Research - (2020) Advances in Dental Surgery
Relationship between Serum and Salivary Estimation of Glucose and TNF-α in Patients with Type-II Diabetes
Vedavalli Subramanian, Muthukumar Santhanakrishnan*, Nizar Ahmed, M Ganesh and P Kennedy Kumar
*Correspondence: Muthukumar Santhanakrishnan, Department of Periodontology, Sri Ramachandra Institute of Higher Education and Research, India, Email:
Abstract
Background: The relationship between diabetes mellitus and chronic periodontitis has gained immense significance amongst researchers worldwide. Diabetes mellitus and periodontitis has an established bidirectional relationship. Evidence suggest that in people with type II diabetes, periodontitis is associated with higher blood glucose levels and it worsens the complications of diabetes mellitus. Materials and Methods: This study was carried out in 18 subjects from the Outpatient Department of Periodontology, Sri Ramachandra Institute of Higher Education and Research, Chennai. Study was approved by the Institutional Ethical Committee. The selected subjects included Type 2 diabetes mellitus (T2DM) patients with chronic periodontitis (CP). They were provided non-surgical periodontal therapy (NSPT), scaling. Blood and saliva samples were collected from the subjects on the day of NSPT, baseline and after one month for estimation of glucose and TNF-α levels. Results: After NSPT, scaling there was a significant decrease in salivary and serum glucose levels (P<0.05) and an increase in the salivary and serum TNF alpha levels, though not statistically significant. Conclusion: This study proved saliva to be a useful indicator for glucose estimation. It also indicated that NSPT, scaling relieved the periodontal inflammatory status in T2DM patients with CP and were reflected by an improvement in the glycemic control as well. Nevertheless, there was no reduction in TNF – alpha levels following treatment.
Keywords
Diabetes mellitus, Chronic periodontitis, Salivary glucose level, Serum glucose level, TNF–alpha
Introduction
Diabetes mellitus is a clinically and genetically associated metabolic disease characterized by abnormally high glucose in blood, patients will be known to have insulin resistance and B-cell dysfunction due to prolonged demand placed by insulin resistance. Periodontal disease is a chronic inflammatory condition characterized by destruction of the periodontal tissues, resulting in loss of connective tissue attachment, loss of alveolar bone and the formation of pathological pockets around the diseased teeth. Moreover, the periodontal signs and symptoms are now recognized as the "sixth complication" of diabetes. Salivary glucose is increased in T2DM. The effect of periodontal infections on diabetes mellitus is potentially explained by the resulting increase in levels of the systemic proinflammatory mediator, TNF-α which exacerbates insulin resistance. TNF-α causes decreased insulin-derived peripheral uptake of glucose by intensifying the serine phosphorylation of insulin receptor substrate 1 (IRS-1) which thereby converts IRS-1 and blocks the activity of insulin receptor tyrosine kinase [1]. Evidence suggests that non-surgical periodontal therapy may improve the glycemic control and relieve periodontal inflammatory conditions, in addition to reducing insulin antagonizing adipokines in diabetic patients with chronic periodontitis. The present study aimed at evaluating the salivary estimation of glucose as compared to serum estimation of glucose as salivary estimation of glucose is beneficial, because it is an easy, non-invasive, painless, aseptic method of sample collection which requires limited training, minimal armamentarium and lesser patient compliance. It overcomes the disadvantages of blood glucose estimation which involves the use of sharps, tendency of small bruise or mild soreness at the puncture site and patient fear to injections. This study also assessed the efficacy of non-surgical periodontal therapy, scaling in type II diabetes mellitus patients with chronic periodontitis in alleviating the inflammatory burden and insulin antagonizing adipokine, TNF- α in saliva and serum of the patients.
Materials and Methods
The study was conducted in the Outpatient Department of Periodontology, Sri Ramachandra Institute of Higher Education and Research, Chennai. Sample size calculation was done based on single mean paired t test with a power of 90% and α error of 5% and a total of 18 patients within the age range of 30-65 years with type II diabetes mellitus and chronic periodontitis were recruited for this study following the specific inclusion and exclusion criteria. The study was approved by the Institutional ethical committee (REF: CSP/19/SEP/80/302). The patients were detailed about the study protocol and signed an informed consent before a detailed case history was taken and sample collection was done.
Inclusion criteria
Subjects in the chosen age range diagnosed with chronic periodontitis according to the criteria for diagnosing chronic [2], clinical attachment level (CAL) of ≥2 mm, periodontal pocket depth (PPD) of ≥4 mm in more than 30% of sites.
Patients diagnosed with type II diabetes mellitus according to the ADA criteria for Type II diabetes mellitus [3], the diagnosis of diabetes was made if HbA1c ≥6.5% (48 mmol/mol) or fasting plasma glucose of ≥126 mg/dl; fasting is defined as no caloric intake for at least 8 hours or two hour plasma glucose of ≥200 mg/dl or a patient with classical symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose of ≥200 mg/dl.
Patients taking oral hypoglycemic drug (Metformin 500 mg).
Subjects without any other systemic diseases.
Patients who have not undergone any periodontal therapy for the past 6 months.
Exclusion criteria
Patients with known systemic disease other than type II diabetes and patients with diabetes related complications.
Patients with HbA1C more than 10%.
Patients on external insulin therapy.
Patients who use tobacco in any form, alcoholics.
Subjects who have taken antibiotics in the past 6 months.
Subjects who have taken analgesics in the past 1 week.
Pregnant and lactating women.
Mentally and physically challenged patients.
The patients were then provided non-surgical periodontal therapy, scaling. The periodontal parameters, i.e. oral hygiene index, probing pocket depth, clinical attachment level, bleeding sites, periodontal inflammed surface area (PISA index) were measured and saliva and serum samples were collected at baseline and one month follow up. Both serum and salivary glucose levels were estimated using the glucose oxidase-peroxidase (GOD-POD) method. Salivary and serum TNF – α levels were analysed by an ELISA kit.
Procedure
A total of 18 patients with chronic periodontitis in accordance with the Armitage et al 1999 criteria for diagnosing chronic periodontitis and Type II diabetes mellitus as per the ADA 2014 criteria were recruited for this study. The patients were requested to report one hour after breakfast for saliva and serum collection.
Periodontal examination
All the participants underwent a full-mouth clinical periodontal examination. The periodontal parameters were recorded at baseline and at one month. Full-mouth clinical measurements of the probing depth, clinical attachment level and bleeding on probing were made at the buccal, lingual, mesiobuccal, mesiolingual, distobuccal and distolingual surfaces of the teeth using a manual probe (PCP-UNC 15; Hu-Friedy Manufacturing, Chicago, IL, USA). Oral hygiene was assessed by means of the Oral hygiene index (OHI-S) by Greene and Vermilion. Periodontal inflammed surface area (PISA) scores were computed using the formulae given by Hujoel.
Collection of saliva samples (Figure 1a)-Saliva was collected by making the patients to rinse his/her mouth with distilled water and insisting them to open the mouth for 5 mins without swallowing and about 2 ml of saliva was collected in sterile eppendorf tubes.
Figure 1a: Collection of saliva samples.
Collection of blood samples (Figure 1b)-4 ml blood was collected by venepuncture in a sterile hemo repellent coated vacutainer employing strict aseptic measures. Blood samples were immediately centrifuged, and the serum was separated for glucose estimation instantly. A part of the serum sample after centrifugation was stored in sterile eppendorf tubes for the assessment of TNF-alpha.
Figure 1b: Collection of blood samples from the antecubital vein, followed by immediate centrifugation and serum separation from the blood sample.
Estimation of glucose levels (Figure 2) - Both serum and salivary glucose levels were estimated using the glucose oxidase-peroxidase (GODFigure POD) method and the values were attained using a semi autoanalyzer.
Figure 2: Estimation of glucose levels using glucose reagents and semi autoanalyzer.
(GOD catalyses the glucose oxidation into gluconic acid and hydrogen peroxide
Hydrogen peroxide+Phenol+4 aminoantipyrine=Quinoneimine
Quinoneimine forms a red colour complex which is read in a colorimeter and the value is obtained) [4].
Estimation of TNF-α levels (Figure 3)-Salivary and serum TNF – α levels were estimated using an ELISA kit.
Figure 3: Estimation of TNF – α using ELISA kit.
Statistical analysis
Results were interpreted using single mean paired t test and data were analysed using the SPSS software package (version 12.0) with the level of significance set at 95% confidence interval.
Results
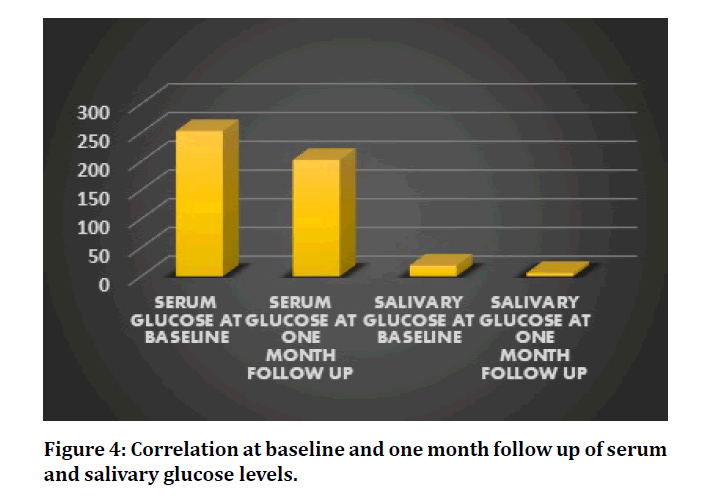
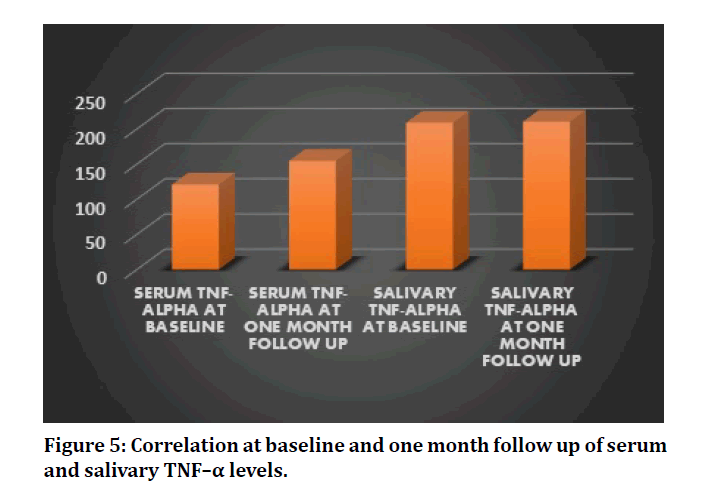
In this study, the glycemic levels of the patients have been ameliorated and has presented a statistically significant reduction in the serum and salivary glucose levels after non-surgical periodontal therapy, scaling at one month follow up (P=0.000 and 0.002 respectively) (Table 1 and Figure 4). Saliva has proven to be a good indicator for measuring glucose levels in diabetes patients. The proinflammatory adipokine, salivary TNF–α and serum TNF-α has raised, post scaling although its value is not statistically significant. (P=0.979 and 0.403 respectively) (Table 2, Figure 5). In addition, among the 18 patients, 14 of these patients revealed substantial reduction in the levels of TNF–α from baseline, post scaling.
Figure 4: Correlation at baseline and one month follow up of serum and salivary glucose levels.
Figure 5: Correlation at baseline and one month follow up of serum and salivary TNF–α levels.
| Parameter | Mean ± std | P Value |
|---|---|---|
| Serum glucose pre-operative | 253.11 ± 42.36 | 0 |
| Serum glucose post-operative | 202.83 ± 48.78 | 0 |
| Salivary glucose pre-operative | 19 ± 16.81 | 0.002 |
| Salivary glucose post-operative | 6 ± 4.52 | 0.002 |
Table 1: Comparison of serum and salivary glucose levels at baseline and follow up.
| Parameter | Mean ± std | P Value |
|---|---|---|
| Serum TNF-α pre-operative | 120.878 ± 15.30 | 0.403 |
| Serum TNF-α post-operative | 154.530 ± 166.53 | 0.403 |
| Salivary TNF-α pre-operative | 208 ± 129.73 | 0.979 |
| Salivary TNF-α post-operative | 210 ± 168.76 | 0.979 |
Table 2: Comparison of serum and salivary TNF- α levels at baseline and follow up.
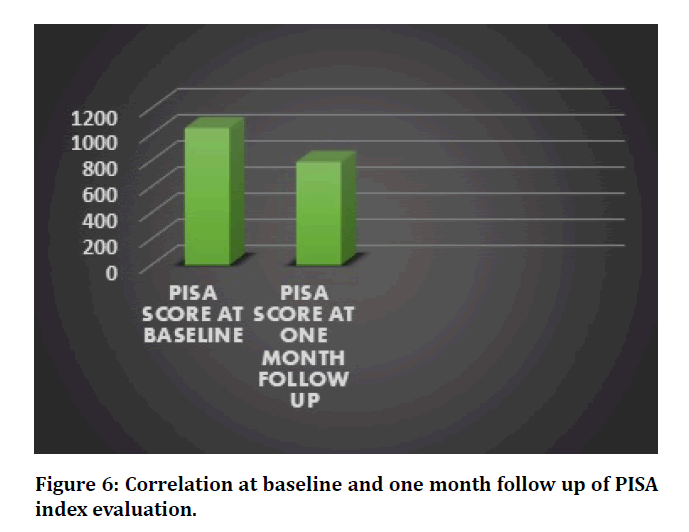
The PISA scores indicated a highly statistically significant reduction in the inflammatory burden of patients with type II diabetes, at one month follow up (P=0.00) (Table 3, Figure 6). Periodontal parameters such as oral hygiene index, probing pocket depth, clinical attachment level and bleeding sites also showed reduction in their values post treatment.
Figure 6: Correlation at baseline and one month follow up of PISA index evaluation.
| Parameter | Mean ± std | P Value |
|---|---|---|
| Pisa pre-operative | 1048 ± 205.50 | 0 |
| Pisa post-operative | 788.88 ± 190.84 | 0 |
Table 3: Comparison between PISA scores at baseline and follow up.
Discussion
Periodontal disease constitutes a chronic bacterial challenge intricating diabetes mellitus [5]. The presence of dental plaque and calculus deposits in the periodontal tissues attribute to periodontitis [6]. In people with Type II diabetes, periodontitis is associated with higher HbA1c levels and exacerbates diabetes complications. The glycemic control of diabetes patients has shown an improvement as a result of treatment of periodontitis, in the short term [7]. In this regard, we carried out this study to ascertain the outcome of non-surgical periodontal therapy, scaling on the levels of pro inflammatory adipokine, TNF- α and glycemic levels in patients with T2DM and CP and the amelioration of glycemic control in diabetic patients, which could be reflected as an absolute evidence of its causal nature. The necessity of a non-invasive and consistent method in detection of diabetes which would pave way for the ease in patients, to keep a track of their glycemic levels instilled us to appraise the efficiency of saliva as an effectual method in detecting type II diabetes mellitus. Saliva being a safe, non-invasive, inexpensive, non-technique sensitive method for collection if evinced to be effective, is a boon to patients who are physically challenged, severely immuno compromised and to those who face a phobia to injections. This study exemplified saliva to be a good indicator for measuring glucose levels in diabetes patients. However, due to the lack of standard salivary glucose levels in literature for estimating the glycemic levels in diabetes patients, the accuracy of using saliva as a noninvasive diagnostic tool remains questionable.
Nonetheless, saliva also possesses certain drawbacks such as contamination by food and drinking substances, increase in breakdown of starch to glucose in physically stressed patients and difficulty in collection in patients with xerostomia. Nevertheless, in the prospect, saliva is envisaged to be an efficacious cost-effective screening tool for detecting diabetes mellitus in a large population with benefits of being noninvasive and convenient for the subjects, with no requirements of specific devices or instruments and expertise training, saving a lot of chair side time.
The results of this study are in accordance with Yamazaki et al. [8] who reported the changes in TNF-alpha levels after periodontal treatment at 2 months follow up to be insignificant. The present study is compatible with the meta analyses by Quan Li et al. [9] which in conclusion supported the effectiveness of non-surgical periodontal therapy in the enhancement of glycemic control in patients with CP and T2 DM and with the meta analyses by Lei Chen at al. [10] which specified non-surgical periodontal treatment in diabetes patients has an evidence of somewhat improved glycemic levels and periodontal inflammation control. On the other hand, the results of the present study is in contrast with Shunqin Wang et al. [11] who stated that PT relieved the periodontal inflammatory status, causing reductions in insulin-antagonizing adipokines, that were reflected by an improvement in the glycemic control in T2DM patients with CP. Taking this into consideration, there was relief in the periodontal inflammatory burden and improvement in the glycemic levels but there was no statistically significant reduction in the insulin antagonising adipokine, TNF – alpha. A probable reason could be that type II diabetes mellitus patients with periodontitis are systemically stressed by the plaque containing bacteria and the increase in circulating inflammatory mediators [12]. Furthermore, the reduction in the salivary glucose levels apart from the serum glucose levels in the present study has manifested the efficacy of saliva as a reliable indicator for detecting diabetes mellitus which is in agreement with a study by Agrawal RP et al. [13] which concluded that an useful index of diabetes mellitus is monitoring of the salivary glucose level. In this cross-sectional study, the periodontal inflammed surface area (PISA) index was also evaluated as it is considered to be an effective measure which quantifies the amount of inflamed periodontal tissue, thereby enumerating the inflammatory burden posed by periodontitis [14] and there is a corroborated dose–response relationship between glycemic levels and PISA in type 2 diabetes patients [15]. This study also showed non-surgical periodontal therapy to bring a significant reduction from the baseline PISA score to one-month post treatment in type II diabetes patients. Clinical measures including OHI, PPD, CAL and bleeding sites also showed substantial reduction in their values post treatment emphasising the importance of non-surgical periodontal therapy in diabetes mellitus patients with chronic periodontitis.
To the best of our knowledge this is the first study which has compared the glycemic levels, the systemic insulin antagonising adipokine, TNF- α in both saliva and serum of type II diabetes patients and the periodontal inflammatory burden to bring about a comprehensive evaluation of non-surgical periodontal therapy, in diabetes mellitus patients with chronic periodontitis.
The results of the present study clearly demonstrated the value of saliva as a propitious diagnostic tool in the detection of diabetes mellitus. However, RCTs with large sample size and long term follow up should be conducted to ascertain the diagnostic validity of salivary glucose level in the early diagnosis of diabetes mellitus.
Conclusion
Saliva has manifested to be an effective noninvasive diagnostic tool in detection of diabetes mellitus.
Non-surgical periodontal therapy, scaling has proved to improve the glycemic levels in serum and saliva of diabetes mellitus patients with chronic periodontitis.
Conflict of Interest
The authors would like to declare that there were no conflicts of interest.
Acknowledgement
The authors would like to thank the patients who have participated in this study.
References
- Plomgaard P, Bouzakri K, Krogh-Madsen R, et al. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 2005; 54:2939-2945.
- Armitage GC. Development of a classification system for periodontal diseases and conditions. Annals Periodontol 1999; 4:1–6.
- American diabetes association standards of medical care in diabetes 2014. Diabetes Care 2014; 37:14-80.
- Ragunathan H, Aswath N, Sarumathi T. Salivary glucose estimation: A non-invasive method. Indian J Dent Sci 2019; 11:25-27
- Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: A two-way relationship. Annals Periodontol 1998; 3:51-61.
- Christgau M, Palitzsch KD, Schmalz G, et al. Healing response to non-surgical periodontal therapy in patients with diabetes mellitus: clinical, microbiological, and immunologic results. J Clin Periodontol 1998; 25:112-124.
- Preshow PM, Bisett SM. Periodontitis and diabetes. Br Dent J 2019.
- Yamazaki K, Honda T, Oda T, et al. Effect of periodontal treatment on the C-reactive protein and proinflammatory cytokine levels in Japanese periodontitis patients. J Periodontal Res 2005; 40:53–58.
- Quan Li, Sha Hao, Jie Fang, et al. Effect of non-surgical periodontaltreatment on glycemic control of patients with diabetes: A meta-analysis of randomised controlled trials. Trials 2015; 16:291-299.
- Lei Chen, Gang Luo, Dongying Xuan, et al. Effects of non-surgical periodontal treatment on clinical response, serum Inflammatory parameters, and metabolic control in patients with type 2 diabetes: A randomized study. J Periodontal 2012; 83:435–443.
- Wang S, Liu J, Zhang J, et al. Glycemic control and adipokines after periodontal therapy in patients with Type 2 diabetes and chronic periodontitis. Braz. Oral Res 2017; 31:90.
- Jennifer Talbert, John Elter, Heather L Jared, et al. The effect of periodontal therapy on tnf-α, IL-6 and metabolic control in type 2 diabetics. J Dent Hygiene 2006; 80:2.
- Agrawal RP, Sharma N, Rathore MS, et al. Non-invasive method for glucose level estimation by saliva. J Diabetes Metabol 2013; 4:266-271.
- Nesse W, Abbas F, van der Ploeg I, et al. Periodontal inflamed surface area: Quantifying in&flammatory burden. J Clin Periodontol 2008; 35:668–673.
- Nesse W, Linde A, Abbas F, et al. Dose–response relationship between periodontal inflamed surface area and HbA1c in type 2 diabetics. J Clin Periodontol 2009; 36:295–300.
Author Info
Vedavalli Subramanian, Muthukumar Santhanakrishnan*, Nizar Ahmed, M Ganesh and P Kennedy Kumar
1Department of Periodontology, Sri Ramachandra Institute of Higher Education and Research, India2Department of Biochemistry, Sri Ramachandra Institute of Higher Education and Research, India
3Department of Microbiology, Sri Ramachandra Institute of Higher Education and Research, India
Citation: Vedavalli Subramanian, Muthukumar Santhanakrishnan, Nizar Ahmed, M Ganesh, P Kennedy Kumar, Relationship between Serum and Salivary Estimation of Glucose and TNF-? in Patients with Type-II Diabetes–A Correlative Study, J Res Med Dent Sci, 2020, 8 (7): 344-349.
Received: 20-Oct-2020 Accepted: 05-Nov-2020 Published: 12-Nov-2020