Research Article - (2022) Volume 10, Issue 8
Salivary Cotinine Cutoff Value as a Mirror for Serum Cotinine in Chronic Smokers
Muayad Hashim Matloob* and Ameena Ryhan Diajil
*Correspondence: Muayad Hashim Matloob, Department of Oral Medicine, College of Dentistry, University of Baghdad, Iraq, Email:
Abstract
Background: Cotinine is used most commonly as a marker to distinguish between tobacco smokers and non-smokers, it has greater sensitivity and specificity than other biochemical tests, Cotinine consider as a major metabolite of nicotine, can be easily detected in various body fluids like blood, saliva, urine.
Objective: The target of this study was to evaluate cotinine concentration in saliva as a mirror for serum cotinine in chronic tobacco smokers, non-invasive, simple with no pain collection procedure when we need large samples are required over a limited period of time.
Material and methods: Study design-One hundred male volunteers, were divided into two groups: the 1st group consisted of 58 smokers, each consumed 20 cigarettes/day for at least 10 years; and the 2nd group consisted of 48 non-smokers who were consider as a control group. The samples were taking from serum and un-stimulated saliva.
Results: In this study showed a significant difference between control and smokers groups, (P>0.05), with cut-off (11.43 ng/mL) for saliva (sensitivity 96% and specificity of (85%) and (98.81) for serum with sensitivity (93%) and specificity of (88%).
Conclusion: Many types of techniques have been used to measure cotinine like, gas chromatography, high performance liquid chromatography and colorimetric assays Immunoassays. Cotinine can be used widely in future compared to other diagnostic tools because of its higher sensitivity, specificity, long half-life. Moreover, it is the best indicator for distinguishing the tobacco smokers from non-smokers, saliva cotinine cut-offs in this study that can be used to distribute smokers and non-smokers with impartially high sensitivity and specificity.
Keywords: Cotinine, Metabolite of nicotine, Gas chromatography, Colorimetric assays, Immunoassays
Introduction
Smoking of tobacco is a main reason for a number of health problems because it contains thousands of harmful, carcinogenic and toxic substance, including cancer, chronic obstructive pulmonary disease, lung fibrosis, and cardiovascular disease, also oral squamous cell carcinoma [1,2].
Cotinine is considering a major metabolite of the tobacco alkaloid nicotine [3], and also for the assessment of Environmental Tobacco Smoking (ETS) exposure [4], because it offers advantages over other compounds (e.g., carbon monoxide and thiocyanate ions) since cotinine levels are not affected by diet or common pollution exposure [5].
It has a longer half-life for that cotinine, measured in saliva, urine, or serum, is a commonly used biomarker of tobacco smoke exposure [3].
In the liver, nicotine is rapidly metabolized to neither cotinine (70-80%) by CYP2A6 and to nor nicotine (5%) by CYP2A6 and CYP2B6. With a half-life of 10-fold longer than that of nicotine (15–19) h for cotinine versus 2-3 h for nicotine [6]. Cotinine reflects the actual uptake, known as internal dose of tobacco smoke [7].
Saliva cotinine: Saliva is more bio-monitoring schemes than plasma, as its collection is non-invasive, easy and well tolerated collection procedure when many samples are required over a limited period [3,4], and considered as a biomarker pool in which, safe and non-invasive source to guide knowing systematic [7,8].
As cotinine is considering a weak base, it is transported from the blood into the saliva by passive diffusion, no additional binding requiring to other molecules. Moreover, several studies have shown that saliva/plasma cotinine concentration ratio is above unity and is almost constant, in spite of variations in salivary flow [10]. For these reasons, salivary cotinine can supply an accurate picture of plasma levels, without requiring blood samples collection.
Cotinine is occasionally used as an indicator for smoking. Active smokers typically have levels of cotinine that are higher (10-500 ng/ml) than those of non-smokers (1-10 ng/ml) [6].
Concerning saliva cotinine concentration is defined as the cut point to distinguish between smokers and non-smokers, the Society for Research on Nicotine and Tobacco has recommended a value of 15 ng/mL in plasma or saliva [11].
Materials and Methods
Cross-sectional study performed on 100 males (Age 20-40 years) who were divided into two groups’ (smokers) and control group (non-smokers). The study done in Babylon city (The blood bank), Ethical approval for the study and informed patients consent obtained, and each patient will filled a case sheet questionnaire:
Including age, name, and systemic diseases.
Inclusion criteria include male with age between 20-40 years and heavy smoker (20 cigarette/day) at least and duration ≥ 10 years.
Serum: Five ml of blood was aspirated from median cubital vein of each subject, serum allow clotting for (10-20) minutes at room temperature. Then centrifuge at (2000-3000) RPM (99 hettich zentrifugen centrifuge) for 10 minutes and supernatant serum was aspirated then frozen at -2°C until analysis.
Saliva: The participants were refrained from drinking or eating for about (30 min) before to collection of saliva, and then they asked to rinse their mouth with water 5 minutes before the collection. 3 ml of whole un-stimulated saliva were collected in a 15 ml polypropylene autoclavable sterile centrifuge tube (99 Hettich zentrifugen centrifuge) while it was kept in ice then be analysis according to (Human Cotinine Enzyme-Linked Immunosorbent Assay ELISA kit (serum and saliva) Cat. NO EA0155Hu).
Statistical analysis: All statistical calculation was performed using SPSS Version 21.0. USA) and Microsoft Excel (2010, Microsoft Corp. USA). All the results were expressed as ± SEM. Independent sample t-test was used to evaluate significant differences between the groups, p<0.05 was considered statistically significant. Roc curve analysis has been carried out to asses' specificity and sensivity.
Results
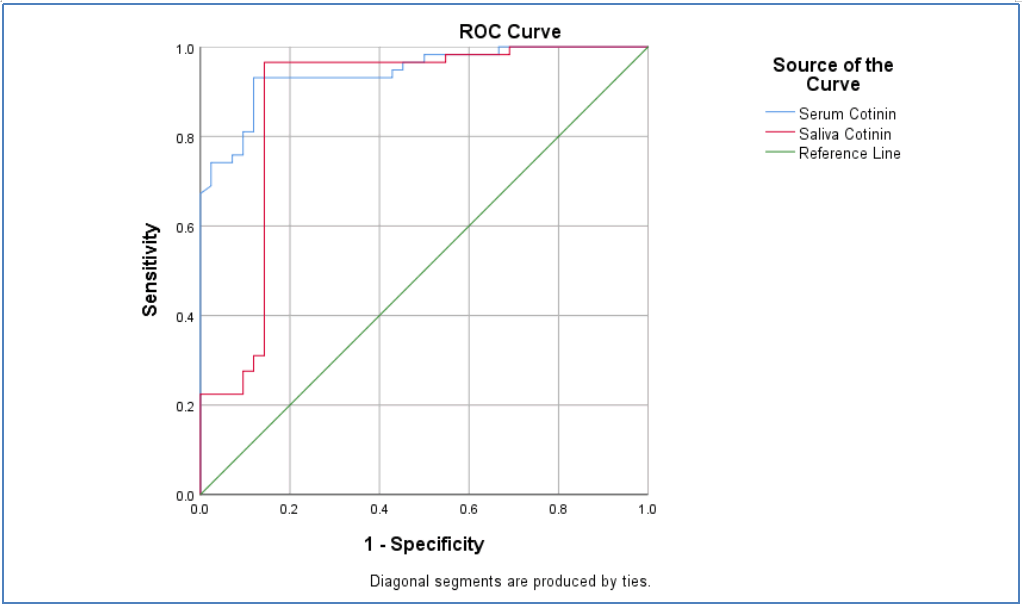
In this study, as shown in Table 1, that Area Under the Curve (AUC) for saliva and serum cotinine (0.876) and (0.943) respectively.
| Test Result Variable(s) | AUC | P-value |
|---|---|---|
| Serum Cotinine | 0.943 | 0 |
| Saliva Cotinine | 0.876 | 0 |
Table 1: Saliva and serum cotinine values performance analysis by ROC curve.
The cut-off point for salivary cotinine was (11.43 ng/mL) with sensitivity of (96%) and specificity of (85%), Serum cotinine was with the cut off value of (98.81) with sensitivity (93%) and specificity of (88%); as show in Figure 1.
Figure 1: ROC curve assessment to evaluate cotinine level performance as a diagnostic marker (saliva and serum cotinine).
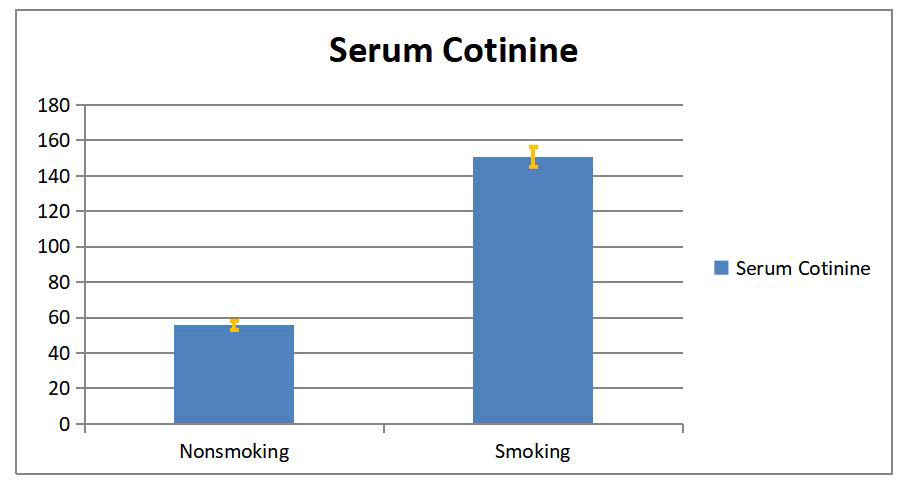
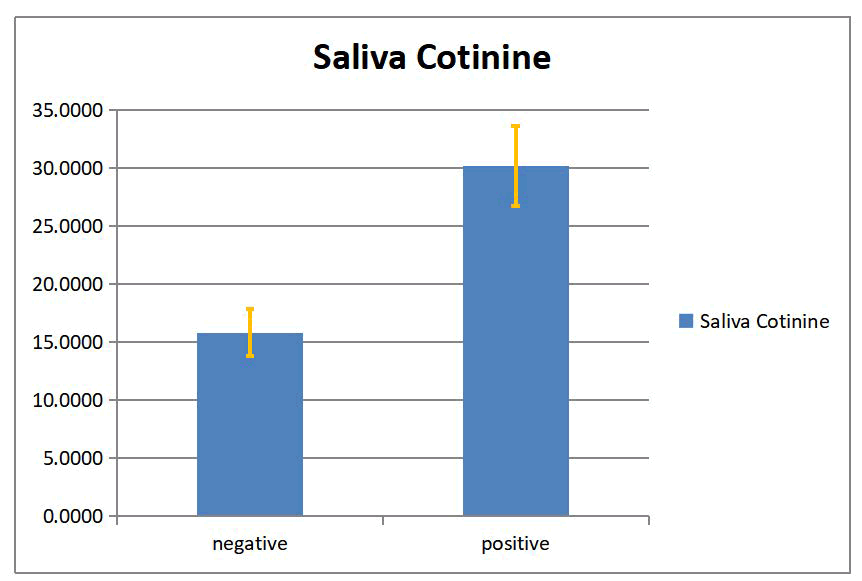
In this study, a significant difference between smokers and non-smokers groups in serum and salivary cotinine with (p <0.05) was found as show on in Tables 2 and 3, Figures 2 and 3.
| Serum Cotinine | Group | N | Mean | Mean Difference | SD | SEM | t | P. Value |
|---|---|---|---|---|---|---|---|---|
| Nonsmoking | 42 | 55.59 | -95.08 | 16.73 | 2.58 | -13.676 | 0 | |
| Smoking | 58 | 150.68 | 49.16 | 5.46 |
Table 2: The serum cotinine level expressed as mean ± SEM in smokers and non-smokers groups.
| Saliva Cotinine | Group | N | Mean | Mean Difference | SD | SEM | t | P. Value |
|---|---|---|---|---|---|---|---|---|
| Nonsmoking | 42 | 6.865 | -28.57 | 1.65 | 0.254 | -9.93 | 0 | |
| Smoking | 58 | 35.432 | 21.82 | 2.865 |
Table 3: The serum cotinine level expressed as mean ± SEM in smokers and non-smokers groups.
Figure 2: Serum cotinine level (mean ± SEM) for smoking and nonsmoking groups.
Figure 3: Saliva cotinine level (mean ± SEM) for smoking and non-smoking groups.
There is relationship between increasing cotinine level in serum and saliva of smoker group than non-smoker group as in Table 4.
| Mean | Std. Deviation | N | Pearson correlation | P. Value | |
|---|---|---|---|---|---|
| Serum cotinine | 110.7415 | 61.09051 | 100 | 0.886 | 0 |
| Saliva cotinine | 23.4337 | 21.81757 | 100 |
Table 4: Association between serum and saliva cotinine.
Relationship between cotinine level in serum and saliva of smoker group only as show in Table 5.
| Descriptive Statistics | |||
|---|---|---|---|
| Mean | Std. Deviation | N | |
| Age | 33.67 | 5.303 | 58 |
| Serum Cotinine | 150.6767 | 49.16035 | 58 |
| Saliva Cotinine | 35.4377 | 21.8137 | 58 |
Table 5: Serum and saliva cotinine (smoker group).
Discussion
Tobacco is a harmful, addictive chemical responsible for many oral diseases and adverse oral conditions. It can be consumed in two forms, smoking and non-smoking. Tobacco consumption in any form is responsible for diseases such as oral cancer, adult periodontal diseases [12]. Substantially all tobacco products contain nicotine in substantial concentration [3].
Cotinine, a major metabolite of nicotine, can be easily detected in various body fluids such as blood, urine and saliva [13].
Cotinine is considered to be the marker of choice for body fluids for the absorption of tobacco smoke; it has been isolated in plasma [14].
At the salivary level, the cotinine concentrations are significantly greater in un-stimulated than in stimulated saliva, the differences being explained by changes in pH with alterations in flow rate; the transfer of cotinine from plasma to saliva is usually via passive diffusion and the pH of saliva is an important influence factor [15]. This is because unionised or uncharged molecules have greater solubility in the lipophilic cellular membranes found in the oral mucosa. The proportion of the unionized form of a drug also depends on the dissociation constant of the compound and the pH of the biological medium. Therefore, the concentration of nicotine and cotinine in saliva will be dependent upon their existence in a unionised form which can be altered by manipulating the pH of the oral cavity [16].
It is most commonly used marker to distinguish between tobacco users and non-users because of its greater sensitivity and specificity than other biochemical tests [17-19].
The Enzyme Linked Immunosorbent Assay (ELISA) stands as a precise method for cotinine estimation as the values are estimated using microtiter wells with enzyme substrate [12,15,20].
The present study showed that there was a different between cotinine concentration in saliva of smokers and non-smokers groups [12]. Also there was a different in cotinine concentration in serum of smokers and non-smokers groups [5,8]. The saliva cotinine cut off was (11.43 ng/mL) with sensitivity (96%) and specificity of (85%), which is the same with other studies [4,6]. The serum cotinine at the cut off value of (98.81) with sensitivity (93%) and specificity of (88%) [21].
Reference
- Kubota T, Yokoyama A. Smoking Behavior and Cessation (Nicotine Addiction): Are Genetic Factors Involved in Smoking Behavior?. Springer, Singapore, 2018; 77–91.
- Mohammed MK, Mohammed LKMHM. Cardamom as a blood pressure lowering natural food supplement in patients with grade one hypertension. Diabetes Care 2020; 1:2.
- Hukkanen J, Jacob P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev 2005; 57:79–115.
- Avila-Tang E. Assessing secondhand smoke using biological markers. Tob Control 2013; 22:164–171.
- Almeida MIGS, BarreirosL, Kolev SD, et al. Salivary Cotinine Assays. Neuroscience of Nicotine, Elsevier 2019; 411–418.
[Crossref]
- LloydA, Gray Place-based outdoor learning and environmental sustainability within Australian Primary Schools. J Sustain Educ 2014; 1.
- Zhang J. Carbon science in 2016: Status, challenges and perspectives. Carbon N Y 2016; 98:708–732.
- Abdullah IM, Abeas KA, Mohamed LK, et al. Evaluation of Shear Bond Strength of Metal Brackets in Total Dryness and Partial Dryness Conditions. Indian J Public Heal Res Dev 2018; 9.
- Abeas KA, Al-MahdiZ, MerzaI, et al. Mutans Streptococci and Removable Orthodontics. Indian J Forensic Med Toxicol 2020; 14:3.
- Perez-OrtunoR, Martinez-SanchezJM, FernándezE,et al. High-throughput wide dynamic range procedure for the simultaneous quantification of nicotine and cotinine in multiple biological matrices using hydrophilic interaction liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 2015; 407:8463–8473.
- Almeida, M Ines GS, et al. Salivary Cotinine Assays. Neuroscience of Nicotine. Academic Press 2019; 411-418.
- RajaM, GargA, Yadav P, et al. Diagnostic methods for detection of cotinine level in tobacco users: a review. J Clin diagnostic Res JCDR 2016; 10:ZE04.
- Kulza M, Wozniak A, Senczuk-Przybylowska M, et al. Saliva cotinine determination using high-performance liquid chromatography with diode-array detection. Przegl Lek 2012; 69:837–840.
- BinnieV, McHughS, MacphersonL, et al. The validation of selfâ?reported smoking status by analysing cotinine levels in stimulated and unstimulated saliva, serum and urine. Oral Dis 2004; 10: 287–293.
- NucaC, Amariei C, Badea V, et al. Salivary cotinine-biomarker of tobacco consumption in the assessment of passive smoking prevalence. Farmacia 2012; 60:662–674.
- RobsonN, BondA, WolffSalivary nicotine and cotinine concentrations in unstimulated and stimulated saliva. African J Pharm Pharmacol 2010; 4:61–65.
- BalharaYPS, JainR. A receiver operated curve-based evaluation of change in sensitivity and specificity of cotinine urinalysis for detecting active tobacco use. J Cancer Res Ther 2013; 9:84.
- Smoking Behavior and Cessation (Nicotine Addiction): Are Genetic Factors Involved in Smoking Behavior
- BenowitzNL, HukkanenJ, JacobNicotine chemistry, metabolism, kinetics and biomarkers. Nicotine Psychopharmacol 2009; 29–60.
- Mohammed MK, EwadhMJ, Hamza Beta trace protein level as a better diagnostic marker of renal impairment in patients with chronic kidney disease, diabetes mellitus, and renal transplants. J Pharm Sci Res 2018; 10:615–618.
- XuX, SuY, FanCotinine concentration in serum correlates with tobacco smoke-induced emphysema in mice. Sci Rep 2014; 4:3864.
Author Info
Muayad Hashim Matloob* and Ameena Ryhan Diajil
Department of Oral Medicine, College of Dentistry, University of Baghdad, IraqCitation: ZMuayad Hashim Matloob, Ameena Ryhan Diajil, Salivary Cotinine Cutoff Value as a Mirror for Serum Cotinine in Chronic Smokers, J Res Med Dent Sci, 2022, 10 (8): 000-000.
Received: 02-Jun-2022, Manuscript No. JRMDS-22-57030; , Pre QC No. JRMDS-22-57030; Editor assigned: 06-Jun-2022, Pre QC No. JRMDS-22-57030; Reviewed: 20-Jun-2022, QC No. JRMDS-22-57030; Revised: 05-Aug-2022, Manuscript No. JRMDS-22-57030; Published: 09-Aug-2022