Research - (2021) Volume 9, Issue 3
Selenium-Yeast Administration Ameliorates the Alterations in Haematological and Oxidative Stress Parameters of Wistar Rats Subjected to Restraint Stress
Okwute M Ochayi1, Ruth Y. Manjak2, Emmanual E Ochai3, Oluwatosin I Oyeniran4 and Senol Dane4*
*Correspondence: Senol Dane, Department of Physiology, Faculty of Basic Medical Sciences, College of Health Sciences, Nile University of Nigeria, Abuja, Nigeria, Email:
Abstract
Introduction: Stress is a classical experience of “wear and tear” on physiological systems emanating from the central nervous system to other body systems. The blood acts as intermediary among the hypothalamic-pituitary-adrenal system and tissues of an organism and is among the first tissues opened to oxidative stress. This study aims to investigate the haematological changes that accompany restraint stress and the ameliorative roles of a selenium-yeast antioxidant. Materials and Methods: Fifteen (15) Wistar rats were separated into three groups of five each (n=5); Group I (normal control), Group II- (selenium yeast (0.1mg/kg) + restrained stress), and Group III (stress control; restraint stress + distilled water 1ml/kg). Restraint stress was by restraint meshes from (9:00 h- 15:00 h) for 21 days, and rats were decapitated afterward. Blood sample (3 mL) was collected by cardiac puncture. Erythrocytes count, leucocytes count, and packed cell volume were evaluated using an automated cell counter and biomarkers of oxidative stress using ELIZA kits. Results: The leukocyte count (cells/μL) increase in Group II (3.9 ± 0.53) when compared to Group III (3.59 ± 0.66) and Group I (4.28 ± 0.64). The RBC counts decreases in Group II (3.84 ± 0.17) when compared to Group I (7.65 ± 0.23) and Group III (32.7 ± 0.18). The PCV (%) increases in Group I (42%) when compared to Group II (39%) and Group III (37%). In biomarkers of oxidative stress the values for Group II SOD (2.08 ± 0.08); CAT (44 ± 0.90); GSH (33 ± 1.2); MDA (2.6 ± 0.09) increases when compared with Group III SOD (1.87 ± 0.08); CAT (34 ± 0.62); GSH (25 ± 0.3); MDA (2.2 ± 0.07). While in Group I the values for MDA (0.1 ± 0.12) significantly decreases when compared with Group II (2.6 ± 0.09) and Group III (2.2 ± 0.07). Conclusion: Restraint stress decreases the values of RBC, WBC, and PCV this might possible be due to its ability to increase lipid peroxidation of the blood cells membrane; however this effect was ameliorated when administered with selenium-yeast antioxidant.
Keywords
Selenium-yeast, Oxidative stress, Restraint stress, Haematology, Wistar rats
Introduction
Stress can be term as a classical experience of “wear and tear” on the physiological system emanating from the central nervous system to other systems of the body; where it results in mental and physical health problems [1]. Over the years, hormones such as cortisol and adrenaline are being tagged as classical stress hormones. These hormones have been implicated in the elicitation of oxidative stress and the creation of free radicals [2]. Hormones are chemical messengers transported through the blood to target organs; hence, the first tissue to feel the impact of stress is the blood [3]. The study of this effect is well encapsulated under the study of blood in health and disease via haematological indices; which have been implicated in the deleterious effects induced by stress.
Stress leads to a decrease in red blood cell count (RBC), leucocytes or white blood cell count (WBC), packed cell volume (PCV), and haemoglobin concentration (Hb) [4]. It also affects platelet volume index (MPV), platelet distribution width (PDW), red blood cell distribution width (RDW), and mean corpuscular haemoglobin concentration (MCHC) [5,6]. Considering the implicating roles of stress hormones in the generation of free radicals and oxidative stress which ripple effect might be the alteration of haematological indices observed in the aforementioned studies [2]. There is a need to consider some natural antioxidants or supplements such as selenium-yeast which is a major constituent of most grains and feeds [7].
Some studies have reported the pivotal role of selenium-yeast as a potent antioxidant. Selenium yeast was observed to improve the antioxidant capacity of aquatic animals such as juvenile Eriocheir Sinensis when exposed to stress [8]. It plays a critical role in elevating the antioxidant status of Tibetan sheep as seen in malondialdehyde (MDA), superoxide dismutase (SOD), and total antioxidant capacity (T-AOC) concentrations [9]. Considering the above roles selenium plays in the improvement of antioxidant capacity both aquatic and terrestrial lives, one has to possibly reason its application on haematological parameters during stress. This study aimed towards investigating the effects of exogenous selenium-yeast on some haematological indices in Wistar rats exposed to stress.
Materials and Methods
Animals
Fifteen Wistar rats weighing between (100-150 g) were employed in this experiment, they were gotten from the Department of Human Physiology, Ahmadu Bello University, Zaria, Nigeria, and housed at the same venue for the experiment where they were given rats’ food and distilled water ad libitum. All handling was under the best standard practiced following the rules of Ahmadu Bello University Animal Handling Committee.
Experimental groups
The Wistar rats used; were randomly separated into three groups, consisting of 5 rats each (n=5) namely; Group I, acted as normal control (NCG); and they remained undisturbed during the study. Group II, Selenium-yeast group (SYG); was pre-treated with selenium-yeast at 0.1mg/ kg before restraint stress. Group III, Stressed group (SG); was induced with restraint stress and administered distilled water at 1ml/kg.
Methods
Group II and Group III animals were subjugated to restraint stress for 6 h between the periods of (9:00 h- 15:00 h) for 21 days. Group III rats were administered with 1 ml/kg of distilled water before subjecting them to restraint stress, while Group II rats were pretreated with 0.1 mg/kg of selenium-yeast before restraint. Restraint stress protocol was according to the principle of Kumar et al., 2007 using a wire mesh of dimensions 8 cm (length) X 4 cm (Breadth) X 4 cm (High) for the experiment, and a key lock and fastener were employed to fix the rats in a restrainer from escaping. After the experiment, the rats were anaesthetized and the blood sample was obtained to assess for haematological indices and markers of oxidative stress.
Haematological indices
The blood samples collected were stored in a heparinized tube after rats been anaesthetized under light anaesthesia. An automated blood analyzer was used; this was programmed to count red blood cells or reticulocytes (RBCs), white blood cells or leukocytes (WBCs), and Packed Cell Volume (PCV) (Abacus Junior Vet 5, Austria).
Oxidative stress markers
Lipid peroxidation level was evaluated via the concentration of the level of thiobarbituric-acid (TBA) reactive substance and malondialdehyde (MDA), using the NWLSSTM MDA assay kit (Northwest Life Sciences Specialties, Product NWK-MDA01, Vancouver WA, specificity: Malondialdehyde, Sensitivity: 0.08 μM). This follows the interaction of MDA with TBA; leading to the formation of an MDA-TBA2 compound that absorbs greatly at a wavelength of 532 nm [10].
Superoxide dismutase (SOD) activity
Superoxide dismutase (SOD) activity in the serum of the Wistar rats was assessed using the NWLSS SOD assay kit (product NWK-SOD02, Specificity: Cu/Zn, Mn, and Fe Superoxide Dismutase, Sensitivity: 5 U/mL). This follows the principle of the suppression of autoxidation of hematoxylin as explained in the study of Martins et al [11].
Catalase (CAT) activity
Catalase activity was evaluated utilizing the NWLSS CAT activity assay kit (Product NWKCAT01, Specificity: Catalase, Sensitivity: 6.0 Catalase/mL). The enzyme activity was evaluated following the standard that catalase consumes peroxidases substrate at a wavelength of 240 nm.
Glutathione peroxidase (GPx) activity
The serum concentration of glutathione peroxidase was evaluated employing NWLSSTMc GPx (GPx1) ELISA assay kit (Enzyme-Linked Immunosorbent Assay) (Product NWKGPX02, specificity: Glutathione peroxidase, Sensitivity: 12.5 pg/ml). In this sample, the GPx concentration is evaluated by juxtaposing the 450 nm absorbance of sample wells to the absorbance of a recognized standard [12].
Statistical analysis
The statistical package used was Graphpad Prism version 5.0. (San Diego California, USA).
Data gotten from the study were presented as Mean ± standard error of the mean and analyzed employing the analysis of variance (ANOVA) with Turkey’s posthoc test. When P-value is < 0.05, it was deemed significant.
Results
Red blood cell (RBC) counts
The result as shown in Figure 1 reveals that there is a significant increase in RBC count in Group I (Normal control) having a value of 4.2 cells/μL when compared to Group III (Stress control group) having a value of 3.7 cells/μL and Group II (selenium-yeast group). However, there was an increase in the RBC counts in Group II (selenium-yeast group) when compared with Group III (stress group), although the increase in statically negligible.
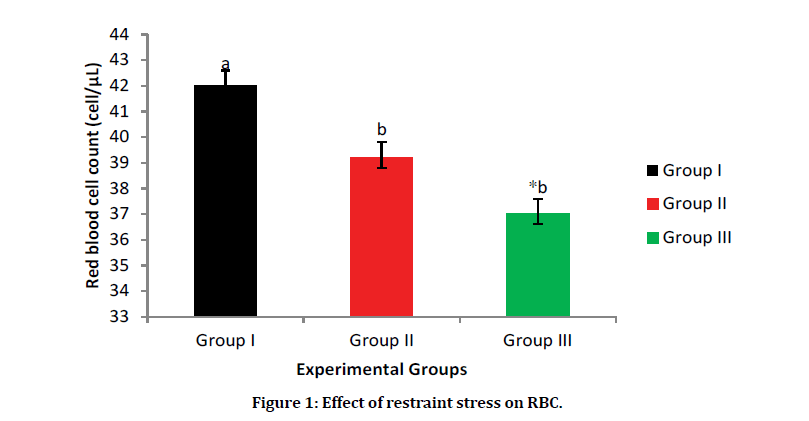
Figure 1: Effect of restraint stress on RBC.
Data are presented as Mean ± SEM. a, b=Means with different superscript letters are significantly different (P<0.05). Group, I=Normal control, Group II=selenium-yeast stress group, Group III=restraint stress group.
White blood cell (WBC) or Leucocyte counts
The WBC counts significantly increase in Group I (normal control) (42.2 cells/L) when compared with Group III (stress control) (39 CELLS/L) and Group II (selenium-yeast group) (37 cells/L). In the stress groups one can say selenium-yeast decreases the deleterious effects of stress on WBC with an increase value of WBC count in Group II when compared with Group III, although not statically significant (Figure 2).
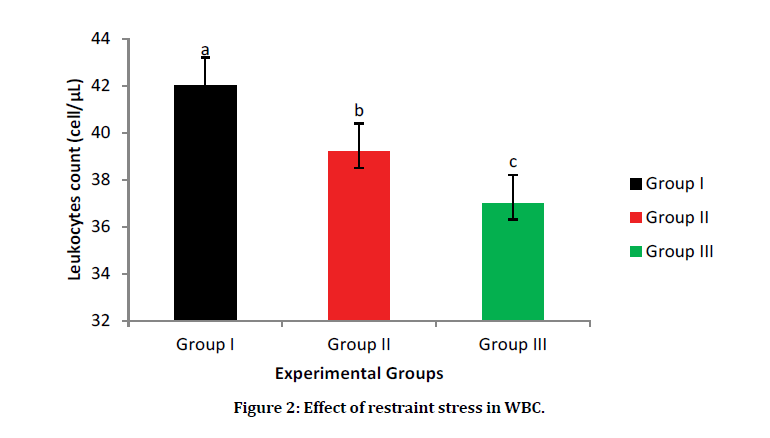
Figure 2: Effect of restraint stress in WBC.
Data are presented as Mean ± SEM. a, b, c=Means with different superscript letters are significantly different (P<0.05). Group, I=Normal control, Group II=selenium-yeast stress group, Group III=restraint stress group.
Packed cell volume (PCV)
The result for PCV shows a statistical increase in Group I (Normal control group) 42% when compared with Group III (Stressed group) 35%. However, there was an increase in the PCV of Group II (Selenium-yeast group) 39% when compared with Group III. This might be a possible case of improvement as a result of the prior administration of selenium-yeast (Figure 3).
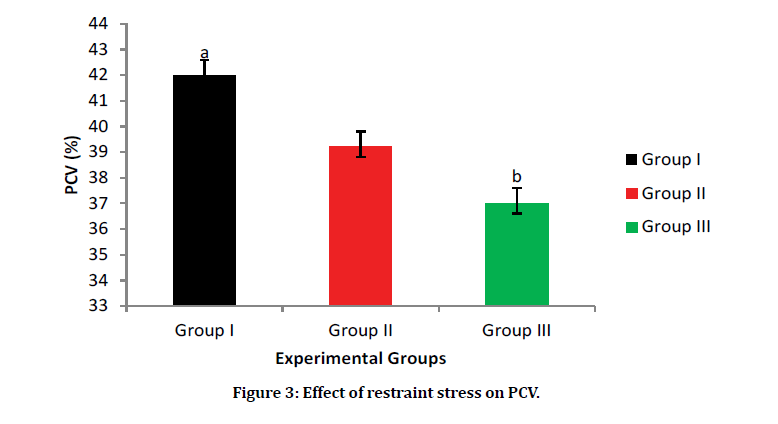
Figure 3: Effect of restraint stress on PCV.
Data are presented as Mean ± SEM. a, b, c= Means with different superscript letters are significantly different (P < 0.05); Group I=normal control, Group II=selenium-yeast stress group, Group III=restraint stress group.
The selenium-yeast has influence on biomarkers of oxidative stress. The mean values increase in Group II for SOD (2.08 ± 0.08); CAT (44 ± 0.90); GSH (33 ± 1.2); MDA (2.6 ± 0.09) when compared with Group III SOD (1.87 ± 0.08); CAT ( 34 ± 0.62); GSH (25 ± 0.3); MDA ( 2.2 ± 0.07). While in Group I the values for MDA (0.1 ± 0.12) significantly decreases when compared with Group II (2.6 ± 0.09) and Group III (2.2 ± 0.07). The result for MDA indicates level of lipid peroxidation (Table 1).
Table 1: Effects of restraint stress and selenium yeast on biomarkers of oxidative stress.
| (IU/L) | Group I | Group II | Group III |
|---|---|---|---|
| SOD | 2.23 ± 0.08a | 2.08 ± 0.08b | 1.87 ± 0.08a |
| CAT | 50 ± 1.10a | 44 ± 0.90b | 34 ± 0.62a |
| GSH | 48 ± 1.2a | 33 ± 1.2b | 25 ± 0.3 |
| MDA | 0.1 ± 0.12b | 2.6 ± 0.09a | 2.2 ± 0.07 |
Data are presented as Mean ± SEM. a, b=means with different superscript letters within rows are significantly different (P<0.05); Group I=normal control, Group II=selenium-yeast stress group, Group III=restraint stress group.
Discussion
Haematological parameters are key indicators of the physiological status of both humans and animals. The effects of stress on red blood cell (RBC) as shown in Figure 1 indicated that stress as a deleterious effects on RBC. The reduction in RBC in the stress group agrees with the findings of a study [13] which stated that stress damages the RBC membrane leading to a reduction in RBC counts. However, this finding disagrees with the report of a recent study [14] which showed that the RBC counts increases during stress; this may be due to the kind of stressor (chemical stressor) applied in their study [14], unlike ours which was a physical stressor. However, the administration of selenium-yeast antioxidant mitigate the decrease observe in the RBC counts induced by stress; this agrees with a study [15], which showed that selenium-yeast at 0.03 g/kg/ BW improves RBC counts in higher animals.
Restraint stress decreases leucocytes counts in Wistar rats. This finding conforms to the report of a study [16], which indicated that oxidative stress alters white blood cell (WBC) structures. Stress is known to reduce WBC count, and this might be due to the sensitivity of B-lymphocytes to glucocorticoid cells and its apoptotic nature [17]. Although findings from this present study shows an increase in the WBC count in the selenium-yeast administered group when compared with the stress group. However, this does not agree with the result of a study [18] which showed that selenium-yeast pretreatment does not affect the total WBC count. Nevertheless, this finding is similar to the result of a study [19] which revealed that seleniumyeast pre-treatment improves WBC counts.
The packed cell volume (PCV) decreases in Group III was the least among the three groups which agreed with the finding from a previous research [20], which showed that stress decreases PCV. However, in the selenium-yeast pre-treated group, there was an improvement in the PCV. This shows that selenium-yeast may decrease the deleterious effects of stress of restraint stress on the PCV.
Considering the haematological changes with response to restraint stress administration, there is a need for further verification on the mechanism by which restraint stress induces these changes, though it has been speculated to be due to its ability to induce oxidative stress. The following biomarkers of oxidative stress were evaluated; MDA, SOD, GPx, and CAT. There exist a significant reduction in SOD, CAT, and GSH in the stress group (SG) relative to the selenium-yeast (SYG) and normal control (NCG). However, the activity of MDA which indicates the level of lipid peroxidation was more in the stressed group compared to the normal control group. These findings correspond to the following studies [21- 23].
Conclusion
Our findings from this study have proven that restraint stress induces oxidative stress, thereby altering RBC, WBC counts, and PCV. These alterations in haematological indices can be ameliorated through pre-treatment with selenium-yeast antioxidants.
Funding
This research was fully funded by authors; we didn’t receive any aid from any other source be it government or nonprofit agencies.
Conflict of Interest
The authors have no conflicts of interest to declare.
Ethical Approval
The ethical approval for this study was obtained from the Animal Ethical Committee of Ahmadu Bello University, Zaria, Nigeria. This study was conducted following the best standard practices of the Animal Ethical Committee.
Author's Contributions
OMO formulated the study. RYM, EEO and OIO conducted the research and provided research materials. OMO, RYM and EEO collected organized, analyzed, and interpreted data. All authors were fully engaged in the actualization of this manuscript.
Acknowledgement
We appreciate the efforts of all laboratory technicians who provided technical support during this study.
References
- McEwen BS, Karatsoreos IN. What Is stress? In stress challenges and immunity in space. Springer Cham 2020; 19-42.
- Chainy GB, Sahoo DK. Hormones and oxidative stress: an overview. Free Radic Res 2020; 54:1-26.
- Mitoh A, Suebe Y, Kashima T, et al. Shear stress evaluation on blood cells using computational fluid dynamics. Biomed Mater Eng 2020: 31:169-178.
- Ikechukwu UR, Ikechukwu ES, Ani OJ, et al. Methanol extract of acanthus montanus (acanthaceae) leaves ameliorates oxidative stress and improves haematological indices in rats. Afr J Pharm Res Dev 2020; 12:26-37.
- Naghipour Hamzekolaei M, Jafarisani M, Farajzadeh A, et al. Changes in mean platelet volume and hematologic indices in patients with panic disorder due to oxidative stress. Brain Behav 2020; 10:e01569.
- Abdel-Moneim A, Zanaty MI, El-Sayed A, et al. Relation between oxidative stress and hematologic abnormalities in children with type 1 diabetes. Can J Diabetes 2020; 44:222-228.
- Khalili M, Chamani M, Amanlou H, et al. The effect of feeding inorganic and organic selenium sources on the hematological blood parameters, reproduction and health of dairy cows in the transition period. Acta Sci 2020; 42:e45371.
- Wang X, Shen Z, Wang C, et al. Dietary supplementation of selenium yeast enhances the antioxidant capacity and immune response of juvenile Eriocheir sinensis under nitrite stress. Fish Shellfish Immunol 2019; 87:22-31.
- Wang Z, Tan Y, Cui X, et al. Effect of different levels of selenium yeast on the antioxidant status, nutrient digestibility, selenium balances and nitrogen metabolism of Tibetan sheep in the Qinghai-Tibetan Plateau. Small Rumin Res 2019; 180:63-69.
- Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med 1990; 9:515-540.
- Martin Jr JP, Dailey M, Sugarman E. Negative and positive assays of superoxide dismutase based on hematoxylin autoxidation. Arch Biochem Biophys 1987; 255:329-336.
- Takebe G, Yarimizu J, Saito Y, et al. A comparative study on the hydroperoxide and thiol specificity of the glutathione peroxidase family and selenoprotein P. J Biol Chem 2002; 277:41254-41258.
- Cyboran-Mikołajczyk SM, Kleszczyńska H, Oszmiański J, et al. Allium ursinum L. leaves components modified the physico-chemical properties of red blood cells protecting them from the effects of oxidative stress. Acta Pol Pharm 2019; 76:483-491.
- Lutnicka H, Bojarski B, Witeska M, et al. Exposure to herbicide linuron results in alterations in hematological profile and stress biomarkers of common carp (Cyprinus carpio). Ecotoxicology 2019; 28:69-75.
- Shareef MA, Mohammed TR, muneeb Alrawi H. Effect of yeast (Saccharomyces cerevisiae) enhanced with selenium or zinc on the hematological characteristics in Iraqi Does. Anbar J Vet Sci 2019; 12:75-81.
- Bila I, Dzydzan O, Brodyak I, et al. Agmatine prevents oxidative-nitrative stress in blood leukocytes under streptozotocin-induced diabetes mellitus. Open Life Sci 2019; 14:299-310.
- Engelsma MY, Hougee S, Nap D, et al. Multiple acute temperature stress affects leucocyte populations and antibody responses in common carp, Cyprinus carpio L. Fish Shellfish Immunol 2003; 15:397-410.
- Calamari L, Abeni F, Bertin G. Metabolic and hematological profiles in mature horses supplemented with different selenium sources and doses. J Anim Sci 2010; 88:650-659.
- Takahashi LS, Biller-Takahashi JD, Mansano CFM, et al. Long-term organic selenium supplementation overcomes the trade-off between immune and antioxidant systems in pacu (Piaractus mesopotamicus). Fish Shellfish Immunol 2017; 60:311-317.
- Rammerstorfer C, Potter GD, Brumbaugh GW, et al. Physiologic responses of acclimatized or non-acclimatized mature reining horses to heat stress: I. Heart rate, respiration rate, lactate, rectal temperature, cortisol and packed cell volume. J Equine Vet Sci 2001; 21: 431-438.
- Amin SN, El-Aidi AA, Zickri MB, et al. Hepatoprotective effect of blocking N-methyl-d-aspartate receptors in male albino rats exposed to acute and repeated restraint stress. Can J Physiol Pharmacol 2017; 95:721-731.
- Batandier C, Poyot T, Marissal-Avry N, et al. Acute emotional stress and high fat/high fructose diet modulate brain oxidative damage through NrF2 and uric acid, in rats. Nutr Res 2020; 79:23-34.
- Zhang H, Wei M, Sun Q, et al. Lycopene ameliorates chronic stress-induced hippocampal injury and subsequent learning and memory dysfunction through inhibiting ROS/JNK signaling pathway in rats. Food Chem Toxicol 2020; 145:111688.
Author Info
Okwute M Ochayi1, Ruth Y. Manjak2, Emmanual E Ochai3, Oluwatosin I Oyeniran4 and Senol Dane4*
1Department of Human Physiology, Faculty of Basic Medical Sciences, BAZE University, Abuja, Nigeria2Department of Human Physiology, Faculty of Medicine, Ahmadu Bello University, Zaria, Nigeria
3Department of Physiology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria
4Department of Physiology, Faculty of Basic Medical Sciences, College of Health Sciences, Nile University of Nigeria, Abuja, Nigeria
Citation: Okwute M Ochayi, Ruth Y Manjak, Emmanual E Ochai, Oluwatosin I Oyeniran, Senol Dane, Selenium-Yeast Administration Ameliorates the Alterations in Haematological and Oxidative Stress Parameters of Wistar Rats Subjected to Restraint Stress, J Res Med Dent Sci, 2021, 9 (3):171-176.
Received: 25-Jan-2021 Accepted: 22-Mar-2021 Published: 29-Mar-2021
