydrazine/hydrazine hydrate in ethanol and glacial acetic acid which refluxed in hot water bath at temperature 50°C for about 2-3 hours, using one pot reaction. To form pyrazoline II (1-6), then antioxidants compounds (a: Eugenol and b: Vanillin) was added; reflex for two hours, cool the reaction mixture in ice water bath until crystal formation is complete. Add ice cold water to the flask and the product was filtered, washed with water and then dried to get pyrazoline antioxidant derivatives, III a (1-6) and III b (1-6). The structures of the newly synthesized pyrazoline antioxidant derivatives have been established on the basis of their spectral data. The synthesized compounds tested for their anti-inflammatory and antimicrobial activity.
Keywords
Furan-2-carbaldehyde, Chalcones, Pyrazoline, Eugenol, Vanillin, Anti-inflammatory activity, Antimicrobial activity
Introduction
Furfural (furan-2-carbaldehyde) has a hetero aromatic furan ring at the C2 position a reactive aldehyde functional group, oily compound, which was soluble in 8.3% at 20°C in water and evaporated together with water also, soluble in most organic solvent, miscible with ethyl ether, has boiling point 161.7°C, in its reactions as an aldehyde, furfural bears a strong similarity to benzaldehyde [1,2]. Thus, it undergoes the cannizzaro reaction in strong aqueous alkali.
Chalcone present nature as C5H12O in pair of stereochemistry forms (cis-1,3-diaryl-2-propen-1-one) and (trans-1,3-diaryl-2-propen-1-one). Chalcones in the advance chemistry important intermediate in the scientific research, it considers the basic for starting to synthesis many compounds as pyrazoline, which consider most important element of the natural products [3]. However, trans more reactive form cis in the reaction, also trans form can found in plants like citrus fruits (apple, tomato) also in licorice, vegetables, tea, etc. [4].
A famous synthesis of chalcones routs was aldol condensation is also known as a claisen schmidt condensation [5-7].
Pyrazoline products nitrogen heterocycles consider rich electron (neutrophil) which help to show a significant part in the various biological activities [8,9].
The reaction of chalcones with hydrazines is definitely the most common technique for the production of 2- pyrazolines [10].
Substituted pyrazoline possess antimicrobial, antioxidant activity, anti-depressant activity, antifungal, anticancer, anti-inflammatory, analgesic [11-23].
All antioxidant will show beneficial in preparation and design new medication in the future due to ability to as eugenol breaks the generation of hydroxyl radicals and stop superoxide anion production and vanillin study excellent scavenger of free radical [24,25].
Major goal is synthesizing pyrzolines derivatives by connecting with different antioxidants (i.e. eugenol and vanillin) increase their desired activities in antibacterial and anti-inflammatory as compared to drugs.
Materials and Methods
Furan-2-carbaldehyde (furfural) 97%, acetophenone derivatives 97% and antioxidants (eugenol, vanillin) from (China) hyper chem company. Organic solvent and other chemical reagent used in reaction from college of the pharmacy, Baghdad University. The reactions monitoring was done by used Thin Layer Chromatography (TLC), the solvent mobile phase system used is petroleum ether: Ethyl acetate (7:3). Melting point electronic apparatus (stuart SMP30) to measure all melting points for synthesis compounds FT-IR spectrophotometer (Japan). 1H-NMR spectra were got on BRUKER model ultra-shield 500 MHz spectrophotometer, as a solvent use Dimethyl Sulfoxide (DMSO).
Chemical synthesis
The Figure 1 illustrates the full reaction pathway to synthesis of intermediates and pyrazolines antioxidants conjugate derivatives.
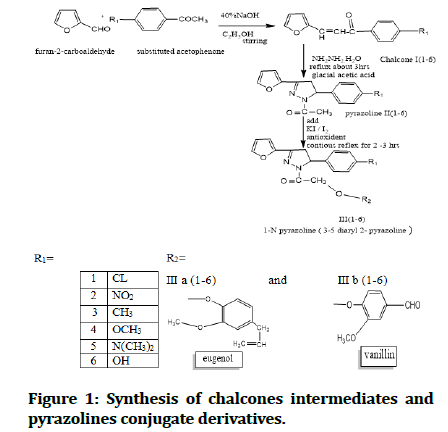
Figure 1: Synthesis of chalcones intermediates and pyrazolines conjugate derivatives.
Synthesis of chalcone derivatives I (1-6)
Synthesis is illustrated in Figure 1. Synthesis of chalcon intermediates and pyrazoline conjugate derivatives.
Solution of substituted acetophenone (0.025 m mole) stirred in ethanol 98% (8 ml) except nitro acetophenone stirred in 30 ml methanol was added 2-furaldehyde (furfural) (3.0 g, 0.025 m mole) in a conical flask (25 ml) then NaOH 40% (4 ml) was added drop wise. The mixture was stirred in an ice cold water bath until it solidified at 20-25℃. Reaction was controlled by TLC. Cold Distal Water (DW) 5 ml was added during the stirring, then solidified mass (E)-3-(furan-2-yl)-1-phenylprop-2-en-1 appearance in Figure 1, this kept in cold condition overnight in refrginater and after that solidified mass separated by filtered wash with cold (DW) and dried on room temperature. In case of hydroxyl acetophenone the reaction mixture neutralized with 5%HCL until appearance precipitates. Dry product and recrystallized using two system solvent ethanol/hexane (3:8 drops). Check formation of chalcones (3-6) by TLC.
The percent yield, physical data and FT-IR characteristic absorption bands are given below for chalcone compounds I (1-6).
• (I-1) 4-Cl (85%), MP=60-66°C, light yellow crystals, C13H9ClO2 (MW=232.66). (FT-IR) (v.cm-1) 1654 (C=O), 1597,1590 (C=C), 1473 (Ar C=C), 1219 (C-OC), 739 (Cl).
• (I-2) 4-NO2 (90%), MP=135-140°C, dark yellow crystals, C13H9NO4 (MW=243.22). (FT-IR) (v.cm-1) 1654 (C=O), 1600,1581 (C=C), 1519 (NO2 Asym) 1469 (Ar C=C), 1323 (NO2 sym), 1219 (C-O-C)
• (I-3) 4-CH3 (75%), MP=59-64°C, creamy crystals, C14H12O2 (MW=212.25) (FT-IR) (v.cm-1) 1651(C=O), 1593, 1550 (C=C), 1477 (Ar C=C), 1229 (C-O-C),
• (I-4) 4-OCH3 (80%), MP=63-66°C, yellow crystals, C14H12O2 (MW=228.25) (FT-IR) (v.cm-1) 1654 (C=O), 1589,1550 (C=C), 1469 (Ar C=C), 1338 (C-H), 1222 (C-O-C),
• (I-5) 4-N(CH3)2 (90%), MP=106-108°C, light orange fluffy, C15H15NO2 (MW=241.29) (FT-IR) (v.cm-1) 1651(C=O), 1604-1577(C=C), 1473 (Ar. C=C), 1377 (C-H ), 1242 (C-O-C),
• (I-6) 4-OH (95%), MP=120-123°C, golden crystals, C13H10O3 (MW=241.29) (FT-IR) (v.cm-1) 1647 (C=O), 1597,1550 (C=C), 1473 (Ar C=C), 1384 (OH bending), 1276 (C-O-C).
Synthesis of pyrazoline derivatives (II 1-6 and final product 1,3,5-trisubstituted-2-pyrazoline (III a (1-6, III b (1-6 using one pot reaction
0.01 m mole of chalcones I (1-6) with hydrazine hydrate (0.02 m mole) and glacial acetic acid (30 ml) were refluxed in hot water bath at temperature 50°C for 2-3 hours using one pot reaction [26-28]. After completion of Reaction and check reaction by TLC. Add 0.01 m mole KI dissolve in 10 ml distill water, the reaction stirring for 1/2 hours [29]. Then add 0.01 m mol antioxidant compound still reflex and stir for 2 hours then gradually decrease the temperature and still stirring in cold water bath for 48 hours until precipitated form and the product was poured into crushed ice. The product put in refreginater overnight then filtrated and separated solid mass, washed with cold water, dried in dry place and recrystallized use two system solvent ethanol/hexane (3:8 drops). Check formation of final product in Figure 1 by TLC.
The 1N-acetyl pyrazolines (II 1-6) percent yield, physical data and FT-IR characteristic absorption bands are given below.
• (II-1) 4-Cl (80%), MP=110-113°C, yellow crystals, C15H13ClN2O2 (MW=288.73) (FT-IR) (v.cm-1) 1654 (C=O), 1610 (Ar C=N), 1562-1500 (Ar C=C), 1249 (CO- C), 1141 (C-N), 1068 (Ar N-N), 740 (Cl)
• (II-2) 4-NO2 (75%), MP=120-126°C, dark yellow crystals, C15H13N3O4 (MW=299.29). (FT–IR) (v.cm-1) 1643 (C=O), 1597 (Ar C=N), 1570-1550 (Ar C=C) 1512 (NO2 asym), 1334 (NO2 sym), 1228 (C-O-C) 1168 (C-N), 1060 (Ar N-N)
• (II-3) 4-CH3 (65%), MP=100-103°C, off white crystals, C16H16N2O2 (MW=268.32) (FT-IR) (v.cm-1) 1654 (C=O), 1608 (Ar C=N),1523 (Ar C=C), 1226 (C-O-C), 1149 (C-N), 1064 (Ar N-N)
• (II-4) 4-OCH3 (80%), MP=96-98°C, yellow crystals, C16H16N2O3 (MW=284.32) (FT-IR) (v.cm-1) 1654 (C=O), 1608-1589 (Ar C=N), 1550 (Ar C=C), 1338 (CH), 1253 (C-O-C), 1149 (C-N), 1076 (Ar N-N)
• (II-5) 4-N(CH3)2 (75%), MP=135-138°C, orange fluffy, C17H19N3O2 (MW=297.36) (FT-IR) (v.cm-1) 1662 (C=O), 1639 (Ar C=N), 1527 (Ar C=C), 1365 (C-H), 1230 (C-O-C), 1149 (C-N), 1064 (Ar N-N)
• (II-6) 4-OH (85%), MP=124-130°C, yellow crystals, C13H10O3(MW=241.29) (FT-IR) (v.cm-1) 3325 (OH), 1654 (C=O), 1631 (Ar C=N), 1573 (Ar C=C), 1226 (CO- C), 1141 (C-N), 1068 (Ar N-N)
For final synthesis compounds III a (1-6), III b (1-6) percent yield, physical data and FT-IR characteristic absorption bands, 1 H NMR are given below.
• (III a-1) (75%), MP=123-126°C, green crystals, C25H21ClN2O4 (MW=448.90) (FT-IR) (v.cm-1) 1654 (C=O), 1593 (C=N), 1562-1500 (Ar C=C), 1273 (OCH3) 1249 (C-O-C), 1141 (C-N), 1068 (Ar N-N), 740 (Cl)
1H-NMR (δ ppm) 6.00-8.00 (10H, m, Ar), 4.80-4.87 (2H, S, OCH2), 3.35-3.74 (2H, d of d, CH2 of pyrazoline), 5.50 (1H, d of d, CH of pyrazoline), 3.86 (3H, s, OCH3), 5.03-5.12 (2H, m, CH2 of vinylic protons), 3.32-3.34 (2H, m, CH2 benzylic allylic protons), 5.91-5.96 (1H, m, CH).
• (III a-2) (80%), MP=145-148°C, green crystals, C25H21N3O6 (MW=459.46) (FT-IR) (v.cm-1) 1666-1651 (C=O), 1597 (C=N), 1558 (Ar C=C), 1504 (NO2-asym), 1338 (NO2–sym), 1284 (OCH3) 1249 (CO- C), 1145 (C-N), 1010 (Ar N-N).
1H-NMR (δ ppm) 6.00-8.29 (10H, m, Ar), 4.77-4.84 (2H, S, OCH2), 3.22-3.62 (2H, d of d, CH2 of pyrazoline), 5.54 (1H, d of d, CH of pyrazoline), 3.86 (3H, s, OCH3), 5.03-5.12 (2H, m, CH2 of vinylic protons), 3.32-3.34 (2H, m, CH2 benzylic allylic protons), 5.91-5.96 (1H, m, CH).
• (III a-3) (80%), MP=110-113°C, brown crystals, C26H24N2O4 (MW=428.49) (FT-IR) (v.cm-1) 1654 (C=O), 1593 (C=N), 1546-1500 (Ar C=C), 1269 (OCH3), 1249 (C-O-C), 1141 (C-N), 1068 (Ar N-N).
1H-NMR (δ ppm) 6.00-8.00 (10H, m, Ar), 4.77-4.84 (2H, S, OCH2), 3.23-3.63 (2H, d of d, CH2 of pyrazoline), 5.45 (1H, d of d, CH of pyrazoline), 3.86 (3H, s, OCH3), 5.03-5.12 (2H, m, CH2 of vinylic protons), 3.32-3.34 (2H, m, CH2 benzylic allylic protons), 5.91-5.96 (1H, m, CH). 2.33(3H, t, CH3).
• (III a-4) (75%), MP=115-119°C, dark brown crystals, C26H24N2O5 (MW=444.49) (FT-IR) (v.cm-1) 1654 (C=O), 1608 (C=N), 1516 (Ar C=C), 1270 (OCH3), 1249 (C-O-C), 1145 (C-N), 1068 (Ar N-N).
1H-NMR (δ ppm) 6.00-8.00 (10H, m, Ar), 4.77-4.84 (2H, S, OCH2), 3.23-3.63 (2H, d of d, CH2 of pyrazoline), 5.45 (1H, d of d, CH of pyrazoline), 3.86 (6H, s, OCH3), 5.03-5.12 (2H, m, CH2 of vinylic protons), 3.32-3.34 (2H, m, CH2 benzylic allylic protons), 5.91-5.96 (1H, m, CH).
• (III a-5) (85%), M.P=140-144°C, light brown crystals, C27H27N3O4 (MW=457.53) (FT-IR) (v.cm-1) 1666 (C=O), 1608 (C=N), 1599-1527 (Ar C=C), 1330 (N(CH3)2), 1273 (OCH3), 1234 (C-O-C), 1153 (C-N), 1064 (Ar N-N)
1H-NMR (δ ppm) 6.00-8.00 (10H, m, Ar), 4.77-4.84 (2H, S, OCH2), 3.23-3.63 (2H, d of d, CH2 of pyrazoline), 5.45 (1H, d of d, CH of pyrazoline), 3.86 (3H, s, OCH3), 5.03-5.12 (2H, m, CH2 of vinylic protons), 3.32-3.34 (2H, m, CH2 benzylic allylic protons), 5.91-5.96 (1H, m, CH), 2.05-2.92 (6H, S, CH3 30 amine).
• (III a-6) (65%), MP=124-126°C, brown crystals, C25H22N2O5 (MW=430.46) (FT-IR) (v.cm-1) 3325 (OH), 1631 (C=O), 1608 (C=N), 1593-1573 (Ar C=C), 1276 (OCH3), 1226 (C-O-C), 1154 (C-N), 1068 (Ar NN).
1H-NMR (δ ppm) 6.00-8.00 (10H, m, Ar), 4.77-4.84 (2H, S, OCH2), 3.23-3.63 (2H, d of d, CH2 of pyrazoline), 5.45 (1H, d of d, CH of pyrazoline), 3.86 (3H, s, OCH3), 5.03-5.12 (2H, m, CH2 of vinylic protons), 3.32-3.34 (2H, m, CH2 benzylic allylic protons), 5.91-5.96 (1H, m, CH), 7.61 (1H, S, OH).
• (III b-1) (60%), MP=90-93°C, light brown crystals, C23H17ClN2O5 (MW=436.85) (FT-IR) (v.cm-1) 1743 (C=O aldehyde), 1654 (C=O), 1593C=N (strin pyraroline), 1500 (AR-C=C), 1273-1026 (C-O aldehyde), 1230(C-O-C), 1141 (C-N), 1014 (Ar N-N), 740 (Cl).
1H-NMR (δ ppm) 6.00-8.00 (10H, m, Ar), 4.83-4.87 (2H, S, OCH2), 3.35-3.74 (2H, d of d, CH2 of pyrazoline), 5.48-5.53 (1H, d of d, CH of pyrazoline), 3.87 (3H, s, OCH3), 9.86 (1H, S, CH aldehyde).
• (III b-2) (60%), MP=90-93°C, light yellow crystals, C23H17N2O5 (MW=447.40) (FT-IR) (v.cm-1) 1743 (C=O aldehyde), 1666 (C=O), 1600C=N (strin pyraroline), 1570 (AR-C=C), 1504 (NO2 asym), 1338 (NO2 sym), 1319-1103 (C-O aldehyde), 1249(C-O-C), 1145 (C-N), 1026 (Ar N-N).
1H-NMR (δ ppm) 6.00-8.28 (10H, m, Ar), 4.83-4.87 (2H, S, OCH2), 3.35-3.74 (2H, d of d, CH2 of pyrazoline), 5.48-5.53 (1H, d of d, CH of pyrazoline), 3.87 (3H, s, OCH3), 9.86 (1H, S, CH aldehyde).
• (III b-3) (65%), MP=120-124°C, brown crystals, C24H20N2O5 (MW=416.43) (FT-IR) (v.cm-1) 1747 (C=O aldehyde), 1654 (C=O), 1612 C=N (strin pyraroline), 1500 (AR-C=C), 1269-1029 (C-O aldehyde), 1207(C-O-C), 1141 (C-N), 1014 (Ar N-N).
1H-NMR (δ ppm) 6.00-8.00 (10H, m, Ar), 4.83-4.87 (2H, S, OCH2), 3.35-3.74 (2H, d of d, CH2 of pyrazoline), 5.48-5.53 (1H, d of d, CH of pyrazoline), 3.87 (3H, s, OCH3), 9.86 (1H, S, CH aldehyde) 2.33 (3H, t, CH3).
• (III b-4) (65%), MP=120-124°C, brown crystals, C24H20N2O5 (M W=416.43) (FT-IR) (v.cm-1) 1747 (C=O aldehyde), 1627 (C=O), 1593 C=N (strin pyraroline), 1519 (AR-C=C), 1280-1014 (C-O aldehyde), 1219 (C-O-C), 1149 (C-N), 1072 (Ar N-N).
1H-NMR (δ ppm) 6.00-8.00 (10H, m, Ar), 4.83-4.87 (2H, S, OCH2), 3.35-3.74 (2H, d of d, CH2 of pyrazoline), 5.48-5.53 (1H, d of d, CH of pyrazoline), 3.87 (3H, s, OCH3), 9.86 (1H, S, CH aldehyde) 3.80 (3H, s, OCH3).
• (III b-5) (85%), MP=155-158°C, dark brown crystals, C25H23N3O5 (MW=445.16) (FT-IR) (v.cm-1) 1739 (C=O aldehyde), 1662-1639 (C=O), 1604 C=N (strin pyraroline), 1527 (AR-C=C), 1280-1064 (C-O aldehyde), 1230(C-O-C), 1195 (N(CH3)2, 1149 (C-N), 1014 (Ar N-N).
1H-NMR (δ ppm) 6.00-8.00 (10H, m, Ar), 4.83-4.87 (2H, S, OCH2), 3.35-3.74 (2H, d of d, CH2 of pyrazoline), 5.48-5.53 (1H, d of d, CH of pyrazoline), 3.87 (3H, s, OCH3), 9.86 (1H, S, CH aldehyde), 2.92(6H, s, CH3 30 amine).
• (III b-6) (65%), MP=185-190°C, light brown crystals, C23H18N2O6 (MW=418.41) (FT-IR) (v.cm-1) 3325 (OH) 1739 (C=O aldehyde), 1631 (C=O), 1605 C=N (strin pyraroline), 1512 (AR-C=C), 1280-1033 (C-O aldehyde), 1249(C-O-C), 1157 (C-N), 1014 (Ar N-N).
1H-NMR (δ ppm) 6.00-8.00 (10H, m, Ar), 4.83-4.87 (2H, S, OCH2), 3.35-3.74 (2H, d of d, CH2 of pyrazoline), 5.48-5.53 (1H, d of d, CH of pyrazoline), 3.87 (3H, s, OCH3), 9.86 (1H, S, CH aldehyde), 7.61(1H, S, OH).
Results and Discussion
Antibacterial and antifungal activity
The final manufactured organic compounds (III a (1-6), III b (1-6)) confirmed to calculate their antimicrobial activity against gram negative, gram positive bacteria and fungi, this evaluation was done using well diffusion method, the standard compounds used as the antibacterial agents were (ciprofloxacin, tetracycline, ceftriaxone and amoxicillin) while an antifungal agent was (fluconazole). DMSO was selected as a solvent and control.
Antibacterial activity detected in Table 1 where (III b3, III b4) have to appearance the strongest and most potent antibacterial activity against gram negative and gram positive bacteria, similar in activity to ciprofloxacin and amoxicillin also similar in activity to ceftriaxone and tetracycline against to Gram positive bacteria [30-32].
| Zone of inhibition in mm | ||||||
|---|---|---|---|---|---|---|
| Compound | Conc. μg/ml | Gram negative | Gram positive | Candida albicans | ||
| E. coli | Pseudomonas aeruginosa | Staph. aurous | Streptococcus progenies | |||
| III a1 | 103 | ---- | ---- | 2 mm | 2 mm | 20 mm |
| III a2 | 103 | ---- | 2 mm | 6 mm | 4 mm | 7 mm |
| III a3 | 103 | 8 mm | 10 mm | 3 mm | 8 mm | 13 mm |
| III a4 | 103 | 4 mm | 6 mm | 3 mm | 6 mm | 15 mm |
| III a5 | 103 | 10 mm | 2 mm | 4 mm | 8 mm | 10 mm |
| III a6 | 103 | ------- | 2 mm | 7 mm | 4 mm | 15 mm |
| III b1 | 103 | 8 mm | ------- | ---- | 6 mm | 2 mm |
| III b2 | 103 | 4 mm | 2 mm | 4 mm | ----- | 4 mm |
| III b3 | 103 | 8 mm | 4 mm | 10 mm | 15 mm | 2 mm |
| III b4 | 103 | 10 mm | 6 mm | 7 mm | 6 mm | 4 mm |
| III b5 | 103 | 2 mm | 4 mm | 7 mm | 2 mm | 2 mm |
| III b6 | 103 | ----- | 7 mm | 6 mm | 10 mm | 5 mm |
| Ciprofloxacin | 103 | 8 mm | 40 mm | 10 mm | 40 mm | |
| Tetracycline | 103 | ----- | 42 mm | ----- | 50 mm | |
| Ceftriaxone | 103 | ----- | 45 mm | 20 mm | 40 mm | |
| Amoxicillin | 103 | 7 mm | 25 mm | 8 mm | 20 mm | |
| Fluconazole | 103 | 20 mm | ||||
| DMSO | Control and solvent | 0 | 0 | 0 | 0 | |
Table 1: Antibacterial and antifungal activity of final synthesized compounds.
(III a2, III a3, III a5, III a6) and (III b1, III b5, III b6) have to appearance moderate antibacterial activity against gram negative and gram positive bacteria similar in activity to ciprofloxacin. All of these synthesis compounds have strong inhibition effect to moderate effect against gram negative and gram positive bacteria due to strong antibacterial effect of vanillin and eugenol in synthesis compounds.
For synthesis compounds (III a1, III a4) and (III b2) have to appearance weak antibacterial activity against gram negative and gram positive bacteria.
Antifungal activity
The final synthesized compounds (III a1-6, III b1-6) tested to estimate their antifungal activity, this estimation done using well diffusion method, the standard compounds used as an antifungal agent (fluconazole) while DMSO was chosen as a solvent and control [33].
As observed in the Table 1 the final compounds (III a1, III a2, III a3, III a4, III a5, III a6) have strong effect and high potent against Candida albicans fungal, they have similar effect to fluconazole due to strong effect of eugenol in synthesis compounds [34].
While compounds (III b1-III b6) have weak activity for compounds combine with vanillin because the aldehyde moiety of vanillin plays a key role in its antifungal activity, but side group position on the benzene ring also influences this activity [35-38].
Evaluation of the anti-inflammatory activity
The anti-inflammatory activity calculated for the final synthesizes compounds (III a1-6, III b1-6) by use method of the paw edema (stimulation of acute inflammation via subcutaneous injection of undiluted white egg to the intra planter side of the left hand paw of the rat). This yields swelling due to plasma extravasations, enlarged tissue by water and plasma protein exudation along with neutrophil extravasations, all of these because the metabolism of arachidonic acid.
This method in vivo has benefits over other methods because of the quick estimate via measuring the swelling at the start and during the course, elevation paw sensitivity for inflammation, without anesthesia, suitable budget value, method proximity to human nature and simple.
Evaluation the effect of diclofenac sodium (standard) versus propylene glycol (control)
At zero time and at 1/2 hour there wasn’t significant difference between control and standard in paw edema reduction. But after 2 hours, 3 hours, 4 hours, 5 hours diclofenac sodium formed a significant percent reduction (P ≤ 0.0001) in paw edema related to the control, as illustrated (Table 2) [39].
| Time (hours) | Thickness of paw (mm) | |
|---|---|---|
| Control | Standard | |
| 0 | 4.48 ± 0.03 | 4.43 ± 0.02 |
| 1/2 | 4.71 ± 0.02 | 4.57 ± 0.02 |
| 1 | 6.05 ± 0.03 | 5.99 ± 0.04 |
| 2 | 7.66 ± 0.5 | 6.1 ± 0.02*** |
| 3 | 8.75 ± 0.03 | 6.02 ± 0.01*** |
| 4 | 7.97 ± 0.02 | 5.52 ± 0.01*** |
| 5 | 7.56 ± 0.11 | 5.24 ± 0.02*** |
| Data are expressed as mean ± SEM of mm paw thickness n=6 (number of animals) Significantly different compared to control: P-value*** ≤ 0.0001 |
||
Table 2: Effect of diclofenac sodium (standard) and propylene glycol (control) on egg white induced paw edema in rats.
Anti-inflammatory effect of tested compounds
Table 3 shows the effect of tested compounds on egg white induced edema as an indicator for their antiinflammatory activity. The intra plantar injection of egg albumin into rat hind paw induces a progressive edema, which was reached maximum (measured by millimeter) after 1 hour of injection. In this study, the intra peritoneal injection of tested compounds produced varies in degree of anti-inflammatory effect. Compounds (III a) 1-6(, III b )1-6) (exhibited comparable effect to that of diclofenac (3 mg/kg, i.p.) also compounds (III a1-6, III b1-6) created major reduction of paw edema with respect to the effect of propylene glycol 50% v/v (control group). There isn’t significant difference between the tested synthesis compounds (III a1-6, III b1-6) and the standard at 0 times, 0.5 and 1 hour.
| Paw Thickness (mm) | Time (hours) | 0 hour | 1/2 hour | 1 hour | 2 hour | 3 hour | 4 hour | 5 hour |
|---|---|---|---|---|---|---|---|---|
| Control | 4.48 ± 0.03 | 4.71 ± 0.02 | 6.05 ± 0.03 | 7.66 ± 0.5 | 8.75 ± 0.03 | 7.97 ± 0.02 | 7.56 ± 0.11 | |
| standard | 4.43 ± 0.02 | 4.57 ± 0.02 | 5.99 ± 0.04 | 6.1 ± 0.02*** | 6.02 ± 0.01*** | 5.52 ± 0.01*** | 5.24 ± 0.02*** | |
| III a1 | 4.43 ± 0.02 | 4.66 ± 0.02 | 6.005 ± 0.04 | 6.14 ± 0.01* | 5.16 ± 0.03*** | 5.07 ± 0.02*** | 4.85 ± 0.04*** | |
| III a2 | 4.60 ± 0.01 | 4.67 ± 0.01 | 5.96 ± 0.01 | 6.65 ± 0.04* | 6.41 ± 0.4* | 6.40 ± 0.01*** | 6.32 ± 0.01** | |
| III a3 | 4.51 ± 0.02 | 4.65 ± 0.02 | 5.94 ± 0.03 | 6.51 ± 0.01* | 6.05 ± 0.01* | 5.1 ± 0.02*** | 4.75 ± 0.01*** | |
| III a4 | 4.54 ± 0.03 | 4.66 ± 0.02 | 5.99 ± 0.03 | 6.99 ± 0.02*** | 6.87 ± 0.01*** | 6.67 ± 0.02*** | 6.54 ± 0.02** | |
| III a5 | 4.46 ± 0.01 | 4.63 ± 0.02 | 5.98 ± 0.02 | 6.72 ± 0.01*** | 6.46 ± 0.03*** | 6.17 ± 0.02*** | 5.96 ± 0.04*** | |
| III a6 | 4.49 ± 0.01 | 4.63 ± 0.02 | 5.93 ± 0.05 | 6.45 ± 0.01* | 6.14 ± 0.01* | 6.06 ± 0.02*** | 5.31 ± 0.01* | |
| III b1 | 4.49 ± 0.01 | 4.64 ± 0.02 | 5.96 ± 0.04 | 6.15 ± 0.01* | 5.74 ± 0.03*** | 5.36 ± 0.02*** | 5.02 ± 0.01* | |
| III b2 | 4.51 ± 0.02 | 4.64 ± 0.02 | 5.94 ± 0.03 | 6.50 ± 0.01* | 6.45 ± 0.01*** | 5.95 ± 0.03* | 5.75 ± 0.01*** | |
| III b3 | 4.45 ± 0.01 | 4.68 ± 0.02 | 6.04 ± 0.03 | 6.23 ± 0.02 * | 6.01 ± 0.04* | 5.76 ± 0.01* | 5.14 ± 0.02* | |
| III b4 | 4.47 ± 0.01 | 4.66 ± 0.01 | 5.98 ± 0.03 | 6.99 ± 0.04*** | 7.11 ± 0.01*** | 6.82 ± 0.02** | 6.59 ± 0.02** | |
| III b5 | 4.45 ± 0.03 | 4.66 ± 0.02 | 5.97 ± 0.04 | 6.89 ± 0.02*** | 7.12 ± 0.02*** | 7.01 ± 0.02** | 6.72 ± 0.02** | |
| III b6 | 4.43 ± 0.02 | 4.66 ± 0.02 | 5.97 ± 0.03 | 6.61 ± 0.01* | 5.81 ± 0.02*** | 5.14 ± 0.01*** | 5.05 ± 0.01* | |
| Data are expressed in mm paw thickness as mean ± SEM n=6 (number of animals), Time (0) is the time of i.p. injection of tested compounds, Time (0.5) is the time of injection of egg white (induction of paw edema), NS=Non-significant, *Significantly different with control (p ≤ 0.05), **Significantly with diclofenac sodium (p ≤ 0.05), ***Significantly different both with control and diclofenac sodium (p ≤ 0.05). |
||||||||
Table 3: Anti-inflammatory activity of control, standard and final synthesis compounds on egg white induced paw edema in rat.
But after 2 to 5 hours, compounds (III a1, III a3) and (III b1, III b6) showed good activities and high significant to reduce paw thickness, more than the standard, where the compounds (III a3, IIIa6) and (III b2, III b3) same effect in reduction of paw thickness when compared with standard, but compounds (III a5, III a2) have moderate effect in reduction of paw thickness and rested compounds (III a4) and (III b4, III b5) when compared to the standard, showed weak activities in reduce paw thickness.
Conclusion
• The chemical synthesis of a new pyrazolines derivatives compounds has been achieved successfully.
• Physical properties like (melting point and description), FT-IR, 1H-NMR spectra have been checked for the identification and characterization of the synthesized compounds and the results confirm their chemical structure.
• In vivo anti-inflammatory evaluation of the synthesized compounds produced a reduction in the paw edema thickness. For compounds (III a3, III a6) and (III b2, III b3) the effect is same comparable to that of the reference drug (diclofenac sodium).
• Compounds (III a2, III a5) showed a significant reduction in the paw edema compare to (diclofenac sodium) whereby, the greatest significant reduction was displayed by compounds (III a1, III a3) and (III b1, III b6).
• The anti-bacterial assessment of the final products with the incorporation of electron donating groups (OCH3 and OH) displays remarkable activity than the electron withdrawing group (Cl).
References
- Alvarez Builla J, Vaquero JJ, Barluenga J. Modern heterocyclic chemistry. John Wiley and Sons, 2011; 4. [Crossref][Googlescholar][Indexed]
- Ozdemir A, Turan Zitouni G, Asım Kaplancıklı Z, et al. Preparation of some pyrazoline derivatives and evaluation of their antifungal activities. J Enzyme Inhib Med Chem 2010; 25:565-571. [Crossref][Googlescholar][Indexed]
- Jacobi PA. Introduction to heterocyclic chemistry. Wiley, 2018. [Googlescholar]
- Revanasiddappa B, Kumar MV, Nayak P, et al. Synthesis, antibacterial and antifungal evaluation of novel pyrazoline derivatives. Res J Pharm Technol 2017; 10:1481. [Crossref][Googlescholar][Indexed]
- Jaiswal P, Pathak D, Bansal H, et al. Chalcone and their heterocyclic analogue: A review article. J Chem Pharm Res 2018; 10:160-173. [Indexed]
- Chidrawar AB. Novel synthesis of bioactive pyrazoline derivatives through reactive chalcones. Int J Res Pharm Chem 2017; 7: 344-347. [Googlescholar]
- Joshi VD, Kshirsagar MD, Sarita SJ. Synthesis and antimicrobial activities of various pyrazolines from chalcones. Int J Chem Tech Res 2012; 4:971-975.
- Yusuf M, Jain P. Synthetic and biological studies of pyrazolines and related heterocyclic compounds. Arab J Chem 2014; 7:553-596. [Crossref][Googlescholar][Indexed]
- Hadi AA. Synthesis and microbial studies of pyrazoline and its derivatives. Department of chemistry, Fergusson College, Pune University, India, 2012.
- Sridhar S, Rajendraprasad Y. Synthesis and analgesic studies of some new 2 pyrazolines. E J Chem 2012; 9:1810-1815. [Crossref][Googlescholar][Indexed]
- Almahdi MM, Saeed AEM, Metwally NHJE. Synthesis and antimicrobial activity of some new pyrazoline derivatives bearing sulfanilamido moiety. Eur J Chem 2019; 10:30-36. [Crossref][Googlescholar][Indexed]
- Sunil K, Rajat K. Synthesis and antimicrobial activity of 5-(substituted-phenyl)-3-(furan-2-yl)-4, 5-dihydro-1H-pyrazole compounds using silver tri fluro methane sulphonate as catalyst. Der Pharm Lett 2018; 10:57-67.
- Prasad YR, Kumar PR, Deepti C, et al. Synthesis and antimicrobial activity of some novel chalcones of 2-hydroxy-1-acetonapthone and 3-acetyl coumarin. E J Chem 2006; 3:236-241. [Crossref][Googlescholar][Indexed]
- Jagadish P, Soni N, Verma AJ. Design, synthesis and in vitro antioxidant activity of 1, 3, 5-trisubstituted-2-pyrazolines derivatives. J Chem 2013; 2013. [Crossref][Googlescholar][Indexed]
- Jois VH, Kalluraya B, Girisha KSJ. Synthesis and antioxidant activity study of pyrazoline carrying arylfuran/thiophene moiety. J Serbian Chem Soc 2014; 79:1469-1475. [Crossref][Googlescholar][Indexed]
- Das N, Dash B, Dhanawat M, et al. Design, synthesis, preliminary pharmacological evaluation and docking studies of pyrazoline derivatives. Chem Pap 2012; 66:67-74. [Crossref][Googlescholar][Indexed]
- Revanasiddappa B, Kumar MV, Kumar HJDU. Synthesis and antidepressant activity of pyrazoline derivatives. Dhaka Univ J Pharm Sci 2020; 19:179-184. [Crossref][Googlescholar][Indexed]
- Karabacak M, Altıntop MD, Ibrahim Ciftci H, et al. Synthesis and evaluation of new pyrazoline derivatives as potential anticancer agents. Molecules 2015; 20:19066-19084. [Crossref][Googlescholar][Indexed]
- Gowramma B, Jubie S, Kalirajan R, et al. Synthesis, anticancer activity of some 1-(bis N, N-(chloroethyl)-amino acetyl)-3,5-disubstituted 1,2-pyrazolines. Int J Pharm Tech Res 2009; 1:347-352. [Googlescholar]
- Bhat KI, Revanasiddappa B, Kumar MV, et al. Synthesis and in-vitro anti-inflammatory activity of new pyrazoline derivatives. Res J Pharm Technol 2018; 11:3969-3972. [Crossref][Googlescholar][Indexed]
- Venkataraman S, Jain S, Shah K, et al. Synthesis and biological activity of some novel pyrazolines. Acta Pol Pharm Drug Res 2010; 67:361-366. [Googlescholar][Indexed]
- Al-Nakeeb MR, Omar TN. Synthesis, characterization and preliminary study of the anti-inflammatory activity of new pyrazoline containing ibuprofen derivatives. Iraqi J Pharm Sci 2019; 28:131-137. [Googlescholar][Indexed]
- Neethu M, Yusuf S. In silico design, synthesis, anti-inflammatory and anticancer evaluation of pyrazoline analogues of vanillin. Int J Pharm Sci Drug Res 2014; 6:128-131. [Googlescholar]
- Reddy ACP, Lokesh B. Studies on the inhibitory effects of curcumin and eugenol on the formation of reactive oxygen species and the oxidation of ferrous iron. Mol Cell Biochem 1994; 137:1-8. [Crossref][Googlescholar][Indexed]
- Mohammed ZB, Omar TN. Chemical design, synthesis and biological evaluation of mutual pro drug of gabapentin with different type of phenolic and alcoholic antioxidants. Syst Rev Pharm 2021; 12:858-868. [Googlescholar][Indexed]
- Salum KA, Alidmat MM, Khairulddean M, et al. Design, synthesis, characterization and cytotoxicity activity evaluation of mono chalcones and new pyrazolines derivatives. J Appl Pharm Sci 2020; 10:020-036. [Googlescholar][Indexed]
- Revanasiddappa BC, Kumar MV, Kumar H. Synthesis and antioxidant activity of novel pyrazoline derivatives. J Database Manag 2018; 10:177. [Crossref][Googlescholar]
- Thirunarayanan G, Sekar KG. Solvent free one pot cyclization and acetylation of chalcones: Synthesis of some 1-acetyl pyrazoles and spectral correlations of 1-(3-(3,4-dimethylphenyl)-5-(substituted phenyl)-4,5-dihydro-1H-pyrazole-1-yl) ethanones. J Saudi Chem Soc 2016; 20:661-672. [Crossref][Googlescholar][Indexed]
- Smith MB. March’s advanced organic chemistry. 8th edition, John Wiley and Sons, Inc. U.S.A, 2020.
- de Sousa D. Analgesic like activity of essential oils constituents. Molecules 2011; 16:2233-2252. [Crossref][Googlescholar][Indexed]
- Bhat Ki, Hussain MM. Synthesis characterization and antimicrobial studies of some substituted pyrazolines from aryloxy acetyl hydrazine. Asian J Chem 2009; 21:3371-3375.
- Fitzgerald DJ, Stratford M, Gasson MJ, et al. Mode of antimicrobial action of vanillin against Escherichia coli, Lactobacillus plantarum and Listeria innocua. J Appl Microbiol 2004; 97:104–113. [Crossref][Googlescholar][Indexed]
- Romero Cortes T, Espana VH, Lopez Perez PA, et al. Antifungal activity of vanilla juice and vanillin against alternaria alternate. CYTA J Food 2019; 17:375-383. [Crossref][Googlescholar][Indexed]
- Goyal S, Tewari G, Pandey H, et al. Exploration of productivity, chemical composition and antioxidant potential of Origanum vulgare L. grown at different geographical locations of Western Himalaya, India. J Chem 2021; 20:21. [Crossref][Googlescholar][Indexed]
- Swathi V, Sudev S, Dhanya K, et al. Review on the CNS activity of pyrazolines. J Med Sci Clin Res 2019; 107:2455-0450. [Crossref]
- Arikan S. Current status of antifungal susceptibility testing methods. Med Mycol 2007: 45; 569-587[Crossref][Googlescholar][Indexed]
- John Mc Murry. Organic chemistry 7th edition, Thomson Learning Inc, USA. 2008; 21:422-429.
- Tong Wong K, Osman H, Parumasivam T, et al. Synthesis, characterization and biological evaluation of new 3,5-disubstituted pyrazoline derivatives as potential anti-Mycobacterium tuberculosis H37Ra Compounds. Molecules 2021; 26:2081. [Crossref][Googlescholar][Indexed]
- Velmurugan V, Surekha S, Kalvikkarasi S, et al. Synthesis, characterization and evaluation of analgesic activity of 3,5-disubstituted pyrazoline derivatives. Int J Pharm Pharm Sci 2012; 4:189-191.
Author Info
Zeinab G Younus* and Tagreed NA Omar
Department of Pharmaceutical Chemistry, College of Pharmacy, University of Baghdad, IraqReceived: 07-Oct-2022, Manuscript No. JRMDS-22-62361; , Pre QC No. JRMDS-22-62361; Editor assigned: 14-Oct-2022, Pre QC No. JRMDS-22-62361; Reviewed: 24-Oct-2022, QC No. JRMDS-22-62361; Revised: 26-Dec-2022, Manuscript No. JRMDS-22-62361; Published: 06-Jan-2023
