Research - (2021) Volume 9, Issue 5
Role of Eosinophil in Oral Squamous Cell Carcinoma in Relation to Stage and Grade
Ahmed Abdul Jabbar Ibrahim* and Layla Sabri Yas
*Correspondence: Ahmed Abdul Jabbar Ibrahim, Department of Oral and maxillofacial pathology, Dental Teaching Hospital, College of Dentistry, University of Baghdad, Iraq, Email:
Abstract
Oral squamous cell carcinoma defined as malignant neoplasm of the oral cavity exhibiting the morphological feature of squamous epithelium and it is the end stage of alteration in the stratified squamous dysplasia when the dysplastic epithelial cell invading the underling connective tissue and reach the basement membranes. Aim: To evaluate the (Eosinophil cell density) in different grades and stages of oral squamous cell carcinoma (OSCC) using Special stain like Giemsa stain. Materials and methods: Seventeenth intraoral histopathologically proven cases of OSCC were selected (9 cases were well-differentiated squamous cell carcinoma (WDSCC), 6 moderately differentiated squamous cell carcinoma (MDSCC), and 2 poorly differentiated squamous cell carcinoma (PDSCC)). One section of 4 µm were taken for each case. All cases were stained using special stain (Giemsa stain) for studying tissue eosinophils. Eosinophil cell density was calculated using the density method. Results: (ECD) was counted &correlated with age, sex, site, grade and stage, a high significant relation between (ECD) was observed with an increasing grade of OSCC from well to poor differentiated oral squamous cell carcinoma. While (ECD) correlation with age, sex, site, and stage were statistically non-significant in all stages. Also a high significant relation bet (ECD) was observed with an increasing grade of OSCC. Conclusion: The findings of the present study highlight the significance of eosinophil counting and that it can be used as an additional morphological parameter in the grading of OSCC which can also be included in the biopsy report.
Keywords
Eosinophil cell density, Oral squamous cell carcinoma, Giemsa stain
Introduction
More than 90 % of oral cancers are squamous cell carcinoma. Its biological behavior is influenced by the host immune cells, such as multifaceted eosinophil’s, associated with wound healing and tissue damage processes. Their presence within a variety of human cancers raises queries about their role. The infiltrations of tumor stroma by eosinophils are believed to play a significant role in progression of the carcinoma and could be either a potential diagnostic tool for stromal invasion or as a prognostic indicator. Its role in cancer remains unclear since in the literature, there are very few studies showing improved prognosis and few contradictory studies showing poor prognosis [1].
Oral squamous cell carcinoma made the tumor epithelium and the surrounding connective tissue stroma. The connective tissue stroma creates the tumor microenvironment (TME) within which varying populations of mesenchymal cells, extracellular matrix and inflammatory cells are found. [2]. TME provides the cross-talk between the tumor cells and the stromal elements such as inflammatory cells and cancer-associated fibroblasts, contributing to the development, growth, invasion, and metastasis of the tumor [3]. Literature suggests six features in the development of cancer. Epidemiological studies, from the beginning of the 19th century, are done to propose the role of inflammation as the seventh feature of cancer. [4].
Inflammation in tumor microenvironment assists in both promotion and growth of tumor. Tumor-associated tissue eosinophilia (TATE) is the term used when eosinophils are detected in a tumor tissue with inflammatory infiltrate. Although carcinogenesis with inflammation is one of the important hallmarks, the exact role of eosinophils remains unclear. Various studies on oral squamous cell carcinoma (OSCC) that focused on eosinophils stated both favorable and unfavorable prognosis in cancer tissue, because of which the exact function of eosinophil’s still remains uncertain [5].
Very few studies are conducted to elicit the role of the inflammatory environment in relation to (Oral potentially malignant disorders) and their progression to cancer. However, these studies have provided an evidence that a high level of immune cell infiltration is prognostic of progressing immune reactivity in premalignant lesions and in cancers.
Tissue eosinophils are granulocytes which come under myeloid progenitor series of immune cells system. Eosinophils were first described as “coarse granule cells.” The eosinophils are 8 μm in diameter and characterized by its bright red granules. Their nuclei are bilobed usually although three or more lobes observed. An eosinophil is a granular leukocyte which is normally present in the gut lining and the bloodstream [6]. They contain proteins that give the body ability to fight infection of parasitic organisms, such as worms. However, in certain diseases, these proteins can damage the body [7].
The term eosinophilia refers to conditions in which abnormally high levels of eosinophil’s are found in either the blood or in body tissues [8], although it is controversial. In this study the counting of eosinophils were correlated with the differences in clinicopathological features of OSCC. The aim of present study was Evaluation of (ECD) in OSCC in relation to age, sex, site, grade and stage by using special stain (Giemsa stain).
Materials and Methods
In this retrospective study the eosinophil cells counting in histopathologic records of 17th OSCC patients who underwent surgery were retrieved from the archives of Oral Pathology laboratory, College of Dentistry Baghdad University, and the histopathological laboratory in Al-Shaheed Ghazi hospital for specialized surgeries which were dated from the period 2014 to 2017. The demographic and histopathologic features of patients were recorded, and descriptive analysis was used for statistical interpretation between them. Evaluation of eosinophil counting cell by using Giemsa staining by counting the eosinophil cells density (ECD).
Giemsa stain preparation
Giemsa's solution is a mixture of methylene blue, eosin, and Azure B. Giemsa staining Giemsa stain is one of the best-known histological stains, coloring the nuclei dark blue and the cytoplasm blue to pink, according to the acidity of the cytoplasmic contents.
The stain is usually prepared from commercially available Giemsa powder. Generation a thin air-dried samples film of the specimen on a microscope slide is fixed in methanol for 10 min. Air-dry until all methanol has evaporated. Stain in coplin jar containing 5% Giemsa stain (diluted in tap water) for 20 min. Wash sample in large beaker filled with tap water until excess Giemsa stain is removed. Air-dry and examine under microscope [9].
Counting procedure
Eosinophils were analyzed quantitatively by counting the total number of eosinophils in Giemsa-stained sections. The Giemsa-stained sections were first seen at low power (×10). Cell counting was then performed under ×40. (Four high-density areas) were selected and software grid (10 × 10) was created with an area of 0.04 mm2 which was calibrated. The cells were counted throughout each of the tissue sections in four representative and consecutive grid fields (×40). The mean of four values was calculated and expressed as mean ± standard deviation (SD) per mm2. The fields were studied in a step ladder fashion and care was taken to prevent the overlapping of fields. The cells extending over other squares were counted in First Square. (Treville Pereira et al, 2018). And eosinophil cell density (ECD) was calculated from each 4 fields using formula mentioned below.
Eosinophil cell density (ECD): No. of eosinophil cells in a field/0.04mm (Area of field i.e., 40X magnification=0.04 mm).
Statistical analysis
Data were revised, coded, and analyzed using the “Statistical Package of Social Science (SPSS) version 26.0.
For presentation of data using:
Mathematical presentation method (Mean and Stander Deviation).
For analysis of data using:
Independent sample t-test.
Simple Linear regression.
The comparison of significant (p-value) in any test was considered as:
Considering P-Value of less than 0.05 (P<0.05) was statistically significant (S), while P- Value of less than 0.01 (P<0.01) was highly statistically significant (HS).
Results
The distribution of study sample according to clinicopathological data was shown in Table 1. Patient age was ranging from 18 to 78years with a (mean ± standard deviation) was (56.29 of ± 15.20) years, the highest proportion of sample was among age group>50years (70.6%) Regarding gender, proportion of males was higher than females (58.8% versus 41.2%) with a male to female ratio of 1.42:1. Regarding the site of the lesion, the most common site of OSCC in our study was the Tongue (29.4%). The most predominant grade is well differentiated grade OSCC was about (52.94%) of lesions, but cases with poorly differentiated OSCC was 11.76%. Concerning the stage of the lesion, the highest proportion is stage IV (70.59%). While N status in this study positive N was (35.3%), while negative N was (64.7%). Regarding T status in the present study (T1-T2) percentage was 29.5% and (T3-T4) percentage was 70.5%.
Table 1: Distribution of study group by age (year), Sex and Site, grade & stage.
| No.(n=17) | (100%) | ||
|---|---|---|---|
| Age (year) | (≤ 50) | 5 | 29.4 |
| (>50) | 12 | 70.6 | |
| Sex | Male | 10 | 58.8 |
| Female | 7 | 41.2 | |
| Site | Anterior part of palate | 1 | 5.9 |
| Buccal mucosa | 4 | 23.5 | |
| Floor of the mouth | 4 | 23.5 | |
| Lower lip | 1 | 5.9 | |
| Mandible | 1 | 5.9 | |
| Maxilla with Teeth | 1 | 5.9 | |
| Tongue | 5 | 29.4 | |
| Grade | Well | 9 | 52.9 |
| Moderately | 6 | 35.3 | |
| Poorly | 2 | 11.8 | |
| Stage | I (1st) | 2 | 11.8 |
| II (2nd) | 1 | 5.9 | |
| III (3rd) | 2 | 11.8 | |
| IV (4th) | 12 | 70.6 | |
| N Status | Positive | 6 | 35.3 |
| Negative | 11 | 64.7 | |
| T Status | T1-T2 | 5 | 29.4 |
| T3 –T4 | 12 | 70.6 | |
Table 2 shows the Mean value of ECD according to age group (≤50 year) was (0.50 ± 0.04), while Mean value according to age group (>50 year) was (0.43 ± 0.13). The relationship between ECD and age was non-significant statistically (P=0.276).
Table 2: Comparison between eosinophil cell density (ECD) and age, sex, site, grade & stage.
| N | Mean ±Sd. | Sig. Test | ||
|---|---|---|---|---|
| Age (year) | (≤50) | 5 | 0.50±0.04 | P=0.276 P>0.05 (Non-Significant) |
| (>50) | 12 | 0.43±0.13 | ||
| Sex | Male | 10 | 0.45±0.11 | P=0.839 P>0.05 (Non- Significant) |
| Female | 7 | 0.44±0.14 | ||
| Site | Anterior part of palate | 1 | 0.56±0.00 | P=0.643 P>0.05 (Non- Significant) |
| Buccal mucosa | 4 | 0.47±0.06 | ||
| Floor of the mouth | 4 | 0.45±0.08 | ||
| Lower lip | 1 | 0.40±0.00 | ||
| Mandible | 1 | 0.16±0.00 | ||
| Maxilla with Teeth | 1 | 0.60±0.00 | ||
| Tongue | 5 | 0.44±0.17 | ||
| Grade | Well | 9 | 0.22±0.09 | P=0.001 P<0.01 (High- Significant) |
| Moderately | 6 | 0.40±0.03 | ||
| Poorly | 2 | 0.52±0.06 | ||
| Stage | I (1st) | 2 | 0.52±0.11 | P=0.518 P>0.05 (Non- Significant) |
| II (2nd) | 1 | 0.40±0.00 | ||
| III (3rd) | 2 | 0.46 ±0.09 | ||
| IV (4th) | 12 | 0.44±0.13 | ||
| T Status | (T1-T2) | 5 | 0.42±0.05 | P= 0.607 [P>0.05 (Non- significant)] |
| (T3-T4) | 12 | 0.46±0.03 | ||
| N Status | Positive | 5 | 0.47±0.05 | P= 0.578 [P>0.05 (Non- significant)] |
| Negative | 12 | 0.43±0.03 | ||
Regarding sex, the Mean value according to Male was (0.45 ± 0.11), while Female Mean value was (0.44 ± 0.14), But statistically the relationship between ECD and sex was non-significant (P=0. 839).
Concerning site, the highest Mean value of ECD according to site in Maxilla with Teeth Mean value was (0.60 ± 0.00), while the lowest mean value in Lower lip Mean value was (0.40 ± 0.00). The relationship between ECD and site was nonsignificant statistically (P=0.643).
The Mean value of ECD according to grade (WDSCC) was (0.22 ± 0.06), while in Moderate grade Mean value was (0.40 ± 0.03), and (Poor) grade Mean value was (0.52 ± 0.09). The relationship between ECD and grades were high significant statistically (P=0.001).
According to stage, the Mean value of I (1st) stage was (0.52 ± 0.11), while II (2nd) stage Mean value was (0.40 ± 0.00), while Mean value of III (3rd) was (0.46 ± 0.09), otherwise Mean value of IV (4th) stage was (0.44 ± 0.13). The relationship between ECD and all stages was non-significant statistically (P=0.518).
Regarding T status, the Mean value of (T1-T2) was (0.42 ± 0.05), while Mean of (T3-T4) was (0.46 ± 0.03). The relationship between ECD and T status was statistically non-significant (P=0.607). While N status in this study, Mean Value of (ECD) higher in positive case (0.47 ± 0.05) than negative was (0.43 ± 0.03), but the relationship was statistically non-significant (P=0.578) (Figure 1).
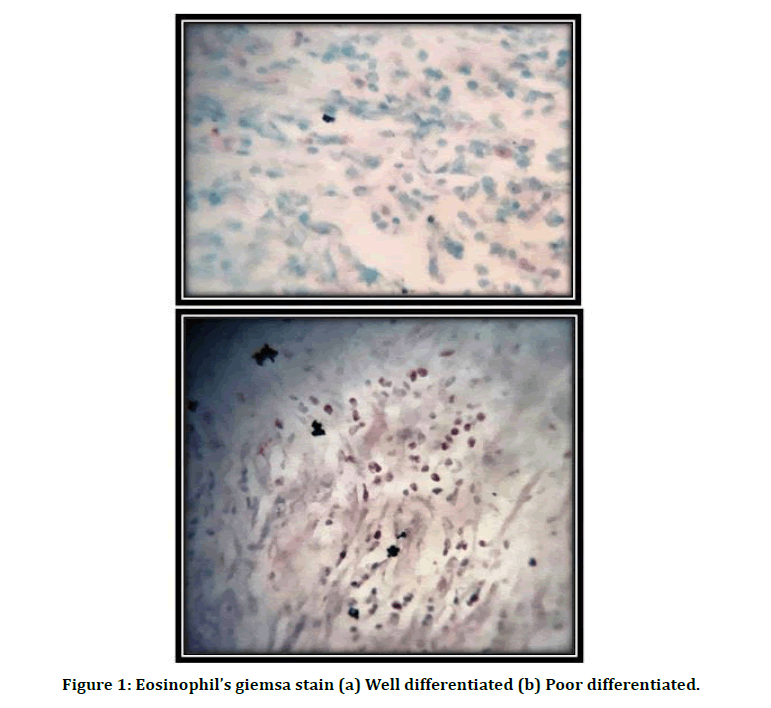
Figure 1: Eosinophil’s giemsa stain (a) Well differentiated (b) Poor differentiated.
Discussion
The pathogenesis of invasive oral cancer is not only based on the genetic changes in the tumor cells but also based on the absolute communication between the tumor cells, inflammatory cells, endothelial cells, fibroblasts, and other stromal cells [10]. In. view of inflammation-associated carcinogenesis, the role of eosinophils in tumor cytotoxicity is not well assumed but has been characterized as more potent than other inflammatory cells in tumor-associated cytotoxic reaction [11,12].
Based on the above facts, this study is based on the ambiguity in functional role of eosinophils in oral squamous cell carcinoma as they are hypothesized as being immunologically directed against tumor cells as well as in lowering the immune response facilitating the tumor growth. Oral squamous cell carcinoma has considered for a long time as a tumor of elderly patient and that may be explained by the prolonged exposure to environmental carcinogens [13] or impaired immune system [14]. In this study, the age of patients was mostly above 50 years of age with mean age of 57.1 years old and the most affected age group was (60-70) years and that analogy to the results were reported by many Iraqi studies [15-20], as well as foreign studies that founded more than 90% of OSCC were above 40 years [21-23]. However, 29.41 % (5 cases) of patient younger than 50 years old and that agreement with some studies which showed the increase of incidence among the younger population [24- 26]. Among these young cases (under 50 years) 2 cases affect females and this agreed with other studies [27,28].
The male to female ratio in this study was 1.42:1, and that identical to most studies which reported that the OSCC affect males more than females [29-31]. The global OSCC M:F ratio is about 5.5:2.5, ranging from 1.2:1 [32] to 3.02:1 [33], such range is similar to most arab nations [34].
Concerning the site distribution, in the current study, the tongue comprised most of the cases (29.41%), followed by the floor of the Mouth and buccal mucosa that represent. (23.53%) of cases, this finding is in accordance with most of published literature, where the tongue was the most common [34-38], nevertheless in contrast with other studies where buccal mucosa was the most common location, especially in south Asian [33].
While in this study we show that stage IV is the highest stage which was agreement with other studies [39,40]. It was obvious that almost all the cases were seen in an advanced stage, the delay was found to be mainly due to ignorance of the patients, and delay in diagnosis and referral by dental practitioners. More than half of the study sample was revealed to be of the advanced stage IV, which was in accordance with the study of [39,41] denoting an aggressive behavior of the tumor and delay in the diagnosis. Regarding the histopathological grading, the well differentiated OSCC is the most predominance one in this study (70.6%) which agree with many previous studies [39,40]. While disagree with several studies [17,19,42,43] who reported (moderately differentiated) grade as a most common. Moreover [44] reported that (poor differentiated) grade was most common.
Differences among these studies may be due to the sample size and to criteria of analysis. Regarding to Distribution of Eosinophil cell density (ECD) by clinicopathological parameters there is no significant relation between ECD and age >50 and <50 also. And non-significant with sex and site. Distribution of Eosinophil cell density (ECD) by Grade: High significant between ECD and all Grades of OSCC. Some researchers employ grade as a part of the risk-assessment to predict prognosis and survival [45].
And this result in accordance with Kargahi et al. [46] reported that the number of eosinophil’s progressively inc. eased from mild to severe at different levels of dysplastic mucosa and from well differentiation to poor differentiation in squamous cell carcinoma. Also, this study is in agreement with [5] suggesting a statistically significant increase in the mean value of tumor eosinophil count from well differentiated to poor differentiated.
Our result was disagreeing with [47] was showed that no correlation was noted between the eosinophilic infiltration and the histologic grades of OSCC. However, this may be because the distribution of OSCC cases according to histologic grades was unequal and not consistent. A conceivable explanation for the disparity in the results of various studies could be due to the lack of a standard criteria for grading TATE that is universally followed and use of biopsy specimens that run the risk of being unrepresentative. This study disagrees with other study [1].
Distribution of Eosinophil cell density (ECD) by Stage: non-significant. This study show increase in all stages but non-significant in the eosinophil count. Others showed absent/mild eosinophils in early clinical stages, i.e., stages I and stage II while significantly higher eosinophilia was seen in stages III and IV, suggesting increased eosinophilia with increasing clinical stages. In the present study, eosinophils were found in most cases of OSCC. There was elevated but not significant Eosinophil count in stages III and IV, so increased eosinophilia was associated with T3 and T4, suggestive of increased eosinophil count with increased primary tumor size [48] have also reported an increase in eosinophil count with an increase in the tumor size. Accordingly, eosinophil’s can be regarded as an indicator of a developing malignancy along with other indicators, and they possibly can be used to develop the prognosis for the disease [46].
Financial Disclosure
There is no financial disclosure.
Conflicts of Interest
None to declare.
Ethical Clearance
All experimental protocols were approved under the College of Dentistry University of Baghdad and all experiments were carried out in accordance with approved guidelines.
References
- Shrestha P, Narayan K, Kumar V, et al. Tumor-associated tissue eosinophilia in oral squamous cell carcinoma–A predictable biological behavior. J Global Oral Health 2020; 3:3-8.
- Curry JM, Sprandio J, Cognetti D, et al. Tumor microenvironment in head and neck squamous cell carcinoma. In Seminars in oncology 2014; 41:217-234.
- Koontongkaew S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J Cancer 2013; 4:66.
- Colotta F, Allavena P, Sica A, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009; 30:1073-1081.
- Deepthi G, Kulkarni PG, Nandan SR. Eosinophils: An imperative histopathological prognostic indicator for oral squamous cell carcinoma. J Oral Maxillofac Pathol 2019; 23:307.
- Potter M. Wintrobe's clinical hematology. Richard Lee G, Foerster J, Lukens J, et al. (Eds) Williams & Wilkins. 1998.
- Weller PF, Goetzl EJ. The human eosinophil: Roles in host defense and tissue injury. Am J Pathol 1980; 100:791.
- Saraswathi TR, Nalinkumar S, Ranganathan K, et al. Eosinophils in health and disease: An overview. J Oral Maxillofac Pathol 2003; 7:31.
- Fraser ST, Isern J, Baron MH. Use of transgenic fluorescent reporter mouse lines to monitor hematopoietic and erythroid development during embryogenesis. Methods Enzymol 2010; 476:403-27.
- Choi S, Myers JN. Molecular pathogenesis of oral squamous cell carcinoma: Implications for therapy. J Dent Res 2008; 87:14-32.
- Lorena SC, Dorta RG, Landman G, et al. Morphometric analysis of the tumor associated tissue eosinophilia in the oral squamous cell carcinoma using different staining techniques. Histology and histopathology. 2003.
- Tadbir AA, Ashraf MJ, Sardari Y. Prognostic significance of stromal eosinophilic infiltration in oral squamous cell carcinoma. J Craniofac Surg 2009; 20:287-289.
- Soames JV, Southam JC. Oral pathology. Oxford University Press. 2005.
- Hakim FT, Flomerfelt FA, Boyiadzis M, et al. Aging, immunity and cancer. Current Opinion Immunol 2004; 16:151-156.
- Mohammed AA. The expression of CD34 and podoplanin as biological markers of angiogenesis and lymphangiogensis in oral and cutaneous squamous cell carcinoma. A comparative study MSc thesis in Oral Pathology. Department of Oral Diagnosis. Baghdad University 2008.
- Sarkis SA, Abdullah BH, Majeed BA, et al. Immunohistochemical expression of epidermal growth factor receptor (EGFR) in oral squamous cell carcinoma in relation to proliferation, apoptosis, angiogenesis and lymphangiogenesis. Head Neck Oncol 2010; 2:1-8.
- Shareef KN, Majeed AH. Immunohistochemical expression of Basic fibroblast growth factor-2 and Heparanase in oral squamous cell carcinoma. J Baghdad College Dent 2013; 25:94-98.
- Neamah AS, Majeed AH. Cell surface expression of 70 KDa heat shock proteins and P21 in normal oral mucosa, oral epithelial dysplasia and squamous cell carcinoma (An Immunohistochemical Study). J Baghdad College Dent 2016; 28:56-60.
- Taha IA, Younis WH. Clinicopathological analysis of oral squamous cell carcinoma in Iraq During period (2001-2013). J Baghdad College Dent 2015; 27:58-65.
- Al-Kawaz AB. Oral squamous cell carcinoma in Iraq: Clinical analysis. Mustansiriya Dent J 2018; 7:100-105.
- Liebertz DJ, Lechner MG, Masood R, et al. Establishment and characterization of a novel head and neck squamous cell carcinoma cell line USC-HN1. Head Neck Oncol 2010; 2:1-4.
- Neville BW, Damm DD, Chi AC, et al. Oral and maxillofacial pathology.4th edition, Elsevier Health Sciences, Canada 2016.
- Smitha T, Mohan C, Hemavathy S. Clinicopathological features of oral squamous cell carcinoma: A hospital –based retrospective study. J NTR Univ Health Sci 2017; 6:29-34.
- Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: Increasing trends in the US population ages 20–44 years. Cancer 2005; 103:1843-1849.
- Sharma P, Saxena S, Aggarwal P. Trends in the epidemiology of oral squamous cell carcinoma in Western UP: An institutional study. Indian J Dent Res 2010; 21:316.
- Ali-Murad AH. Epithelial mesenchymal transition biomarkers expression (β- catenin, MMP1, Fibronectin, SNAIL1, and TWIST2) in oral squamous cell carcinoma in relation to histopathological prognostic factors. Phd. Thesis in Oral Pathology, Baghdad University 2014.
- Syedmukith R, Ahmedmuji BR, Bastian TS. Oral squamous cell carcinoma in elderly vs young patients: a comparative analysis using stnmp staging system. Oral Maxillofac Pathol J 2014; 5.
- Al-Qahtani K, Brousseau V, Islam T. Prognosis of patients less than 40 years of age with Squamous cell carcinoma of Oral Tongue. Int J Head Neack Surg 2015; 6:53-56 .
- Al-Rawi NH, AL-Talaban NG. Squmous cell carcinoma of the oral cavity: A case series analysis of clinical presentation and histological grading of 1,425 cases from Iraq. Clin Oral Investigations 2007; 12:15-18.
- Hatif ML. Fluorescent in situ hybridization evaluation of epidermal growth factor receptor and cyclin d1 genes in oral squamous cell carcinoma. A master thesis in oral pathology, department of oral diagnosis, college of dentistry, university of Baghdad 2012.
- Gadbail AR, Chaudhary M, Gawande M, et al. Oral squamous cell carcinoma in the background of oral submucous fibrosis is a distinct clinicopathological entity with better prognosis. J Oral Pathol Med 2017; 46:448-453.
- Rodrigues PC, Miguel MC, Bagordakis E, et al. Clinicopathological prognostic factors of oral tongue squamous cell carcinoma: A retrospective study of 202 cases. Int J Oral Maxillofac Surg 2014; 43:795-801.
- Dissanayaka WL, Pitiyage G, Kumarasiri PV, et al. Clinical and histopathologic parameters in survival of oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 113:518-525.
- Al-Jaber A, Al-Nasser L, El-Metwally A. Epidemiology of oral cancer in Arab countries. Saudi Med J 2016; 37:249.
- Jerjes W, Upile T, Petrie A, et al. Clinicopathological parameters, recurrence, locoregional and distant metastasis in 115 T1-T2 oral squamous cell carcinoma patients. Head Neck Oncol 2010; 2:1-21.
- Pires FR, Ramos AB, Oliveira JB, et al. Oral squamous cell carcinoma: Clinicopathological features from 346 cases from a single oral pathology service during an 8-year period. J Applied Oral Sci 2013; 21:460-467.
- Maleki D, Ghojazadeh M, Mahmoudi SS, et al. Epidemiology of oral cancer in Iran: a systematic review. Asian Pacific J Cancer Prevention 2015; 16:5427-5432.
- Buchakjian MR, Tasche KK, Robinson RA, et al. Association of main specimen and tumor bed margin status with local recurrence and survival in oral cancer surgery. Otolaryngol Head Neck Surg 2016; 142:1191-1198.
- Rasheed FS, Abdullah BH. Perineural invasion in oral squamous cell carcinoma in relation to tumor depth. J Baghdad College Dent 2019; 31:1-6.
- Bdewi A, Mohamed Labib A, Drobie B, et al. Evaluation of CD68 in oral squamous cell carcinoma and their relation with clinicopathological parameters–An immunohistochemical study. Int J Current Microbiol Applied Sci 2020; 9:3832-3839.
- Ribeiro AC, Silva AR, Simonato LE, et al. Clinical and histopathological analysis of oral squamous cell carcinoma in young people: A descriptive study in Brazilians. British J Oral Maxillofac Surg 2009; 47:95-98.
- Roxana MI. Angiogenisis role in lips squamous cell carcinoma progression: Clinical, histopathological and immunohistochemical study. Doctoral thesis, University of medicine and pharmacy, Craiova, Romania 2014.
- Beena VT, Binisree SS, Ayswarya T, et al. Oral squamous cell carcinoma in patients younger than 40 years: A 10-year retrospective study. Int J Scientific Study 2016; 4:150-153.
- Abdulai AE, Nuamah IK. Squamous cell carcinoma of the oral cavity and oropharynx in ghanaians: A study of histopathological charts over 20 years. World J Surg Med Radiation Oncol 2013; 2.
- Elaiwy O, El Ansari W, AlKhalil M, et al. Epidemiology and pathology of oral squamous cell carcinoma in a multi-ethnic population: Retrospective study of 154 cases over 7 years in Qatar. Annals Med Surg 2020; 60:195-200.
- Razavi SM, Deyhimi P, Homayouni S. Comparative evaluation of eosinophils in normal mucosa, dysplastic mucosa and oral squamous cell carcinoma with hematoxylin-eosin, Congo red, and EMR1 immunohistochemical staining techniques. Electronic Physician 2015; 7:1019.
- Joshi PS, Kaijkar MS. A histochemical study of tissue eosinophilia in oral squamous cell carcinoma using Congo red staining. Dent Res J 2013; 10:784.
- Peter CD, Shashidara R, Haragannavar VC, et al. Assessment of tumour associated tissue eosinophilia (TATE) in oral squamous cell carcinoma using carbol chromotrope stain. Int J Odontostomat 2015; 9:91-95.
Author Info
Ahmed Abdul Jabbar Ibrahim* and Layla Sabri Yas
Department of Oral and maxillofacial pathology, Dental Teaching Hospital, College of Dentistry, University of Baghdad, IraqCitation: Sundus Abdul-Alhussain Jasim, Shorouq M Abass, Effect of Alum Disinfectant Solutions on Some Properties of a Heat-Cured Acrylic Resin, J Res Med Dent Sci, 2021, 9 (5):132-139.
Received: 20-Mar-2021 Accepted: 13-May-2021 Published: 31-May-2021
