Research - (2022) Volume 10, Issue 7
Sealing Ability and Biocompatibility of Different Bioceramic Based Sealers
Tara H Haji1*, Bahar J Selivany2 and Abdulhaq A Suliman3,4
*Correspondence: Tara H Haji, Kurdistan Board for Medical Specialist, Iraq, Email:
Abstract
Introduction: The effectiveness of root canal therapy in endodontic practice is largely determined by achieving a compact fluid-tight closure at the apical end of the root canal, which inhibits irritant entry and buildup, which leads to a biological breakdown of the attachment mechanism and failure. During obturation, root canal sealers are used in conjunction with gutta percha to fill voids and seal root canals. Root canal sealers come in a variety of shapes and sizes, each with its own set. Aim: Biocompatibility was tested on animal models, and sealing ability was assessed using Scanning Electronic Microscope. Materials &methods: This study utilized two Bioceramic sealers (BioRoot RCS and Meta Biomed bio_ ceramic sealer (CeraSeal RCS) and compared the findings to a control of Zinc oxide eugenol sealer. Biocompatibility was determined by examining histopathological biopsy specimens collected from rabbits. Each rabbit had four dentin tubes implanted into the subcutaneous tissues, one for BioRoot RCS, one for CeraSeal RCS, and one for ZOE RCS, with the fourth tube being empty. Histological sections were stained with haematoxylin and eosin and assessed with light microscope. Extracted human single canal premolars were used to test the sealing ability. The root canals were divided into 3 sections (coronal, middle, and apical). SEM was used to assess the adhesion quality at the sealer-dentin interface. Results: BioRoot and CeraSeal sealers have excellent sealing adaptation and biocompatibility, as well as rapid tissue recovery, while ZOE sealers have a slower recovery of inflammatory reaction results when compared to bioroot and ceraseal sealers, as well as less sealing adaptation than the two other bioceramic sealers. Conclusion: In general, all sealers tested were biocompatible and capable of sealing or adhesion.
Keywords
Bioceramic, Biocompatibility, Sealing ability, SEM, Root canal sealers
Introduction
Endodontic treatment is a set of procedures used to treat an infected canal in a tooth, with the aim of ending the infectious process and preventing new microbial [1]. The use of root canal sealers to execute root canal filling in obturation procedures is essential in preventing treatment failure [2]. As a result, these materials should contain a set of characteristics that allow for successful endodontic filling. The most significant characteristics are: (a) having a hermetic seal, (b) Antibacterial activity (or at least not encouraging bacterial development), (c) being biocompatible and not irritating to radicular tissue, (d) not staining tooth structure [3]. Scientific and technology advancements have allowed materials to improve equipment and materials in a range of fields over time [4], particularly in endodontic, thus providing superior physical results like sealing ability to the root canal dentin [3].Notably in endodontics, therefore delivering superior physical effects such as root canal dentin sealing ability [3], The ability to seal is determined by the material's resistance to microleakage through thickness [5,6], and the long-term success of endodontic therapy is determined by full filling after root canal obturation [7]. As a result of poor contact in the middle of the gutta-percha and the sealer, as well as the dentin, microleakage is one of the most common source of endodontic failure [8-10]. Stereomicroscopy, scanning electron microscopy (SEM), and leakage tests have all been used to assess a sealant's adaptability to dentin [11,12]. In this investigation, SEM will be used to assess the sealing ability. Sealers' adhesion ability is being scored as either very good, good, or acceptable [13]. Several materials have been developed, and they can be classified into the following classes based on their chemical composition and structure: Bioactive endodontic sealers, resin-based endodontic sealers, silicone-based endodontic sealers, calcium hydroxidebased endodontic sealers [12,14,15]. CeraSeal (CS) (MetaBiomed Co., Cheongju, Korea) is an antibacterial calcium-silicate-based premixed substance that never shrinks and has a high pH level [16]. There is also a BioRoot BC sealer (Septodont -France) that's a tricalcium silicate bioactive sealer that comes in powder and liquid form [17]. It possesses excellent characteristics [18,19]. Along with ZOE sealer, these two bioceramic sealers will be used in this investigation. Biocompatibility is the most important features of root canal sealers since they come into close contact with periradicular tissues [5]. This biocompatibility refers to the ability to elicit an appropriate host response in a specific application; that is, it does not cause an adverse reaction when it comes into contact with the tissue, which can be determined by looking for cell infiltration or vascular changes, as well as determining the severity of the inflammatory response [19,20]. The materials are implanted via surgery in these studies, and the body's response to tissue injury begins with inflammation and progresses via wound healing mechanisms [21-24]. If a normal healing process is followed, the implanted substance can be considered biocompatible [20,22]. In certain investigations, the materials to be examined may be directly injected subcutaneously, while in others, the materials should be implanted in tubes, such as a dentin tube or a polyethylene tube [25,26]. The materials that will be tested in this investigation will be inserted into dentin tubes. The canal will be enlarged with proTaper files (Dentsply Maillefer, Ballaigues, Switzerland) and Gates Glidden burs (Dentsply Maillefer, Ballaigues, Switzerland) to a thickness of 0.5-1 mm for the dentin tube. The apical foramina will be expanded to a diameter of approximately 2 mm. The material-filled tube will be injected subcutaneously into the tissue [27]. The reaction will be monitored once the specimen has been implanted for 96-10-21 days. The number of inflammatory cells and fibrous tissue development as a result of the sealers will be scored histologically [28].
Material and Methods
Biocompatibility test
Thirty healthy male rabbits weighing 350±50 gm from the local area were used. All animals were implanted with four dentin tubes. Each tube was prepared from roots derived from the palatal and distal roots of the molars. Protaper files and Gate Glidden burs from( Dentsply Maillefer, Ballaigues, Switzerland) were used for preparation of the dentin tube, resulting in an exterior wall thickness of 0.5–1 mm. The apical foramina were enlarged to an approximate diameter of 2 mm. each dentine tube was irrigated with 3 % sodium hypochlorite and 18% (ethylene diamine tetra acetic acid)(EDTA), rinsed with distilled water, and then autoclaved.
Each dentin tube was disinfected in 2.2 % glutaraldehyde for 12 hours before surgery and sterilized in an autoclave. There were 3 groups of rabbits separated according to study time (96-hrs, 10, and 21 days), each of the 10 rabbits was implanted with four dentin tubes subcutaneously. Anesthesia was given to the animals at a rate of 0.001 mg/kg body weight [19]. 10% Ketamine Hydrochloride (Cheminova, Mexico-DF) was used to achieve this effect and it was delivered intraperitoneally associated with Xylazine Hydrochloride (10 mg/kg). After shaving and disinfection of surgical sites. For placement of tubes in the anterior and posterior sections of the dorsum four roughly 4mm incisions were made with a number 15 scalpel blade (Denti-Lab, Mexico City, Mexico). The tubes were longitudinally implanted in animals, with the first three tubes filled with root canal sealers and fourth one left unfilled. 6.0 nylon thread was used for suturing (Ethicon, Mexico D.F.).Animals were euthanized with anesthetic overdose and slaughtered at 96 hours, 10 days, and 21 days (Ketamine hydrochloride, Cheminova, Mexico D.F.) was used. Excision biopsy of areas surrounding the implants was used to evaluate the tissue reaction to implanted materials. Tubes were dislodged from sections without touching tissue extremities using an incision on the longitudinal axis. The samples were fixed in 10% formalin for two days. Specimens were placed in paraffin blocks. Serial sections of 4 μm thickness were developed by a rotary microtome for further staining with hematoxylin and eosin stain (H&E).
A microscope at 400x and 100x magnification was used for evaluation. The presence of inflammatory cells such as polymorph nuclear neutrophils, lymphocytes, macrophages, eosinophil’s and giant cells close to the tube opening was noted with edema, ulceration and granulation tissue formation also. Fibrous capsule thickness was assessed close to the tube opening. -Inflammatory response scored as [19,28]:
1: Few or absence (0_5)
2: Mild (5_25)
3: Moderate (25_125)
4: Severe (up to 125) inflammatory cells. For fibrous capsule, thickness scored either thin fibrous capsule when less than 150 μm and thick when more or equal to 150 μm [19,28].
The samples were examined by a histopathologists, the data was collected and analyzed statistically.
Sealing ability test
Thirty comparable in size, single-rooted human mandibular premolars were taken from patients in the clinic for this study, after obtaining verbal informed agreement for the use of these teeth in the research. Within 2 hours, 5.25% sodium hypochlorite was used to disinfect all of the teeth. Until further testing, the teeth were stored in disinfectant solution (0.1%) thymol crystals. Preoperative radiographs in the mesiodistal and buccolingual directions were taken to establish the existence of a single root canal that is free of root caries, resorption, or calcification. The crowns of all teeth were removed at the cementoenamel junction, and each root was set to about twelve mm in length. A #10 K-File (Dentsply Maillefer, Ballaige, Switzerland) was placed into the root canal until reaches the apex. The working length was set by subtracting 0.5 mm from this length. Instrumentation of all teeth done to a size of 40/06 using a crown-down approach. Between each instrument, 2 mL 2.5 % NaOCl was used for irrigation. To remove the smear layer, a final 1-minute rinse was conducted with 2 mL 2.5% NaOCl, 2ml 17% EDTA (Ethylene diaminete tra acetic Acid, Patterson Dental Supply, Fort Worth, Texas, USA), and 10 mL distilled water. Root canal sealers were mixed according to the manufacturer's recommendations and placed into the canal using a size 40 lentulo spiral (Produits Dentaires SA, Vevey, Switzerland) to distribute sealer equally throughout the canal while using the single cone technique for obturation. According to the following groups:
Group 1: Roots were filled using CeraSeal Root Canal Sealer with 40/06 gutta-percha.
Group 2: Roots were filled using BioRoot canal Sealer with 40/06 gutta-percha.
Group 3: roots were filled with Zinc oxide eugenol canal sealer with 40/06 gutta-percha.
The roots were kept at 37°C and 100% humidity for 5 days after filling to permit the sealer to be fully set.
Roots were sectioned horizontally in the labiolingual direction and separated into apical (0–4mm), middle(4- 8mm), and coronal (8–12 mm) sections and prepared to be examined using SEM Sections were vacuum dried, gold-cover, and then inspected using a scanning electron microscope (SEM) (Carl Zeiss NTS GmbH, Oberkochen, Germany). At a magnification of 2000x, the penetration of sealers into the dentinal tubules and adaptation of each sealer to the dentin were evaluated from the coronal to apical ends, and microphotographs were taken. If a gap presents between the materials and dentin, it was measured by the J image software program.
Scores of sealing ability [13]
1: Very good adhesion; contact line on the sealer-dentine interface with no gaps.
2: Good adhesion; with a curved contact line on the sealer-dentine interface and minor gaps between sealer and dentine wall.
3: Acceptable adhesion; gaps were frequently discovered between sealer and dentine wall, and the sealers-dentine interface had an indistinct and very curved contact line.
Results
Biocompatibility
The severity of the inflammatory response (inflammatory cell scores) in all experimental groups was studied histopathological and statistically during the subcutaneous implantation period.
Control group
At 96hrs, a severe reaction was noted, the tissue was invaded with Dense eosinophil aggregates throughout the wall indicating allergic reaction as shown in (Figure 1A, and 1B). At 10 days, the severity of the reaction was moderate to mild and the tissue was marked with some areas of congested blood vessels. At 21 day (Figure 1C), mild inflammation showing small edema (yellow arrow) within inflammatory infiltrates.
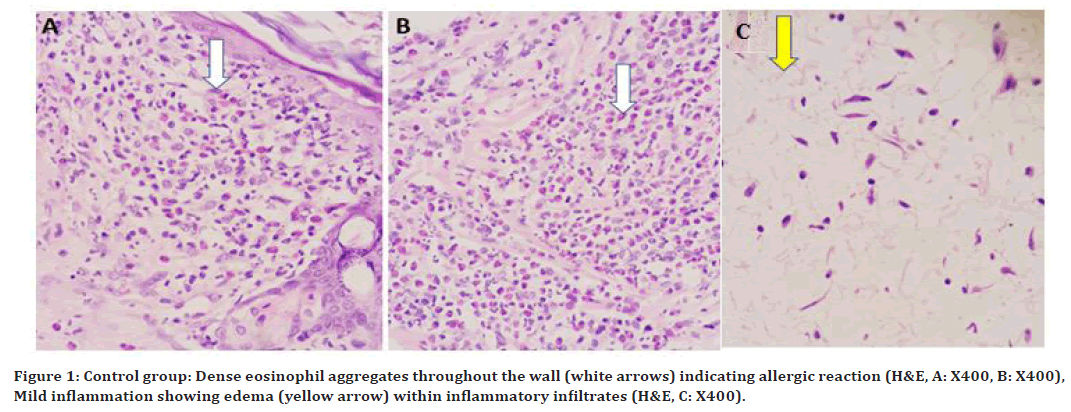
Figure 1. Control group: Dense eosinophil aggregates throughout the wall (white arrows) indicating allergic reaction (H&E, A: X400, B: X400), Mild inflammation showing edema (yellow arrow) within inflammatory infiltrates (H&E, C: X400).
Cera Seal RCS
At 96hrs (Figure 2A and 2B), a severe inflammatory reaction was noted, with beginning of granulation tissue. The severity of the inflammatory reaction was moderate to mild on the 10th-day as shown in. On the 21 Granulation tissue formation showing proliferating blood vessels (white arrows) and fibroblasts (yellow arrows), as shown in (Figure 2C).
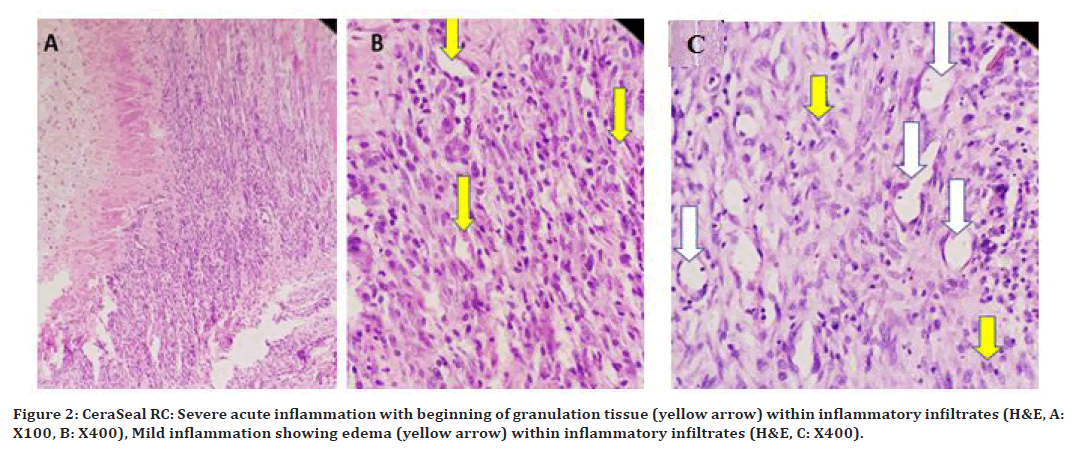
Figure 2. CeraSeal RC: Severe acute inflammation with beginning of granulation tissue (yellow arrow) within inflammatory infiltrates (H&E, A: X100, B: X400), Mild inflammation showing edema (yellow arrow) within inflammatory infiltrates (H&E, C: X400).
Zinc oxide eugenol RCS
At 96hrs (Figure 3A) ulceration was noted with severe suppurative inflammation throughout the wall was seen in (Figure 3 B and 3C). At 10days, the inflammatory reaction was still severe. At 21 days, dilated blood vessels were detected with a considerable number of chronic inflammatory cells.
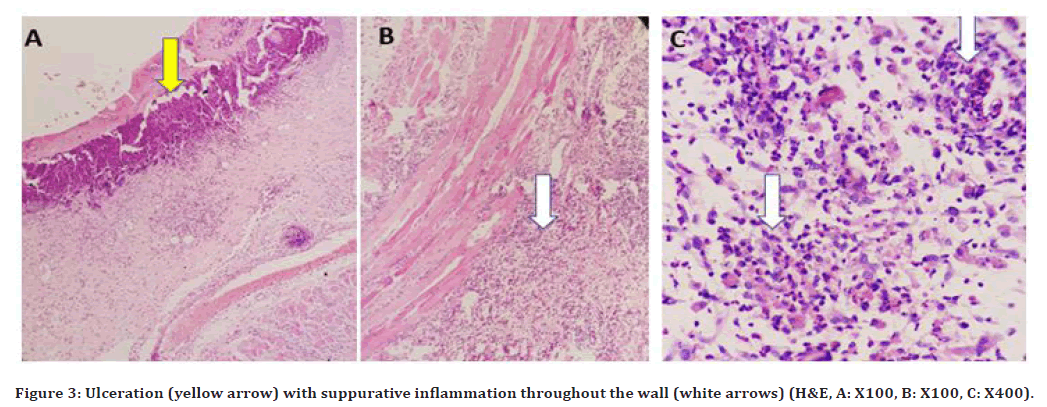
Figure 3. Ulceration (yellow arrow) with suppurative inflammation throughout the wall (white arrows) (H&E, A: X100, B: X100, C: X400).
BioRoot RCS
At 96hrs, a severe to moderate inflammatory reaction was noted. On 10 days, the inflammatory reaction was moderate with edema, and few dilated blood vessels was observed as shown in( Figure 4 A and 4B), with small dilated blood vessels. On 21day, a milder inflammatory reaction with blood vessles formation as shown in Figure 4C.
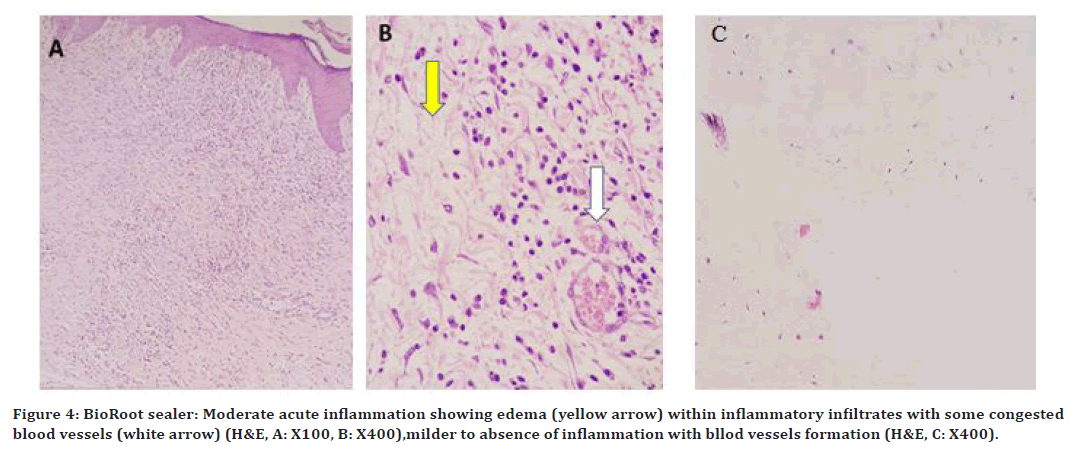
Figure 4. BioRoot sealer: Moderate acute inflammation showing edema (yellow arrow) within inflammatory infiltrates with some congested blood vessels (white arrow) (H&E, A: X100, B: X400),milder to absence of inflammation with bllod vessels formation (H&E, C: X400).
Statistical analysis
The mean values and standard deviation were calculated using the obtained data; finding the statistically significant difference between the three groups studied using SPSS version 20 (Table 1) displays the sample numbers in each period and each group, and there was a statistically significant difference between zinc oxide– eugenol and CeraSeal, BioRoot with P 0.05 using ANOVA test and LSD test in (Table 1) at 2nd, 3rd study period. At 96hrs, according to a histological investigation, all of the sealers examined had substantial inflammatory cell infiltration. There was no variance between the groups (p 0.647). BioRoot RCS exhibited quick recovery, a moderate to mild inflammatory response at 10 days, which was equivalent to control. CeraSeal RCS also showed good recovery, but zinc oxid eugenol RCS exhibited greater inflammation than control and other two experimental root canal sealers, but there were statistically significant differences between the study periods (p=0.000). At 21 days, over time, the inflammatory response considerably subsided for all tested groups at (p=0.000). A significant difference was noted in zinc oxide when compared to control, BioRoot, and CeraSeal RCS.
| Periods | Groups | N | Mean Cells count | Std. Error Cells | Std. Deviation Cells | P - Value | LSD (Post Hoc) | Conclusion |
|---|---|---|---|---|---|---|---|---|
| 4 days | bioroot | 10 | 125.6 | 4.67 | 14.79 | 0.647 | - | No significant differences |
| Ceraseal | 10 | 122.3 | 8.98 | 28.43 | - | |||
| ZoE | 10 | 138.1 | 5.17 | 16.37 | - | |||
| Control | 10 | 109.2 | 10.76 | 34.04 | - | |||
| 10 days | bioroot | 10 | 37 | 10.74 | 33.96 | 0 | - | Significant differences |
| Ceraseal | 10 | 44.3 | 11.47 | 36.28 | - | |||
| ZoE | 10 | 93.2 | 10.68 | 33.79 | * | |||
| Control | 10 | 43.4 | 11.99 | 37.94 | - | |||
| 21 days | bioroot | 10 | 4.2 | 1.22 | 3.88 | 0 | - | Significant differences |
| Ceraseal | 10 | 4.9 | 1.15 | 3.64 | - | |||
| ZoE | 10 | 17.6 | 1.82 | 5.78 | * | |||
| Control | 10 | 3.4 | 0.6 | 1.9 | - |
Table 1: ANOVA and LSD tests for mean Inflammatory cells numbers of the four groups.
Sealing ability
CeraSeal RCS
The interface between CeraSeal RCS with the root canal dentin wall (D) showed very good sealing ability in all parts of the root canal (coronally, middle, apically) with sealer (S) shown in Figure 5A.
BioRoot RCS
The interface between bioroot sealers with the root canal dentin wall showed very good sealing ability in all parts of root canal (coronal, middle, apical) through SEM image analysis at 2000Xmagnification as shown in Figure 5B.
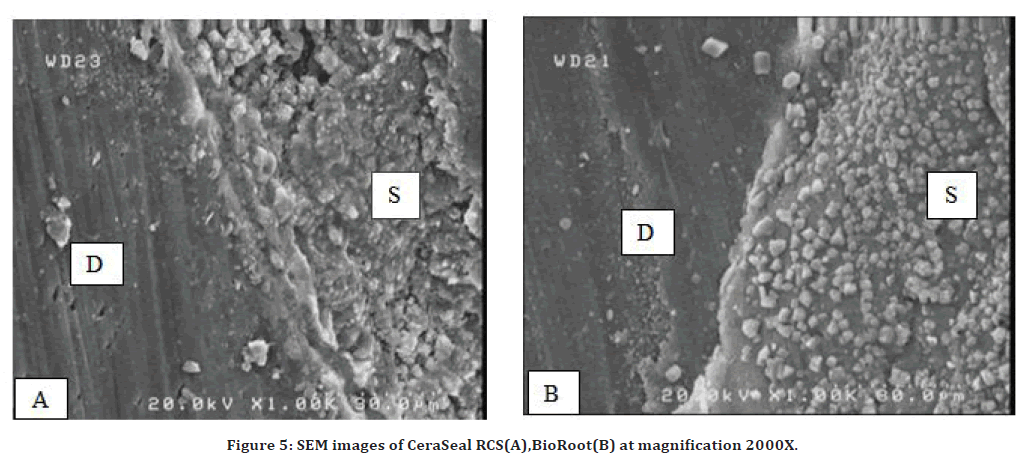
Figure 5. SEM images of CeraSeal RCS(A),BioRoot(B) at magnification 2000X.
Zinc oxide eugenol RCS
The interface between zinc oxide–eugenol with the root canal dentin wall (D) showed acceptable adhesion with the presence of a gap in all parts of the root canals (coronal, middle and apical) as shown in Figure 6.
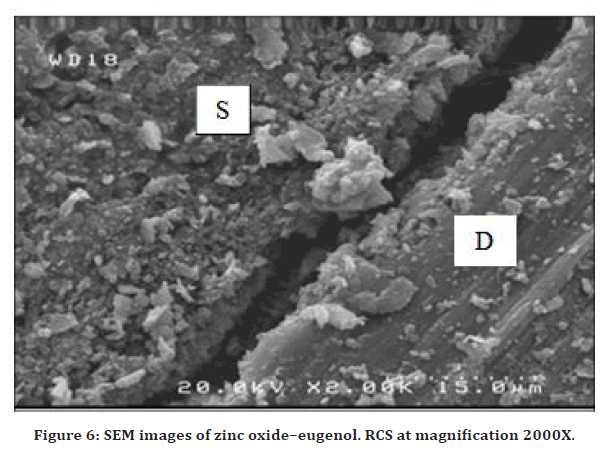
Figure 6. SEM images of zinc oxide–eugenol. RCS at magnification 2000X.
Statistical analysis
The mean values and standard deviation were estimated from the obtained results; find the significant difference between the three groups studied using SPSS version 20 (Table 2) provided in the appendix. There was a statistically significant difference noted between Zinc oxide–eugenol, CeraSeal, and BioRoot RCS with P ≤ 0.05 using ANOVA TEST and LSD TEST in (Table 2). SEM analysis of root canals obturatred with tested root canal sealers demonstrated that their contact to dentin was adequate throughout the root canal. The sealing capacity was improved in the BioRoot RCS and CeraSeal RSC, while Zinc Oxide eugenol RCS had a gap between the dentin wall and the gutta-percha.
| Groups | N | Min | Max. | Mean | STD | Estimated F | P-Value | LSD (Post Hoc) | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Bioroot | 30 | 0 | 1.6 | 0.485 | 0.346 | 5.978 | 0.027 | - | Significant Differences |
| Ceraseal | 30 | 0.14 | 1.2 | 0.625 | 0.285 | - | |||
| ZoE | 30 | 2 | 4.2 | 3.242 | 0.579 | * |
Table 2: ANOVA and LSD tests for the tests RCS groups.
Discussion
Hermetic sealing is a significant aspect in the success of root canal treatment [5]. 58 % of incomplete obturation was linked to treatment failure. Therefor, disinfection and 3D obturation are required. [7]. The current in vitro SEM analysis revealed that in BioRoot RCS, The bioactive tricalcium silicate sealer material that promotes dentinal mineralization and the physiological process of bone growth [29]. Showed a very good seal with dentin and gutta percha [30], as well as creating a favorable environment for periapical healing [29]. As a result, the construction of mean relative gaps was the smallest. thorough studies using three ingredients( coronal, middle,apical) than Zinc oxide eugenol and CeraSeal RCS. On the other hand, the CeraSeal RCS is a calcium silicate sealer bioactive material. Gap percentages were found to be significantly smaller than Zinc oxide eugenol RCS. This finding could be linked to the impacts of increased flow ability and filling capacity [17]. Because of the comparatively high solubility of the Zinc oxide eugenol RCS, its sealing properties were inferior in contrast to other test sealers; hence, the adhesion between Gutta Percha and ZOE-Eugenol is weak, especially after mixing, but also in a set condition [24]. Sealers with favor qualities, such as adhesion and flexibility, should be used, resulting in two favorable outcomes. The first canal sealing was established because the higher sealer interfered with the dentin wall. Second, germs in the canal should be avoided [8]. The purpose of this research was to compare the BioRoot RCS and CeraSeal RCS in vivo biocompatibility to Zinc oxide eugenol RCS. Three observation time intervals were used to examine the intensity of the inflammatory reaction of the tested materials. Rabbit subcutaneous implantation is the most common, reliable, and controlled method for determining the biocompatibility of materials [19]. This followed ISO guidelines for evaluating both short- and medium-term inflammatory responses by employing distinct time intervals [20]. According to the findings of the histological examination, inflammatory responses were seen after 96 hours of observation in both the experimental and control (empty tube) groups. The experimental groups initially presented a severe inflammatory reaction with a thin fibrous capsule, and a significant difference was not observed among the groups (p > 0.05). The surgical trauma of the incision and the physical presence of the tubes may have caused the initial inflammatory reaction in the controls [22]. Similar to previous test groups, a strong inflammatory reaction was seen in the BioRoot RCS at 96 hours. Materials made of calcium silicate are known to release calcium ions when they come into contact with tissue fluids. Thus, the rising alkaline pH after setting could be responsible for the initially significant inflammatory response (96 hrs.). Additionally, the heat produced during the setting process encourages the recruitment of inflammatory cells, which releases cytokines [23]. However, the inflammation reduced rapidly over time, a well-defined fibrous capsule was found especially at the end of 21 days due to the biocompatibility of calcium silicate. Thus, the biocompatibility of BioRoot may be related to the Ca2+ release and give it more alkaline than Zinc oxide eugenol [24]. Cerseal initially had a severe inflammatory response (96hrs) that could be allocated to the high level of pH upon setting. Due to it is low cytotoxicity that assessed by other studies, the CeraSeal RCS inflammatory reaction reduces rapidly [5], it has the potential to allow cell growth due to the release of calcium ions [22]. In the case of Zinc oxide eugenol RCS, when the sealer comes into contact with the tissue due to its resin-based composition, it generates a strong inflammatory response [21]. In terms of the tissue reaction after 21 days, there was a considerable reduction in inflammation intensity. Biocompatibility in vivo study showed that BioRoot RCS and CeraSeal had better biocompatibility than Zinc oxide eugenol RCS in 10days and 21 days. In general, all sealers tested were biocompatible and capable of sealing or adhesion.
Conclusion
However, since this study has limitations as a sealing ability examined by SEM, in the future, it will be necessary to examine it by using a modern device such as a confocal laser microscope. For biocompatibility in the future, model studies can use contemporary sealer materials in the canal of tooth of the dog will be required to enhance the rationale for the new materials and methodologies.
References
- https://evolve.elsevier.com/cs/product/9780323185875?role=student
- Johnson W, Kulild JC, Tay F. Obturation of the cleaned and shaped root canal system. In: Hargreaves KM, Berman LH. Cohen’s pathways of the pulp. Elsevier St. Louis, MO, USA: 2016; 280–322.
- Grossman L. Endodontics. 11th Ed. Lea & Febiger; Philadelphia, PA, USA, 1988.
- Kishen A, Peters OA, Zehnder M, et al. Advances in endodontics: Potential applications in clinical practice. J Conserv Dent 2016; 19:199-206.
- Al-Haddad A, Abu Kasim NH, Che Ab Aziz ZA. Interfacial adaptation and thickness of bioceramic-based root canal sealers. Dent Mater J 2015; 34:516–521.
- Hovland EJ, Dumsha TC. Leakage evaluation in vitro of the root canal sealer cement sealapex. Int Endod J 1985; 18:179-182.
- Camilleri J, Gandolfi MG, Siboni F, et al. Dynamic sealing ability of MTA root canal sealer. Int Endod J 2011; 44:9–21.
- Nair U, Ghattas S, Saber M, et al. A comparative evaluation of the sealing ability of 2 root-end filling materials: an in vitro leakage study using Enterococcus faecalis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011; 112:74-77.
- Trope M, Chow E, Nissan R. In vitro endotoxin penetration of coronally unsealed endodontically treated teeth. Endod Dent Traumatol 1995; 11:90-94.
- Drukteinis S, Peciuliene V, Maneliene R, et al. In vitro study of microbial leakage in roots filled with endorez sealer/endorez points and ah plus sealer/conventional gutta-percha points. Stomatol 2009; 11:21-25.
- Peters OA, Laib A, Rüegsegger P, et al. Three-dimensional analysis of root canal geometry by high-resolution computed tomography. J Dent Res 2000; 79:1405-1409.
- Gomes-Filho JE, Moreira JV, Watanabe S, et al. Sealability of MTA and calcium hydroxidecontaining sealers. J Appl Oral Sci 2012; 20:347.
- Ray H, Seltzer S. A new glass ionomer root canal sealer. J Endod 1991; 17:598.
- Khandelwal D, Ballal NV. Recent advances in root canal sealers. Int J Clin Dent 2016; 9:183–194.
- Gambarini G, Seracchiani M, Zanza A, et al. Influence of shaft length on torsional behavior of endodontic nickel–titanium instruments. Odontology 2021; 109:568-573.
- López-García S, Myong-Hyun B, Lozano A, et al. Cytocompatibility, bioactivity potential, and ion release of three premixed calcium silicate-based sealers. Clin Oral investigat 2020; 24:1749-1759.
- Kharouf N, Arntz Y, Eid A, et al. Physicochemical and antibacterial properties of novel, premixed calcium silicate-based sealer compared to powder–liquid bioceramic sealer. J Clin Med 2020; 9:3096.
- Raghavendra SS, Jadhav GR, Gathani KM, et al. Bioceramics in endodontics–a review. J Istanbul University Faculty Dent 2017; 51:128.
- Santos JM, Pereira S, Sequeira DB, et al. Biocompatibility of a bioceramic silicone-based sealer in subcutaneous tissue. J Oral Sci 2019; 61:171.
- Ricucci D, Langeland K. Apical limit of root canal instrumentation and obturation, Part 2. A histological study. Int Endod J 1998; 31:394-409.
- Simsek N, Akinci L, Gecor O, et al. Biocompatibility of a new epoxy resin-based root canal sealer in subcutaneous tissue of rat. Eur J Dent 2015; 9:31-35.
- Cosme-Silva L, Gomes-Filho JE, Benetti F, et al. Biocompatibility and Immunohistochemical evaluation of a new calcium silicatebased cement, Bio-C pulpo. Int Endod J 2019; 52:689-700.
- Pinheiro LS, Iglesias JE, Boijink D, et al. Cell viability and tissue reaction of NeoMTA plus: An in vitro and in vivo study. J Endod 2018; 44:1140-1145.
- Tyagi S, Mishra P, Tyagi P. Evolution of root canal sealers: An insight story. Eur J Gen Dent 2013; 2:199-218.
- Bernath M, Szabo J. Tissue reaction initiated by different sealers. Int Endod J 2003; 36:256-261.
- El-Mansy LH, Ali MM, Hassan RE, et al. Evaluation of the biocompatibility of a recent bioceramic root canal sealer (BioRoot™ RCS). J Endod 2017; 43:774-778.
- Holland R, de Souza V, Nery MJ, et al. Reaction of dogs' teeth to root canal filling with mineral trioxide aggregate or a glass ionomer sealer. J Endod 1999; 25:728.
- Mori GG, Teixeira LM, de Oliveira DL, et al. Biocompatibility evaluation of biodentine in subcutaneous tissue of rats. J Endod 2014; 40:1485.
- Camps J, Jeanneau C, El Ayachi I, et al. Bioactivity of a calcium silicate–based endodontic cement (BioRoot RCS): Interactions with human periodontal ligament cells in vitro. J Endod 2015; 41:1469-1473.
- Xuereb M, Vella P, Damidot D, et al. In situ assessment of the setting of tricalcium silicate–based sealers using a dentin pressure model. J Endod 2015; 41:111-124.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Tara H Haji1*, Bahar J Selivany2 and Abdulhaq A Suliman3,4
1Kurdistan Board for Medical Specialist, Iraq2College of Dentistry, University of Duhok, Duhok, Iraq
3College of Dentistry, Ajman University, United Arab Emirates
4Centre of Medical and Bio-allied Health Sciences Research, Ajman University, United Arab Emirates
Received: 21-Jun-2022, Manuscript No. JRMDS-22-67121; , Pre QC No. JRMDS-22-67121 (PQ); Editor assigned: 23-Jun-2022, Pre QC No. JRMDS-22-67121 (PQ); Reviewed: 08-Jul-2022, QC No. JRMDS-22-67121; Revised: 13-Jul-2022, Manuscript No. JRMDS-22-67121 (R); Published: 20-Jul-2022
