Research - (2022) Dentistry and Medical Research
Review on Structure, Characteristics, and Applications of Cross-linked Porous Polyimides
Lakhwinder Singh1, Durgesh Wadhwa2 and Manpreet Sedha3*
*Correspondence: Manpreet Sedha, RIMT University, Mandi Gobindgarh, Punjab, India, Email:
Abstract
Porous polyimides are a kind of permeable organic polymer that is interesting to study (POPs). They have good physical and chemical stability, large surface areas, and energy storage capacities, and they are formed by simple poly condensation processes. The monomers employed in polymer synthesis are intimately linked to the characteristics of the polymer. In the instance of polyimides, various methods are used to generate the required cross-linked and porous PIs from polytypic amines and anhydrides. A large collection of linkers (di anhydrides) and core (amines) starting materials allows for the creation of a broad variety of cross linked pPIs. This research compares and contrasts the unique properties and actions both of semi crystalline pPIs. Furthermore, their uses in energy hydro and isolation, electricity generation storage, catalysis, drug administration, and sensors are explored. Lastly, the paper provides an overview of progress in the area of pPIs since 2010, and a prediction and suggestions for further research in applications.
Keywords
Amines, Applications, Cross linkage, Organic polymers, Poly-condensation
Introduction
Borget and Renshaw were the first to synthesize and publish polyimide (PI) polymers in 1908. There are two types of PIs (Polyimides): aliphatic and aromatic [1]. Commercialization of the first aromatic PI Due to molecular packaging and polarity, benzene PIs have greater mechanical, thermal, and chemical durability than aliphatic PIs, whereas aliphatic PIs have different solubility’s, dielectric loss characteristic, and high optical visibility. PIs (Polyimides) have achieved extensive usage in the fields of air transport, aviation, microsystems, gasification, vesicles, fuel cells, battery packs, memory devices, optoelectronic maneuvers, biomedicine, hydrogels, and the matrix material in composites/hybrid substances (Figure 1) [2].
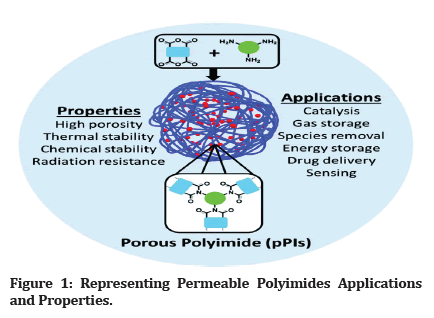
Figure 1: Representing Permeable Polyimides Applications and Properties.
Polyimides with aliphatic/alicyclic content
The three types of PIs are entirely PIs, partially-aliphatic/ alicyclic PIs, or fully-aliphatic/alicyclic PIs. Among the alicyclic / aromatic (Al)-containing polyimides examined are completely and least in part, with promising findings. Bicycle [2.2], tetra carboxylic di anhydrides with a poly alicyclic structure, and tetra carboxylic di anhydrides with a poly alicyclic structure were utilized in that study [2].
Commercialization of polyimides containing aliphatic/alicyclic atoms
Deny the reality that alkanes PIs, that include fully- and partly, have potential utility in a range of sectors, there are just a few fully- and partly now accessible. The fundamental physical characteristics of two commercial completely and partly Al-PIs and Neopulim Grade-S is used for gas separation processes and stretchy optoelectronic devices, Matrimid 5218 and Neopulim Grade-S have been extensively utilized. In addition, certain orientation films for liquid crystal-display strategies are partially-Al-PIs, which are made from 1, 2, 3, 4 cyclobutane tetra carboxylic di anhydride and different diamine. CBDA-based partially-Al-PIs are known for their exceptional flexibility, temperature resistance, and transparency [3].
Chemical structure
Monomer structure
Monomers employed in polymer synthesis are intimately linked to the characteristics of the polymer. In the instance of polyimides, various methods are used to generate the required cross-linked and porous PIs from polytypic amines and anhydrides. (Figure 2) depicts the architectures of amine monomers. Imide bonds link PICOFs, which are chemically and thermally stable and offer a variety of skeletons and pores.
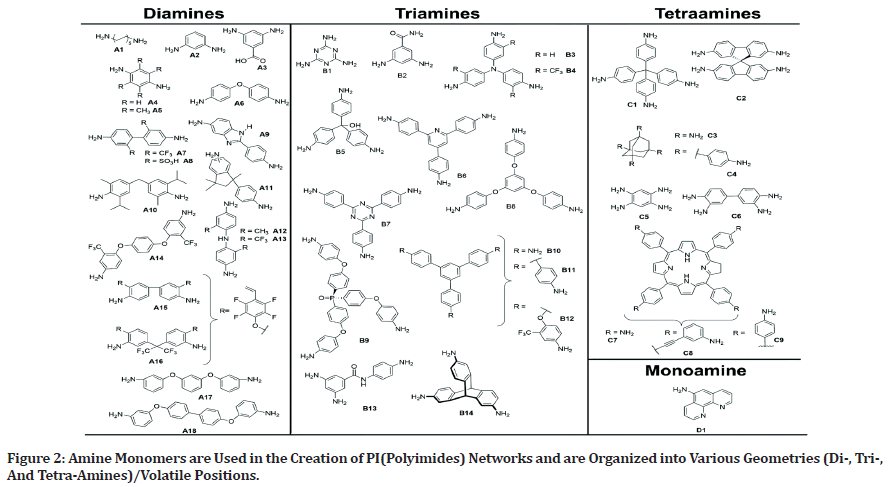
Figure 2: Amine Monomers are Used in the Creation of PI(Polyimides) Networks and are Organized into Various Geometries (Di-, Tri-, And Tetra-Amines)/Volatile Positions.
The heating of PI-COFs materials inside a closed Pyrex tubes may take up to 5–7 days in (Figure 3). This technique, which itself is extensively used in the conventional COFs synthesis, are commonly used synthesis methodology for PI-COFs material formation. Combustion takes place in a small heat bearable glass tube under the high pressure and temperatures. Charge up alcohols and catalysts, and the necessary PI monomers, comprise the reaction system. They use chemical solvents to modify the solubility of the monomers and a suitable catalyst to increase the reaction rate, as well as a correct temperature to enhance the imine’s closure reaction.
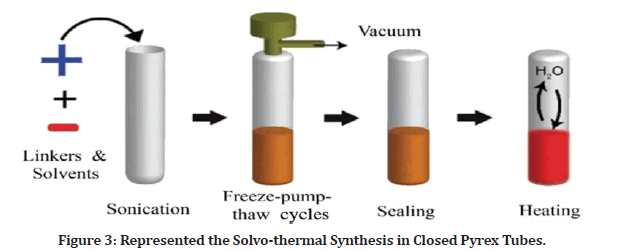
Figure 3: Represented the Solvo-thermal Synthesis in Closed Pyrex Tubes.
This synthesis technique differs from earlier amorphous porous PI synthesis methods. The monomer is fully dissolved in the previously produced Polyimides, resulting in fast and irreversible polymer and the formation of amorphous materials [4,5].
Properties
Thermal stability
Due to the excellent mechanical characteristics, polymer aerogels have gotten a lot of interest. Melamine formaldehyde, polyurethane, polyimide, and polyamide are only a few of the materials studied. These substances not only offer excellent thermal insulating properties, but they also show promise in a variety of fields, such as particle size reduction. Adsorb, gas adsorbents, oil– water segregation, building supplies, and so on are all examples of adsorbent. Polyimide stands out among polymeric material due to its superior mechanical properties, heat resistance, and rapid phase changes.
Polyimide aerogels can be classed as straight or cross linked depending on the linking process used. The mechanical and physical properties of the nonlinear polyimide aerogels are equivalent to that of known function polymer-enhanced aerogels of comparable densities; however these commonly shrink throughout the manufacturing. Wet gelation is a chemically bridging event whereby an oligomer produces covalently bonded networks in a solvent, while drying is contains similar reaction whereby an oligomer generates a covalently bonded network in a liquid. The skeletal of aerogels is made up of this cross-linked architecture; to form an aerogel, the solvents in the damp gel pore is eliminated, and shrinking, collapsing, or split of the skeletons should be avoided if possible during the drying. CO2 supercritical drying is now the most effective technique. A low shrinkage polyimide aerogel and high porosity may be made using this technique [6].
Porosity
Pores of sizes ranging from nanometers to millimeters may be found in cross-linked polymers. Many factors, such as the carbon precursor and crosslinking agent agents employed in feeding or the sample's past thermal history, change the formation (closed or interconnected) and form (fibrillar networks, honeycomb-like, spherical, cylindrical, channel-like, etc.) of the these holes. Porous characteristics such as pore diameter, connectivity of superficial, pore stations, as well as the physicochemical character of the whole barrier all have a role in the adsorption capabilities of micro porous materials for biological fumes and airs.
According findings, hydrophobic alteration of porosity zeolites and activated carbons may substantially enhance organic vapor absorption and competing adsorption of water under humid circumstances. Naphthalene is hydrophobic group, as is widely known. Furthermore, compared to other building blocks often used to make, naphthalene having a high electron-delocalization impact that is beneficial for increasing attraction for aromatic fumes. The addition the naphthalene to MOPs is anticipated that enhance privileged adsorption of hazardous benzene and the compounds over additional air and water vapor in this respect [7].
Capacity for storing energy and associated characteristics
For electrical storage uses, polymers having an elongated -conjugated support with large surface area with good micro porosity are beneficial. Conjugation is beneficial in terms of electrochemical stability as well as electronic conductivity. Figure 4 shows the use of two commonly produced polyimides, pyromellitic dianhydride as polymeric basic components for dianhydride-based polyimides. The particle aromatic amines and triazine rings of the polymers may cooperate with Na ions to escalation of storage space.
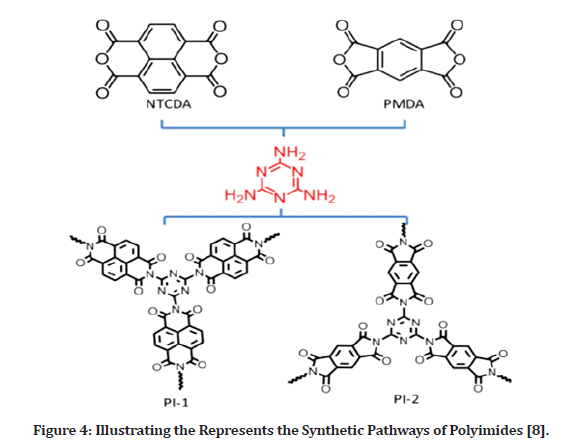
Figure 4: Illustrating the Represents the Synthetic Pathways of Polyimides [8].
Melamine amino-groups arranged in three orientations may efficiently form a three-dimensional cross-linked network. Due to the solvent evaporation, the bioactive granule cross link in deep at high temperatures to produce an extremely reliable and conjugated superstructure, which may provide a viable solution to the aforementioned challenges. As its electrical stability and electrical properties improve, such substances may supply more reactive functional groups for expedite electrons and ions electrode–electrolyte interface passage throughout irreversible sodiation processes. The electro-chemical experiment results showed that NIBs had a large capacity, rapid rechargeable anode and steady cycle [8].
Applications
Gas storage and separation
Gas storage and separation chemical processing drug administration power storage and sensors among other applications, synthetic materials with molecule gauge absorbency consisting of one, two, and 3-dimensional network have gotten a lot of interest recently. Covalent bonding connections among highly organized organic construction blocks are used to create covalent organic frameworks, which have a large surface area and welldefined Nano pores [9].
Heterogeneous catalyst
For first time, Shultz et al. produced a bi - functional heath rings with an aromatic component that possessed anhydride functionalities that can be utilized to catalyze the precipitation of tetra mine C1 utilizing the described. This resulted in the formation of polyimide net, is then metalized at the methoxy rings with manganese (ii) or iron (ii). The synthetic plastic was employed as a reusable catalyst for styrene oxidation utilizing iodosylbenzene as the air; nevertheless, reuse was only partly successful, with bulk of the enzymatic characteristics being lost following three cycles. So because nucleophilic institutes in protein sequences have excellent photocatalytic characteristics for the moisture of CO2 to epoxides, it is anticipated that adequately built and designed pPI catalysts will undertake well with this reaction; however, to our knowledge, these hopeful elements were yet to be explored for this implementation [10].
Review of Literature
Z. Wang and G. Li explored poly-merisation naphthalene- 1,4,5,8-tetracarboxylic dianhydride using tetrakis (4-aminophenyl)methane, 1,3,5-tris(4-aminophenyl) benzene, and tris (4-aminophenyl)amine, and found three polyimide range of network topologies. The resultant monomers having a high surface area and the pore diameter of around 6 microns on average. It has separation ratios of 759.3, 40.3, and 13.8, for benzene over nitrogen, liquid, and cyclohexane, and therefore can collect 90.5 wt% toluene vapors. The presence quantities of propyl units in the networks is attributed to high capacities and selectivity of toluene vapor, since naphthalene is very weakly water soluble and has a -electron-delocalization effect. CO2 uptake capacity in polymer, are irreversible and reach 12.3 wt%, at 88.6 and 12.9 compared to other micro - porous organic polymers. In accordance to the aforesaid investigational findings, the vibrating diameters, polarity, and specific heat of vapors and gases, as well as the stereo arrangement of net endpoint, porous properties, and wettability of the pores of micro porous polyimides, were investigated and described [7].
M. Hasegawa looked into the creation of new hightemperature polymeric materials that may be used as foundation surface in picture display systems. This paper presents novel colorless polyimides that may be solutionprocessed and have super linear thermal expansion coefficients. The principles of PI film colour scheme are briefly mentioned, to evident the obstacles of realistic looking excellent optical responsibility, a really high Tg, a really low CTE, and excellent film tenacity, like the impact of processing parameters on film colour scheme, as well as the physiochemical variables able to dominate the low CTE characteristics of the subsequent PI films. One strategy to attain these desired properties might be to use quasi PI systems with linear chain topologies. Semi-cycloaliphatic produced from cycloaliphatic diamines, and has difficulties with precursor’s polymer, cyclodehydration, and film formation. Whenever the cycloaliphatic diamine trans-1, 4-cyclohexanediamine (t-CHDA) is utilized, a serious concern arises: salt development during in the early phases of precursor’s polymer, which can either halt the polymerization or significantly extend the response time [3].
According to Y. Zang and his colleagues the PI-COF has got a lot of interest and is widely used in the fields of catalysts, gas separation, and storage, despite the fact that it was still in its early development stages throughout the previous decade. As a consequence, the purpose of this research is to give a comprehensive understanding of PI-COFs' recent evolution. This work reviews the evolution of PI-COF from three different perspectives: regulated organization design, synthesis process, and use. To begin, utilizing network chemistry design guidelines, the topologies kind of PI-COF, as the volume and form of the created holes, are analyzed in the terms of distinct organic monomers [4].
A. Roy and his Collogues investigated about the noncovalent have indeed been produced via the condensation polymerization route. 13C NMR and FTIR investigations were used to study the production and molecular connectivities of these polymers. Prior to adsorption experiments, FESEM and HRTEM images were used to confirm the presence of holes in these polymers. The pyromellitic diimide containing benzimidazole COF has the largest larger specific surface area among some of the COFs presented here, 1 along with an also compatible with our numerical simulations? The binding affinity of naphthalene diimide as well as the diaminobenzidinecontaining COF with CO2 is higher than the other two COFs, at 127.87 cc g-1 at 195 K. BIBDZ, on the other hand, demonstrated a specific capacitance =88.4 F g-1 in 1M H3PO4 electrolytic solution at 0.5 A g-1 current density, and also outstanding specific capacitance preservation after 5000 voltammetric charge and discharge cycles [9].
Discussion
Although pPIs may be made without the use of solvents to prevent insolubility and incompatibility of the solvents. The fundamental challenge in the production of PI-COFs is building very crystal PIs with really regular and robust frames. In the end, it will be vital to employ the concept of framework biochemistry to control crystal formation, achieve architectural predictions, and manage the manufacture of material requirements. There are just a few distinct kinds of artificial crystal-like PI-COFs available right now, particularly at the singlecrystal equal.
Comparable microporous polyimide systems with homogeneous micropores was generated using naphthalene- 1,4,5,8-tetracarboxylic dianhydride in a polymerisation with tetra(4-aminophenyl)methane, 1,3,5-tris(4- aminophenyl)benzene or tris(4-aminophenyl)amine,. Chemical and physical properties were described using FTIR spectras, solid-state 13C MAS NMR spectra/ CP or chemical investigations. The monomers are crystalline and have a high chemical stability, with a maximal losing the weight of 550 degrees Celsius. The BET area of the three monomers ranges 291 to 721 m2 g -1, depends on stereo arrangement of the show's net nodes.
Two covalently natural polymers were produced by polymerizing pyromellitic dianhydride or naphthalene dianhydride using melamine. Electric eliminating chemicals (dianhydride and triazine) in the polymer developed might increase the storage ability of Na-ions substantially.Melamine's three amino acids, which are oriented in separate directions, may be used to create a three-dimensional extended connected network that has high durability or rate capability as an electrode in NIBs. After 100 starts charging cycles, the capacity of PI-1 could be preserved at 176.6 mAh g-1 at a current of 500 mA g-1. Even at a abundant higher current density of 5 A g-1, an irreversible capability of 88.8 mAh g-1 is achieved. The bigger linked naphthalene monomers in PI-1 form a more robust and conductive network, improving electrocatalytic activity.
Conclusion
One consistent feature that emerged from the research included in this review was the capability and opportunity to regulate the characteristics and functionality of the produced PIs. Wherever possible, they tried to emphasize elements of intentional design and demonstrate the impact of careful selection of raw material and their functionality, as well as synthesis conditions, on the applications we've selected. However, the wider area of porosity functional materials, including especially PIs, has a number of difficulties related to precise control of characteristics and function. The PIs may be made without the use of solvents to prevent insolubility and incompatible of the developing and cross-linked polymer chains with reaction solvents. We believe that as a group of substances, pPIs offer a number of intriguing methods to add to solutions to a wide range of global issues.
References
- Bogert MT, Renshaw RR. 4-amino-0phthalic acid and some of its derivatives. JACS 1908; 30:1135-44.
- Fan W, Zhang X, Zhang Y, et al. Lightweight, strong, and super-thermal insulating polyimide composite aerogels under high temperature. Compos Sci Technol 2019; 173:47-52.
- Hasegawa M. Development of solution-processable, optically transparent polyimides with ultra-low linear coefficients of thermal expansion. Polymers 2017; 9:520.
- Zhang Y, Huang Z, Ruan B, et al. Design and synthesis of polyimide covalent organic frameworks. Macromol Rapid Commun 2020; 41:2000402.
- Diercks CS, Yaghi OM. The atom, the molecule, and the covalent organic framework. Science 2017; 355:eaal1585.
- Meador MA, Alemán CR, Hanson K, et al. Polyimide aerogels with amide cross-links: a low cost alternative for mechanically strong polymer aerogels. ACS Appl Mater Interfaces 2015; 7:1240-1249.
- Li G, Wang Z. Naphthalene-based microporous polyimides: adsorption behavior of CO2 and toxic organic vapors and their separation from other gases. J Phys Chem 2013; 117:24428-37.
- Li Z, Zhou J, Xu R, et al. Synthesis of three dimensional extended conjugated polyimide and application as sodium-ion battery anode. Chem Eng J 2016; 287:516-22.
- Roy A, Mondal S, Halder A, et al. Benzimidazole linked arylimide based covalent organic framework as gas adsorbing and electrode materials for supercapacitor application. Eur Polym J 2017; 93:448-5.
- Shultz AM, Farha OK, Hupp JT, et al. Synthesis of catalytically active porous organic polymers from metalloporphyrin building blocks. Chem Sci 2011; 2:686-9.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Lakhwinder Singh1, Durgesh Wadhwa2 and Manpreet Sedha3*
1Department of Chemistry, Faculty of Science, SGT University, Gurugram, Haryana, India2SBAS, Sanskriti University, Mathura, Uttar Pradesh, India
3RIMT University, Mandi Gobindgarh, Punjab, India
Citation: Lakhwinder Singh, Durgesh Wadhwa, Manpreet Sedha, Review on Structure, Characteristics, and Applications of Cross-linked Porous Polyimides, J Res Med Dent Sci, 2022, 10 (5): 111-115.
Received: 04-Apr-2022, Manuscript No. JRMDS-22-54072; , Pre QC No. JRMDS-22-54072 (PQ); Editor assigned: 06-Apr-2022, Pre QC No. JRMDS-22-54072 (PQ); Reviewed: 20-Apr-2022, QC No. JRMDS-22-54072; Revised: 25-Apr-2022, Manuscript No. JRMDS-22-54072 (R); Published: 02-May-2022
