Research - (2021) Volume 9, Issue 5
Prevalence of Helicobacter Pylori in Alcoholic and Non-Alcoholic Gastritis Patients
*Correspondence: Chitraleka Saikumar, Department of Microbiology, Sree Balaji Medical College and Hospital Affiliated to Bharath Institute of Higher Education and Research, India, Email:
Abstract
The prevalence of Hylicobactor pylori was investigated among the patients admitted to Department of Microbiology, Sree Balaji Medical College and Hospital, Chennai. The majority of cases out of a study population of 109 patients were in the age group of 30-39 years. Abdominal pain was the predominant symptom in the acid peptic disease. Antral gastritis was the commonest endoscopic finding observed during the course of the study. Rapid Urease Test was positive in 82.6% of the samples, where 78.9% of the cases were positive within the first 20 minutes. Rapid Urease Test and Giemsa stain results where 1n harmony with each other. RUT showed about 79% sensitivity and 88% specificity. Culturefor H. pylori was positive only 1n 23.9 % of the cases. H. pylori infection was comparatively more 1n nonalcoholic group. Many males among the study population, being alcoholics, were predisposed to H. pylori infection. This contradictory statement could be contributed to inclusion of females in the study and all of them happen to be non-alcoholics.
Keywords
Hylicobactor pylori, Gastritis, Alcohol consumption, Enteric cancer, Urease test
Introduction
Gastritis is an inflammation of the gastric mucosa. Many different illnesses and irritants acting either alone or in combination can trigger the inflammation of gastritis. In addition to causing gastritis, Helicobacter pylori infections have been linked to the development peptic ulcer disease, open sores inside the stomach or part of the small intestine. However, many people have H. pylori in their stomach and have no symptoms. Chemical and environmental irritants can damage the stomach lining and cause gastritis. Common irritants include alcohol; cigarette smoke; aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs). Excessive consumption of alcohol is one of the most common causes of gastritis. This is because alcohol is a toxin that irritates the mucous lining of the stomach, so that it becomes inflamed. This inflammation is more likely to occur if the individual regularly consumes alcohol. When people frequently drink alcohol, the stomach does not get the chance to recover from the irritation to the lining. Ethyl alcohol can directly damage the mucosa of the alimentary tract. Continuous consumption of alcohol predisposes to atrophic inflammation of the gastric mucosa, and the appearance of this type of inflammatory changes is related to the duration of addiction. It is now well accepted that the most common stomach disease, peptic ulcer disease, is an infectious disease, and all consensus conferences agree that the causative agent, H. pylori, must be treated with antibiotics [1]. It 1s now established that H. pylori infection induces several gastrointestinal complications, ranging from mild gastritis to peptic ulcers [2] and even gastric malignancies, such that the International Agency for Research on Cancer (IARC) has declared this pathogen as an independent carcinogen. In addition, the etiologic association of this infection with an increasing number of disorders including cardiovascular diseases [3], and metabolic syndrome [4] are being investigated. Therefore, it is of utmost essence to detect the infection and pursue with eradication therapy and follow up. In the general population. The prevalence of H. pylori. In alcoholic and non-alcoholic gastritis patients has been elicited. In the present study three parameters namely RUT, Culture and sensitivity and histopathological studies were used for detection of H. pylori in the gastric biopsy samples. The present study aimed to study the prevalence of H. pylori in alcoholic and non-alcoholic gastritis patients.
Materials and Methods
The study was conducted with the approval from the institutional Ethical Committee, SBMCH, and Chennai-44. Permission to conduct the study was sought from the respective hospital authorities. This is a prospective cross-sectional study conducted over a period of eighteen months from May 2012 - October 2013. Outpatients and inpatients, of both sexes in the age group 20 - 70 years, based on the following criteria were included in the study.
Inclusion criteria
✓ Patients with complaints suggestive of upper gastrointestinal diseases in Alcoholic and Non-Alcoholic Gastritis.
✓ Patients with antral gastritis, duodenitis, gastric ulcer and duodenal ulcer.
✓ Patients who were not on antibiotics, proton pump inhibitor or Helicobacter eradication therapy within 1 month prior to inclusion in this study.
Exclusion criteria
✓ Patients with previous gastric surgery.
✓ Patients with active bleeding.
✓ Gastric carcinoma.
The details of complete history, clinical features of the patients to be subjected to endoscopy were obtained. Preinvasive procedure preparation for Oesophago-gastroduodenoscopy was performed as per norms. Biopsy tissue was collected from the gastric antrum of the patient and the specimens were submitted to RUT, Gram stain, giemsa stain, culture and sensitivity and Histopathological study. A patient with H.pylori infection was defined as those patients who were positive for at least two out of the evaluation tests [5,6]. The biopsy Samples were derived from the study patients and the following tests were done. Rapid Urease Test [7-9] the culture test 80, confirmatory test [10]. Crush cytology [11] were done (Figures 1A-Figure 1J).
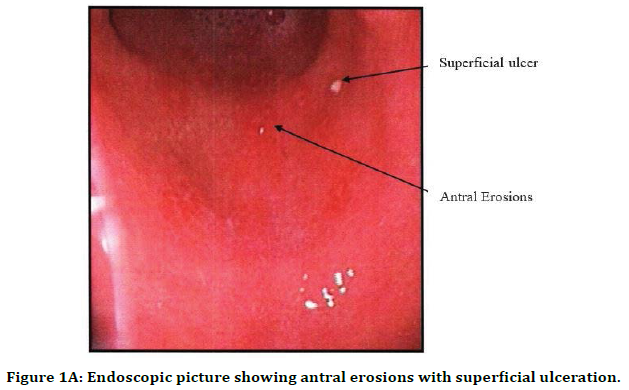
Figure 1A. Endoscopic picture showing antral erosions with superficial ulceration.
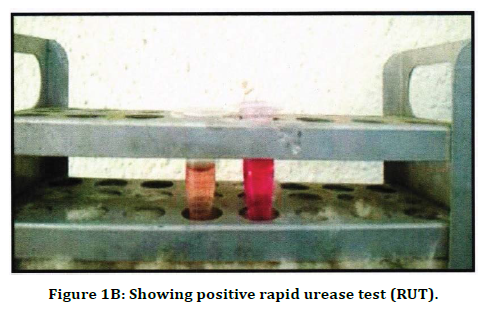
Figure 1B. Showing positive rapid urease test (RUT).
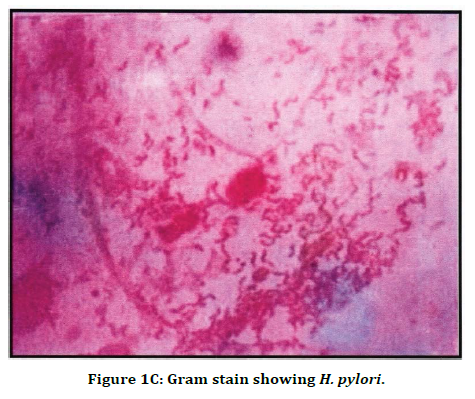
Figure 1C. Gram stain showing H. pylori.
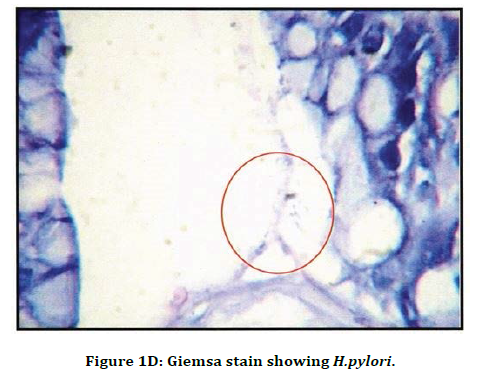
Figure 1D. Giemsa stain showing H.pylori.
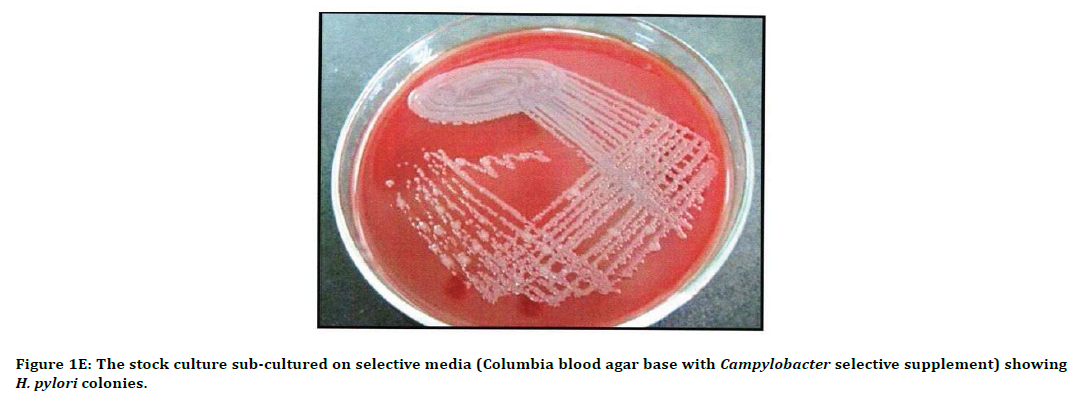
Figure 1E. The stock culture sub-cultured on selective media (Columbia blood agar base with Campylobacter selective supplement) showing H. pylori colonies.
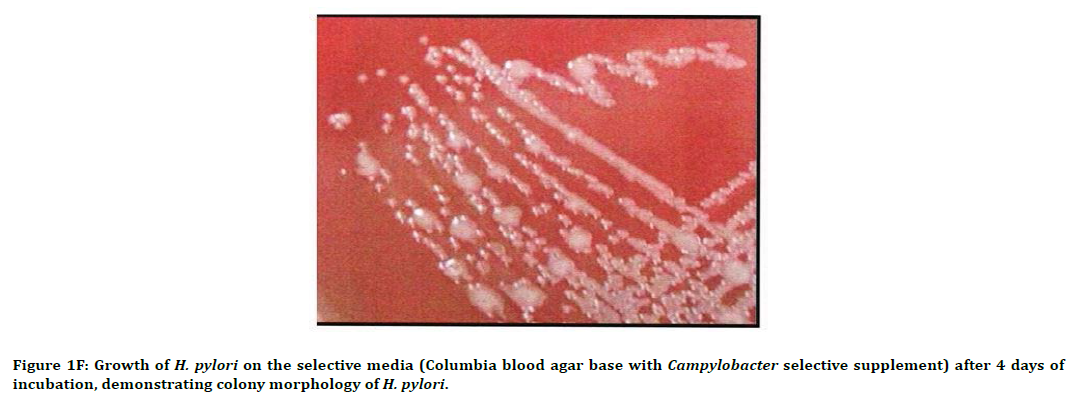
Figure 1F. Growth of H. pylori on the selective media (Columbia blood agar base with Campylobacter selective supplement) after 4 days of incubation, demonstrating colony morphology of H. pylori.
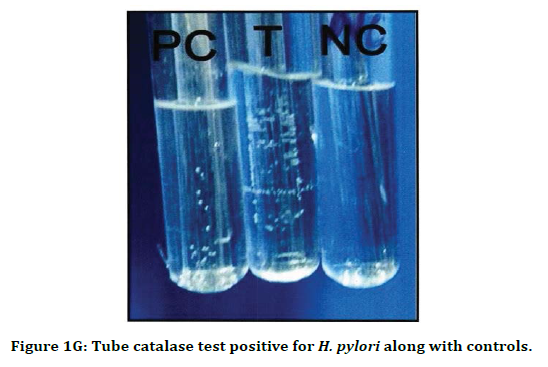
Figure 1G. Tube catalase test positive for H. pylori along with controls.
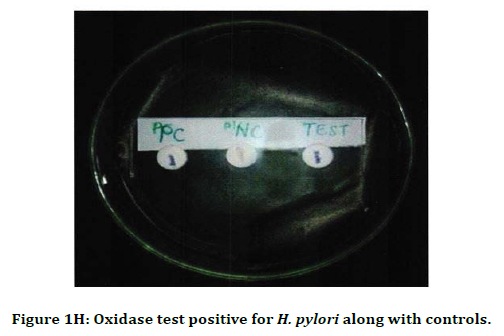
Figure 1H. Oxidase test positive for H. pylori along with controls.
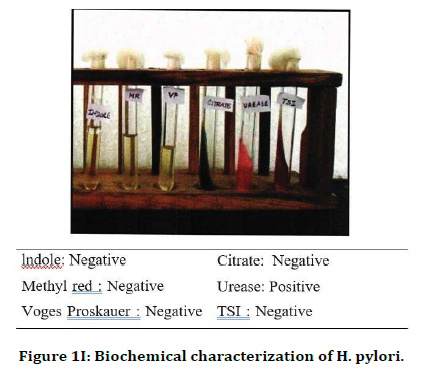
Figure 1I. Biochemical characterization of H. pylori.
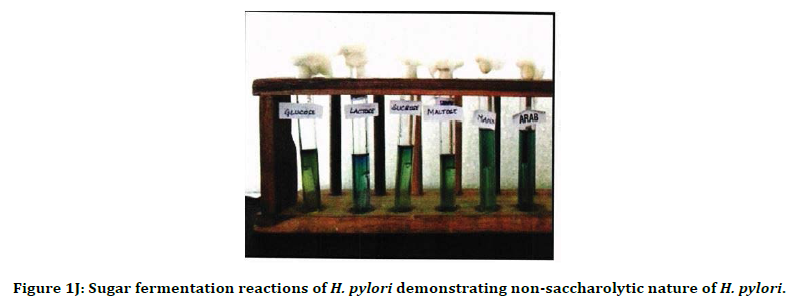
Figure 1J. Sugar fermentation reactions of H. pylori demonstrating non-saccharolytic nature of H. pylori.
Results
A total of 109 endoscopic biopsy samples were collected from alcoholic and non-alcoholic patients to detect the presence of H. pylori using conventional methods. The sample collected was processed immediately without any delay. RUT was done immediately in the endoscopic suite and the positive colour change was documented. Positive cases were further processed for staining, histopathological studies, culture, and sensitivity. Out of 109 cases 70 (Table 1) were male (64.2%) and 39 were female (35.8%). Abdominal pain was the predominant symptom (72.5%) the Acid peptic disease (Table 2). The results showed that 56(51.4%) were nonalcoholic gastritis patients and 53(48.6%) were alcoholic gastritis patients (Figure 2).
| Age | Male | Female | Total |
|---|---|---|---|
| 20-29 | 16 | 7 | 23 (21.1%) |
| 30-39 | 15 | 9 | 24 (22.0%) |
| 40-49 | 17 | 6 | 23 (21.1%) |
| 50-59 | 15 | 7 | 22 (20.2%) |
| >60 | 7 | 10 | 17 (15.6%) |
| Total | 70 | 39 | 109 (100%) |
Table 1: Distribution of gender in the study population (n=l09).
| Symptoms | Male | Female | Total |
|---|---|---|---|
| Abdominal pain | 60 | 19 | 79 (72.5%) |
| Dyspepsia | 14 | 10 | 24 (22%) |
| Vomiting | 5 | 1 | 6 (5.5%) |
| Total | 79 | 30 | 109 (100%) |
Table 2: Symptoms and sex distribution in relation to clinical diagnosis.
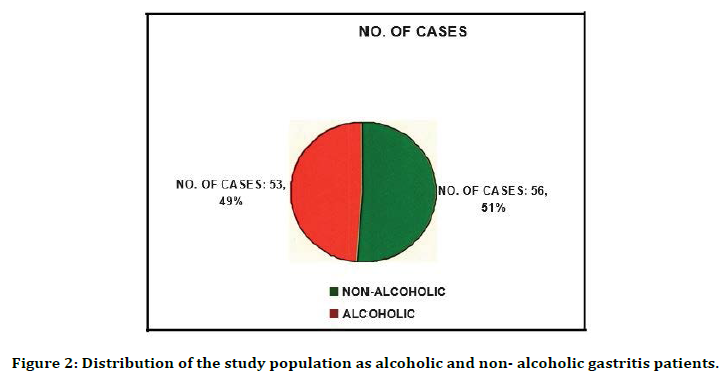
Figure 2. Distribution of the study population as alcoholic and non- alcoholic gastritis patients.
Table 3 and Figure 3 shows that 64 patients had Antral gastritis (58.7%), 33 patients had Duodenitis (30.3%), 10 patients had Duodenal ulcer (9.2%) , 2 patients had Gastric ulcer (1.8%).
| Endoscopic diagnosis | Total | Rapid urease test positive |
|---|---|---|
| Antral gastritis | 64 | 50 |
| Duodenitis | 33 | 29 |
| Duodenal ulcer | 10 | 10 |
| Gastric ulcer | 2 | 1 |
| Total | 109 | 90 |
Table 3: Rapid Urease positivity vs. Endoscopic diagnosis.
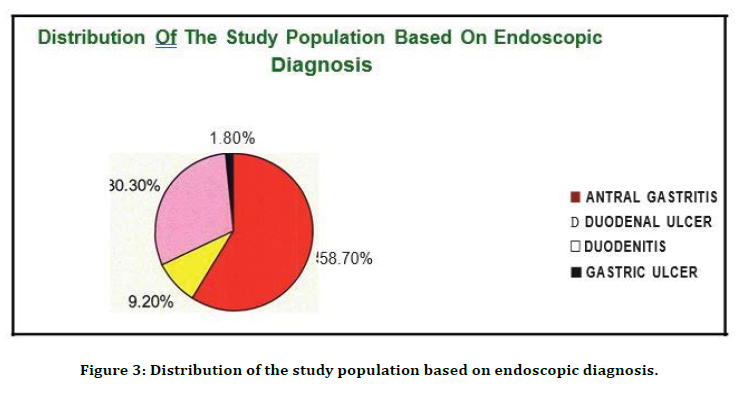
Figure 3. Distribution of the study population based on endoscopic diagnosis.
82.6 % of the cases (n=109) were positive by Rapid urease test. 41.1% of the alcoholic gastritis cases and 58. 9% of the non-alcoholic gastritis cases were positive by rapid urease test (Table 4 and Figure 4). Maximum positive colour change was detected (Table. 5 and Figure. 5) first 5 minutes (42.2%), Giemsa staining was positive in 82. 6% of the cases.
| Total | Rapid urease test positive | Percentage | |
|---|---|---|---|
| Alcoholic gastritis | 53 | 37 | 41.10% |
| Non-alcoholic gastritis | 56 | 53 | 58.90% |
| Total | 109 | 90 | 100 |
Table 4: Rapid urease test positivity in alcoholic and nonalcoholic gastritis.
| Giemsa stain | Rapid urease test positive | Rapid urease test negative | Percentage |
|---|---|---|---|
| Positive | 76 | 14 | 82.60% |
| Negative | 14 | 5 | 17.40% |
| Total | 90 | 19 | 100% |
Table 5: Positivity of Helicobacter pylori by Giemsa stain.
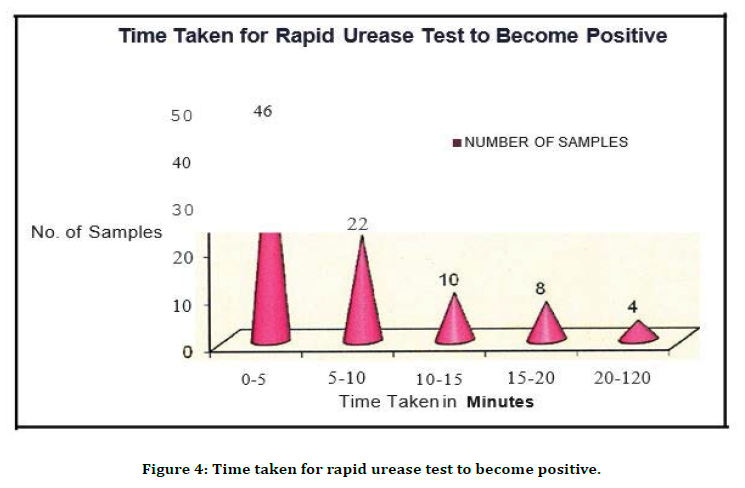
Figure 4. Time taken for rapid urease test to become positive.
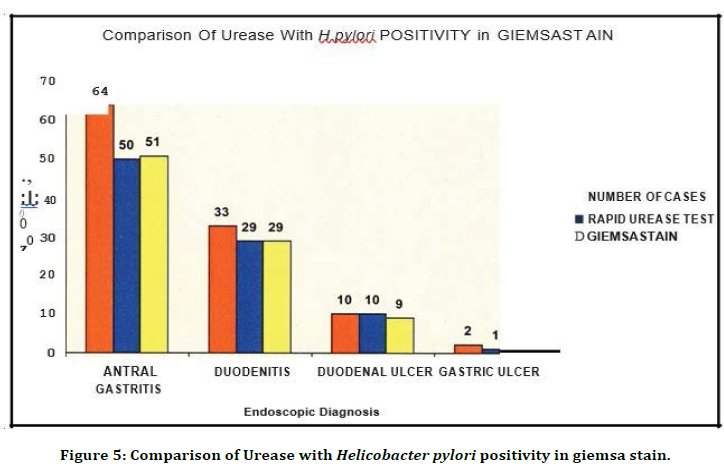
Figure 5. Comparison of Urease with Helicobacter pylori positivity in giemsa stain.
Out of 64 gastritis cases, RUT was positive 50 cases and giemsa stain was positive in 51 cases. Out of 33 duodenitis cases, RUT was positive m 29 cases and giemsa stain was positive in 29 cases. Out of 10 duodenal ulcer cases, RUT was positive m 10 cases and giemsa stain was positive in 9 cases. Out of 2 gastric ulcer cases, RUT was positive in 1 case and giemsa stain was positive in 1 case .This show Rapid Urease and Giemsa gave comparable results (Figure. 6). Of the 109 cases, 23.9% of the cases were positive by culture.
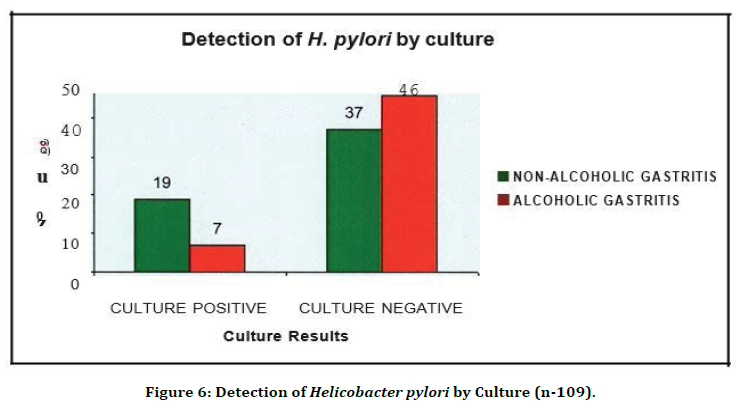
Figure 6. Detection of Helicobacter pylori by Culture (n-109).
Discussion
H. pylori is responsible for one of the world's most common bacterial infections. The significant role of H. pylori in the etiology of gastric disease is now undisputed. Many factors like low socioeconomic conditions such as overcrowding, poor sanitation, close contact with infected persons, food habits, environmental factors, smoking, and alcohol consumption appear to be associated with colonization and infection of H. pylori in humans.
The prevalence of H. pylori also vanes according to age, gender, geographical location (urban/ rural) and educational level [12]. The present cross-sectional study was conducted to know the prevalence of H. pylori in Alcoholic and nonalcoholic gastritis patients in our population of SBMCH, Chennai.
This study is based on using conventional methods for detecting H. pylori infection. Biopsy based tests namely RUT, histopathological examination, bacterial culture and sensitivity were used. The conclusion from the study gives correlation of the conventional methods with endoscopic diagnosis and the association of risk factors with H. pylori positivity. This study included a total of 109 patients (presenting abdominal pain as the predominant symptom) in alcoholic and non-alcoholic gastritis patients. Among this 70 (64.2%) were males and 39 (35.8%) were females (Table 1). The maximum number of patients in the study was in the age group 30-39 (22.0%). In the study conducted by Nair et al [13] out of 13 6 patients, 116 were Male and 20 where female is comparable to the present study which also showed males were more affected than females.
Among the patients with peptic ulcer disease the predominant symptom was abdominal pain in 72.5%, dyspepsia 1n 22.5% and vomiting 5.5%, which showed abdominal pain was the predominant symptom among patient with acid peptic disease. Distribution of the study population as alcoholic and non-alcoholic Gastritis patients revealed that 56 (51.4%) were non-alcoholic gastritis patients and 53 (48.6%) were alcoholic gastritis patients. Distribution of the study population based on endoscopic diagnosis revealed that Antral Gastritis accounted for 64 (58.7%), Duodenitis in 33 (30.3%), Dueodenal Ulcer in 10 (9.2%) and Gastric Ulcer in 2 cases (1.8%). All the 109 samples were subjected to RUT, Gram staining, Giemsa staining, Histopathological study, Culture and Sensitivity. Out of 109 samples studied by RUT, 90 (82.6%) were positive (Table 3). Of the cases, 46 (42 .2 %) were positive in first 5 minutes, 22 (20 .2 %) were positive in 10 minutes, 10 (9.2%) were positive in 15 minutes, 8 (7.3%) were positive in 20 minutes and 4 (3.7%) in 2 hours. In the present study 78.9% of the cases were positive within 20 minutes. This correlates well with Marshall et al. [14] using a CLO test reported that 75% of the positive test is detected within 20 minutes, 92% at 3 hours and 98% at 24 hours.
The positivity of Rapid Urease was observed in alcoholic and non-alcoholic gastritis patients. In the total samples 53 (48.6%) were alcoholic and 56 (51.4%) were non-alcoholic. In the present study, in the non-alcoholic group out of 56 cases, 39 were female and 17 were male. In the alcoholic group, 53 were male and there were no female patients in this group. Out of these, 37 (41.1%) of the alcoholic gastritis cases and 53 (58. 9%) of the non-alcoholic gastritis cases were positive by RUT (Table 4). This shows H. pylori positivity is less in alcoholic group. This is comparable with [15-17]. These studies also showed H. pylori infection was lesser in alcoholic group. In considering the limitations in the present study like alcoholic group was little less 53 (48.6%) than non-alcoholic group 56 (51.4%) and there were no female patients in the alcoholic group, which lead to difficulty in comparison.
After adjustment of gender, age and significant RUT positivity (<5 ruins) and Giemsa stain positivity, 9 males in alcoholic group showed H. pylori positivity and 7 males in non-alcoholic group showed H. pylori positivity. This is correlated with Zhang et al. [18]. 2010 which showed H. pylori infection was more 1n alcoholic group than non-alcoholic group. This relates that alcohol consumption has association with H. pylori infection and facilitates its colonization and infectivity.
Association of H. pylori with gastrointestinal diseases 1s undisputed. Risk factors association with H. pylori infection is not conclusive and there are studies under progress in relation to this. In our present study, alcohol consumption showed less significant association with H. pylori infection. The direct Gram-stained smears in our study showed positivity of 60% which is comparable with Anjana et al. [19] reported 72.3% other studies reported value ranging between 44- 74%.
In the present study Giemsa staining of the crushed smear was positive in 82.6% of the smear studied. RUT and Giemsa staining gave comparable results. The positive cases were subjected to Haemotoxylin and Eosin staining by our Pathologist. Total of 67 cases were positive by H/E. This is correlated with studies by Aarti et al. [20] and Madan et al. [21] which revealed Giemsa technique showed greater positivity than H/E staining. Culture positivity in the present study was 23.9% Arora et al. [22] reported cultural positivity of 28%. The low isolation rate may be due to the distribution of H. pylori. 1n gastric mucosa, its fastidious nature, administration of antibiotics (for other infection) and PPI (39) [23]. Anti-microbial susceptibility was performed by disc diffusion method and the isolates were susceptible to all the antimicrobials tested (amoxicillin, clarithromycin, metronidazole, ciprofloxacin, erythromycin and tetracycline) in the present study.
Conclusion
A preponderance of H. pylori infection was noted in patients with antral gastritis. H. pylori infection was more in non-alcoholic group than in alcoholic group. Alcohol consumption showed less significant association with H. pylori infection. The simple and inexpensive Rapid Urease Test and Giemsa stain detected the maximum number of positive cases. Culture for H. pylori was low, revealing that isolation of H. pylori by culture is possible in reference laboratories.
In general, H. pylori have become the commonest infection associated with gastrointestinal diseases. This may be due to the advanced, accurate and more rapid diagnostic facilities available now. The most simple, rapid, and inexpensive tests like RUT and Giemsa staining can be considered along with the endoscopic clinical findings to diagnose H. pylori infection at an earlier stage. H. pylori eradication therapy can be started and followed up to prevent further complications and development of gastric carcinoma.
Funding
No funding sources.
Ethical Approval
The study was approved by the Institutional Ethics Committee.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
The encouragement and support from Bharath Institute of Higher Education and Research, Chennai India is gratefully acknowledged for providing the laboratory facilities to carry out the research work.
References
- Warren JR, Marshall BJ. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983; 1273-1275.
- Nomura A, Stemmerman GN, Chyou P, et al. H. pylori infection and the risk for duodenal and gastric ulceration. Ann Intern Med 1994; 120; 977-981.
- Franceschi F, Leo D, Fini A. Helicobacter pylori infection and ischemic heart disease: An overview of the general literature. Dig Liv Dis 2005; 37:301-308.
- Gunji T, Matsuhashi N, Sato H, et al. Helicobacter pylori infection is significantly associated with metabolic syndrome in the Japanese population. Am J Gastroenterol 2008; 103:3005-30010.
- Montgomery EA, Martin DF, Peura DA, Rapid diagnosis of Campylobacter pylori by Grams' s stain. Am J Clin Path 1988; 90:606-09.
- Sengupta S, Saraswathi K, Varaiya A et al. Helicobacter pylori in duodenal ulcer disease and its eradication. Indian J Med Micro biol 2002; 20:163-64.
- Vinci S Jones, Kate V, Ananthakrishnan et al. Standardization of urease test for detection of H. pylori. Indian J Med Microbiol 1997; 15:181-183.
- Marshall BJ, McGechie DB, Rogers PA ,et al. Rapid urease test in the management of Campylobacter; pyloridis-associated gastritis. Am J Gasterenterol 1987; 82:200-210.
- Raul V Destura, Eternity D, Leahj et al. Laboratory diagnosis and susceptibility profile of H. pylori in the Philiphines. Annals Clin Microbiol Antimicrob 2003; 1-6.
- Pliccolomini R, Di Bonaventura, Festi D, et al. optimal combination of media for primary isolation of H. pylori from gastric biopsy specimens. J Clin Micro biol 1997; 35:1541-1544.
- http://investigations.debtwire.com/cgi-bin/file.php?article=koneman_color_atlas_and_textbook_of_diagnostic_microbiology_6th_edition_pdf&code=d409e07cd8b9b221d36d7211fb75aff8
- Graham DY, Malaty HM, Evans D, et al. Epidemology of H. pylori in an asymptomatic population in the United States. Effect of age, race, socio economic status. Gastroenterology 1991; 100:1495-501
- Nair D, Uppal B, Kar P, et al. Immune response to H. pylori in gastroduodenal disorders. Indian J Med Microbiol 1997; 15:33-35.
- Marshall BJ, McGechie DB, Rogers PA, et al. Rapid urease test in the management of Campylobacter pyloridis-associated gastritis. Am J Gasterenterol 1987; 82:200-210.
- Brenner H, Bode G, Adler G, et al. Alcohol as a gastric disinfectant? The complex relationship between alcohol consumption and current Helicobacter pylori infection. Epidemiology 2001; 12:209-214.
- Gao, L., Melanie N, Chirista, et al. Alcohol consumption and chronic atrophic gastritis: Population-based study among 9,444 older adults from Germany. J Gastroenterol Hepatol 2001; 15:271-276.
- Murray LJ, Lane AJ, Harvay IM, et al. Inverse relationship between alcohol consumption and active Helicobacter pylori infection the Bristol Helicobacter project. Am J Gastroenterol 2002; 97:2750-2755.
- Zhang L, Guy D, Harry H, et al. Relationship between alcohol consumption and active Helicobacter pylori infection. Alcohol Alcoholism 2010; 45:89-94.
- Anjana M. Comparison of staining methods of impression smears of antral biopsies for the rapid diagnosis of Helicobacter pylori. Indian J Med Microbiol 1998; 16:181-182.
- Aarti Kumar, Rani Bansal, Ved Prakash, et al. Histopathological changes in gastric mucosa colonized by H. pylori. Indian J Pathol Microbial 2006; 49:352-356.
- Madan E, Kemp J, Westblom TU, et al. Evaluation of staining Methods for identifying C. pylori. Am J Clin Pathol Microbiol 1988; 90:450-453.
- Arora U, Aggarwal, Singh K, et al. Comparative evaluation of conventional methods and elisa based igg detection for diagnosis of H. pylori infection in cases of dyspepsia. Indian J Med Microbiol 2003; 21:46-48.
- Iwahi T, Satoh H, Nakao M, et al. Lansoprazole, a novel benzimidazole proton pump inhibitor, and its related compounds have selected activity against H. pylori. Antimicrob Agents Chemother 1991; 35:490-496.
Author Info
Department of Microbiology, Sree Balaji Medical College and Hospital Affiliated to Bharath Institute of Higher Education and Research, Chennai, Tamil Nadu, IndiaCitation: Chitraleka Saikumar, Prevalence of Helicobacter Pylori in Alcoholic and Non-Alcoholic Gastritis Patients, J Res Med Dent Sci, 2021, 9 (5):242-251.
Received: 08-Apr-2021 Accepted: 24-May-2021
