Research Article - (2023) Volume 11, Issue 1
Potential Effect of Manuka Oil Extract (Leptospermum scoparium) against Streptococcus mitis and Streptococcus sanguinis
Abbas S Alkurwi* and Alaa Omran Ali Almosawi
*Correspondence: Abbas S Alkurwi, Department of Oral and Maxillofacial Surgery, College of Dentistry, University of Baghdad, Baghdad, India, Email:
Abstract
Objective: The manuka tree has been used in traditional medicine by maori people in New Zealand to treat a plethora of aliments since thousands of years. It has recently gained attention of medical research because of its broad spectrum antibacterial effect against bacteria due to its β-triketon component. Periodontal diseases are poly microbial infections caused by dental plaque, which is formed by the adherence of early colonizing bacteria to the acquired pellicle covering the non-shedding surfaces in the oral cavity. To this time, chlorhexidine is considered the golden standard in chemical plaque control, however, its reported side effects emerge after a short period of use limiting its benefits Therefore, this study aimed to study the antibacterial potential of manuka essential oil against primary dental colonizers namely Streptococcus mitis and Streptococcus sanguinis.
Materials and methods: Samples of supra gingival plaque were taken from 6 healthy patients with mild to moderate gingivitis. Bacterial Identification was done using polymerase chain reaction alongside with morphological and biochemical tests. Sensitivity testing was done using agar well diffusion on muller hinton agar and then minimum inhibitory concentration was measured using two fold serial broth micro dilutions. The data were analyzed using SPSS (Statistical Package for Social Science) version 25 software and significance of all the statistical tests was determined at p<0.05.
Results: Manuka essential oil aqueous suspension at 20% concentration exhibited a comparable effect to chlorhexidine 0.2% when tested against Streptococcus mitis, while Streptococcus sanguinis showed smaller inhibition zones on muller hinton agar at 20% v/v, but not significantly different than c5. Chlorhexidine 0.2% (p>0.0) minimum in hibitory concentrations for Streptococcus mitis and Streptococcus sanguinis were (0.0625% v/v, 0.0156% v/v) respectively
Conclusion: Within the limitations of this study, manuka essential oil possesses a potent antibacterial effect against Streptococcus mitis and Streptococcus sanguinis in vitro. Further studies are needed to manufacture a safe and chemically stable formula to be used in vivo. However, it can be a promising therapeutic agent to incorporate in the manufacturing of oral care products.
Keywords
Chlorhexidine, Streptococcus sanguinis, β-triketon, Manuka tree, SPSS
Introduction
Periodontal diseases are a leading cause of tooth loss, second only to dental caries, worldwide. The presence of dental plaque is a key etiological cause of both diseases [1]. It is essentially a soft deposit known as biofilm composed of many different bacterial species populating the surfaces of teeth and hard surfaces in the oral cavity. They establish a microbial community by interacting metabolically, physically and molecularly [2,3].
Mature plaque starts by attachment of the acquired pellicle, which consists of mucins, keratins, proline rich proteins, histidine rich proteins and phosphor proteins that act as receptor sites for colonising oral bacteria [4].
Streptococcus and Actinomyces are able to attach readily to the glycoprotein rich salivary pellicle, which covers the tooth, via bacterial surface receptor recognition [5]. These receptors are called adhesins, which in turn make these species the most abundant bacteria during early dental plaque formation. During the first 4 to 8 hours following tooth brushing, Streptococci, such as Streptococcus mitis and Streptococcus sanguinis, compose roughly 60% to 80% of oral bacteria. In addition to the biofilm developed on tooth surfaces, the initial colonists provide new binding sites for the future [6]. This is evident in the metabolism of primary colonizers, which modifies the local environment, facilitating the attachment and proliferation of subsequent colonizing species [7].
Most oral bacteria are removed by mechanical cleaning of teeth. During recolonization, primary colonising bacteria can be observed on the tooth surface after 3 minutes. Initially, little was known about the early stages of bacterial translocation and tooth surface contact. A specific interaction occurs between the adhesin receptors of the primary colonisers and the receptors of the salivary pellicle. As a result of these interactions, bacteria can reliably attach to the tooth surface [8,9].
Dental plaque starts by accumulating at the gingival margin. At this point, it is called supragingival plaque. With time and a lack of plaque control measures, dental plaque builds up and extends to the subgingival area, thus it is called subgingival plaque.
Chlorhexidine is considered, to this day, the gold standard used in chemical plaque control. However, its use is associated with many side effects, including staining of teeth and restorations, enhancement of supragingival calculus formation and displeasing flavor [10].
Natural medicinal herbs are plentiful and can be utilized in place of synthetic pharmaceuticals as stable, safe and biologically active medicine derived from plants. Throughout New Zealand, manuka trees grow in abundance and they've long been utilized in traditional maori medicine for a wide range of ailments. Traditional uses for the tree's bark include skin treatments, sedatives and mouthwash. Boiling the leaves in water or applying them topically to reduce itching is two ways for which the leaves are used [11].
Recent evidence, primarily from in vitro studies, clearly demonstrates a broad spectrum of antibacterial, antiinflammatory, antifungal, antiviral, anti-parasitic/insecticidal and spasmolytic activity. Previous studies showed manuka oil antibacterial potential to against skin and foodborne pathogens. However, limited data is available in regards to its antibacterial action against oral bacteria [12].
To the best of our knowledge, this is the first study that investigates the antibacterial potential of manuka essential oil against primary plaque colonisers (Streptococcus mitis and Streptococcus sanguinis) in vitro.
We hypothesized that manuka EO would demonstrate a strong inhibitory effect against these bacteria.
Materials and Methods
Human sampling
The plaque samples were collected from 6 healthy patients aged 20-35 suffering from mild to moderate gingivitis. Teeth were initially rinsed with a spray of water to remove food remnants, and then the area was isolated with cotton rolls to prevent contamination from saliva. Gracey curettes were used to collect supragingival plaque samples and transfer them to a sterile swab, which was then immersed in transfer medium.
Later, each swab was inoculated into an eppendorf test tube containing Brain Heart Infusion Broth (BHIB) and incubated aerobically for 24 hours at 37°C.
Isolation and culturing of the Streptococcus species
A loop full of sample specimens from BHIB were transferred to mitis salivaris agar supplemented with 1% potassium tellurite using the streaking method. The dishes were incubated for 48 hours at 37°C under aerobic conditions [13].
PCR identification of Streptococcus species
Representative colonies of each species of bacteria were selected from the growth on the Mitis Salivaris Agar with tellurite (MSA) plate. Genomic DNA extraction of bacterial DNA was firstly isolated from the bacteria in accordance with the protocol of ABIO pure extraction. Then, using a quantus fluorometer, the concentration of extracted DNA was determined in order to assess the quality of samples for downstream applications. The primers used in identifying S. mitis and S. sanguinis are shown in Table 1. Macrogen company provided these primers in lyophilized form. Lyophilized primers were dissolved in nuclease free water to give a final concentration of 100 pmol/μl as a stock solution. A working solution of these primers was prepared by adding 10 μl of primer stock solution (stored at -20°C) to 90 μl of nuclease free water to obtain a working primer solution of 10 pmol/μl.
| Primer name | Vol. of nuclease free water (µl) | Concentration (pmol/µl) | Reference |
|---|---|---|---|
| S. mitis_GDH2-F | 300 | 100 | [14] |
| S. mitis_GDH2-R | 300 | 100 | |
| S. sanguinis-F | 300 | 100 | [15] |
| S. sanguinis-R | 300 | 100 |
Table 1: Primers and their preparation.
PCR reactions were set up for 20 μL reactions using a 2 μL DNA template and reaction components from the GoTaq green master mix (Table 2) (Promega; Madison, WI). Reaction conditions were described in Table 3.
| Master mix components | Stock | Unit | Final | Unit | Volume |
| 1 Sample | |||||
| Master mix | 2 | X | 1 | X | 10 |
| Forward primer | 10 | µM | 1 | µM | 1 |
| Reverse primer | 10 | µM | 1 | µM | 1 |
| Nuclease free water | 6 | ||||
| DNA | ng/µl | ng/µl | 2 | ||
| Total volume | 20 | ||||
| Aliquot per single rxn | 18 µl of Master mix per tube and add 2 µl of Template | ||||
| PCR component calculation | Reaction volume/run=20 µl and No. of PCR cycles=30 cycles | ||||
Table 2: Mixing of go taq green master mix components.
| Steps | °C | M:S | Cycle |
|---|---|---|---|
| Initial denaturation | 95 | 5:00 | 1 |
| Denaturation | 95 | 0:30 | 30 |
| Annealing | 51 or 60 | 0:30 | |
| Extension | 72 | 1:00 | |
| Final extension | 82 | 7:00 | 1 |
| Hold | 10 | 10:00 |
Table 3: PCR program settings.
Positive and negative reactions were determined by agarose (1.5%) gel electrophoresis, 5 μl of PCR product were directly loaded to each well in the horizontal agarose gel. Electrical power was turned on at 100 v/ mAmp for 60 min DNA moves from the cathode to plus anode poles. The ethidium bromide stained bands in the gel were visualized using gel imaging system (major science, USA).
Reactions were performed independently three times and gave identical results each time.
Preparation of standard bacterial suspension
4 mL of sterile saline was transferred into a clear polystyrene 12 × 75 mm test tube. A homogenous organism suspension was prepared by transferring several isolated colonies from 18 to 24 hour blood agar plates to the saline tube using a sterile wood stick. The suspension was adjusted to the McFarland standard required by the test (0.5 McF) using the DensiCHEK plus meter.
Preparation of manuka EO aqueous suspension
This is achieved by mixing manuka essential oil (East Cape Te Araroa New Zealand, naturals New Zealand store) with 4% Dimethyl Sulfoxide (DMSO), which acts as an emulsifier to keep the oil suspended in aqueous preparation. Two fold serial dilutions were used to prepare 5 stock solutions (20% v/v, 10% v/v, 5% v/v, 2.5% v/v, 1.25% v/v) used in the study. 20% v/v was obtained by adding 200 μl of essential oil to 800 μl of 4% DMSO.
Experiment one
Antimicrobial effect of manuka oil at different concentrations, on S. sanguinis and S. mitis in vitro.
Test agents
• Manuka EO with 4% DMSO: (20% v/v, 10% v/v, 5%
v/v, 2.5% v/v, 1.25% v/v).
• Chlorhexidine gluconate (CHX) 0.2% (positive
control).
• 3%-4% DMSO was used as a negative control.
Procedure: The agar well diffusion technique was used in this experiment to study the sensitivity of S. mitis and S. sanguinis bacteria to manuka EO on mueller hinton agar [16].
• Muller hinton agar was prepared according to manufacturer’s instructions, then 25 ml was poured into each sterile petri dishes which are left to cool at room temperature.
• For each plate, a sterile swab was immersed into the standard bacterial suspension for each bacterium spread over the plate using the streaking method and left for 20 minutes at room temperature to dry. Following this, five wells of equal size and depth were prepared using a 6 mm cork borer. Each well within the plates was filled with 100 μl of the tested material.
• Plates are incubated in an aerobic environment at 37°C for 24 hour afterwards; each inhibition zone diameter was recorded using a millimeter ruler.
• On a different petri dish, 100 μl of chlorhexidine 0.2% as a positive control and 100 μl of 4% DMSO as a negative control were added into separate wells.
Experiment two: Determination of Minimum Inhibitory Concentration (MIC)
MIC values for each microorganism were measured using two fold serial broth micro dilution [17].
In the first trial, serial dilution started from 50% v/v of manuka essential oil aqueous preparation. However, all wells containing the antibacterial agents came out clear after incubation. Therefore, in the second trial the starting concentration was reduced to 0.25% v/v.
• A volume 100 μl of muller hinton broth was
dispensed using a micropipette into all wells of a
micro titer plate except the negative control. The plate
and lid were labeled.
• 100 μl of manuka EO 0.25% v/v was pipetted into the
wells in column 1 (far left of the plate) without adding
to the first row, which is the blank and sterility
control for the plate scanner.
• Using the micropipette set at 100 μl, the oil was mixed
into the wells in column 1 by sucking up and down
6-8 times.
• Serial dilution was performed by withdrawing 100 μl
from column 1 and adding it to column 2, then
repeating this procedure down to column 10.
Afterwards discarding the last 100 μl from column 10.
• To column 11, 100 μl of 0.2% chlorhexidine was
added to reach a final concentration of 0.1%. Column
12 contained only 100 μl of 4% DMSO as negative
control.
• Utilizing a smaller micropipette, 100 μl of the
standard bacterial suspension was dispensed into all
wells except the first row.
• The plates were incubated at 37°C for 24 hours.
• The plates were then scanned with the GloMax® discover micro plate reader on 600 nm.
• By reading optical density values, the minimum
inhibitory concentration is lowest dose of
antimicrobial that will inhibit bacterial growth after
being sub cultured on antibiotic free media [18].
Determination of MBC
A micropipette was used to extract 100 μl from wells with EO concentrations equal to or greater than the MIC, which were subsequently sub cultured on muller hinton agar using a sterile glass spreader and incubated aerobically for 24 hours.
After 24 hours, the plates were removed and tested for bacterial growth; those that exhibited no growth were designated as having the minimum bactericidal concentration.
Results
Molecular biology results
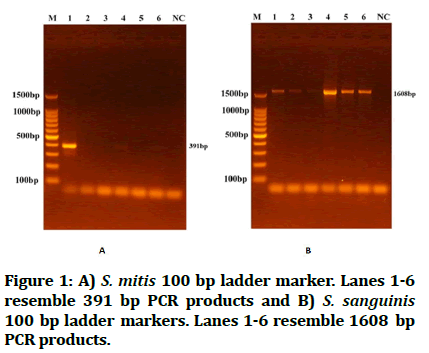
Digital imaging reveals DNA amplification was confirmed by the presence of ethidium bromide stained bands in agarose gel as DNA moves from cathode to anode poles. A DNA size standard for sizing and quantification of double stranded DNA with sizes ranging from 100 to 1500 base pairs. S. mitis was identified by having PCR products near 391 bp, while S. sanguinis PCR product resembled 1608 bp (Figure 1).
Figure 1: A) S. mitis 100 bp ladder marker. Lanes 1-6 resemble 391 bp PCR products and B) S. sanguinis 100 bp ladder markers. Lanes 1-6 resemble 1608 bp PCR products.
Sensitivity of S. mitis and S. sanguinis to manuka oil aqueous suspension (agar well diffusion method)
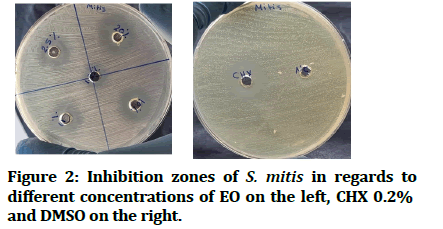
The Maximum (Max) and Minimum (Min) values of the inhibition zones in Millimeters (mm) of the aqueous manuka oil suspension against both bacteria are presented in Tables 4 and 5. It showed an increase in the diameter of the inhibition zone as the EO concentration increased. The aqueous suspension at 20% v/v concentration and CHX showed approximately the same results when tested against S. mitis (the median of the inhibition zone for 20% v/v concentration was 19 mm and for CHX 0.2% was 20 mm) (Figure 2). In the case of S. sanguinis, however, the aqueous suspension at 20% v/v concentration showed smaller inhibition zones in comparison to CHX (the median of inhibition zone for 20% v/v concentration was 15 mm, while that for CHX was 22.5 mm) (Figure 3). DMSO (negative control) showed no inhibition zone for both bacteria.
| Intergroup comparison | ||||||
|---|---|---|---|---|---|---|
| Bacteria | Concentration | N | median | min | max | p value* |
| S. mitis | 20% | 9 | 19 | 19 | 20 | 0.000 |
| 10% | 9 | 17 | 16 | 17 | ||
| 5% | 9 | 15 | 15 | 17 | ||
| 2.50% | 9 | 15 | 14 | 16 | ||
| 1.25% | 9 | 11 | 10 | 12 | ||
| Chx | 9 | 20 | 19 | 21 | ||
| S. sanguinis | 20% | 9 | 15 | 15 | 16 | 0.000 |
| 10% | 9 | 15 | 14 | 16 | ||
| 5% | 9 | 14 | 14 | 15 | ||
| 2.50% | 9 | 13 | 13 | 14 | ||
| 1.25% | 9 | 12 | 11 | 12 | ||
| Chx 0.2% | 9 | 23 | 21 | 23 | ||
Table 4: Inhibition zones of different concentrations of manuka EO. Intergroup comparison using Kruskal wallis test revealed statistically significant difference (p<0.05).
| Pairwise comparisons of groups | ||||
|---|---|---|---|---|
| S. mitis | S. sanguinis | |||
| Groups | Adj. sig.a | Adj. sig.a | ||
| 2.5%-5% | 1 | 1 | ||
| 2.5%-10% | 0.862 | 0.438 | ||
| 2.5%-20% | 0.001 | § | 0.101 | |
| 2.5%-1.25% | 0 | § | 1 | |
| 2.5%-CHX | 0 | § | 0 | § |
| 5%-10% | 1 | 1 | ||
| 5%-20% | 0.016 | 1 | ||
| 5%-1.25% | 0 | § | 0.133 | |
| 5%-CHX | 0 | § | 0.005 | § |
| 10%-20% | 0.66 | 1 | ||
| 10%-1.25% | 0.044 | § | 0.002 | § |
| 10%-CHX | 0.044 | § | 0.255 | |
| 20%-1.25% | 1 | 1 | ||
| 20%-CHX | 1 | 0.945 | ||
| 1.25%-CHX | 1 | 1 | ||
| a= Significance values have been adjusted by the bonferroni correction for multiple tests. §= Statistically significant results. |
||||
Table 5: Shows statistical differences between groups of different concentration of essential oil and chlorhexidine 0.2% against S. mitis and S. sanguinis. No statistically significant difference was noted between mauka EO at 20% v/v group and CHX.
Figure 2: Inhibition zones of S. mitis in regards to different concentrations of EO on the left, CHX 0.2% and DMSO on the right.
Figure 3: Inhibition zones of S. sanguinis in regards to different concentrations of EO on the left, CHX and DMSO on the right.
Determination of minimum inhibitory concentration
Wells inoculated by S. mitis at concentrations less than 0.0625% v/v revealed turbidity. This was confirmed by taking 100 μL from wells with concentration (0.0625% v/v) and sub culturing them on muller hinton agar. For S. sanguinis, the turbidity is present at concentrations less than 0.0156% v/v. This was also confirmed by sub culturing on MHA (Table 6).
| Bacteria | MIC v/v | OD | Versus | P valuea |
|---|---|---|---|---|
| S. mitis | 0.06% | 0.0651 | CHX 0.1% | 0.043 |
| 4% DMSO | 0 | |||
| S. sanguinis | 0.02% | 0.1 | CHX 0.1% | 0.039 |
| 4% DMSO | 0 | |||
| A=Significance values have been adjusted by the bonferroni correction for multiple tests. | ||||
Table 6: Illustrates MIC values of manuka EO in comparison to CHX 0.1%. Kruskal wallis test revealed statistically significant difference between groups (p<0.05).
Determination of minimum bactericidal concentration
MBC for S. mitis and S. sanguinis was 0.125% (v/v) and 0.0313% (v/v) respectively.
Discussion
Primary colonizing bacteria in early plaque formation is mainly composed of Streptococcus viridian group. S. mitis and S. sanguinis are examples of bacteria that have the ability to adhere to non-shedding surfaces of the oral cavity. Subsequently, forming biofilms that are made up of many different bacteria and the ability of these organisms to adhere together appears to be a pathogenic feature [19].
Because of the promising antibacterial properties of L. scoparium essential oil, it is used to lower the bacterial burden in sites susceptible to infection, such as open wounds, to prevent infection. Not only does it have the potential to be employed to manage the microbial population in the oral environment, but now there is now clear evidence of broad spectrum efficacy against a wide range of pathogens due to its β-triketone component responsible for the antibacterial activity [20]. So far, limited data is available on manuka oil’s antibacterial effect on primary dental plaque colonizers. Therefore, it was intended in this study to investigate its effects on Streptococcus mitis and Streptococcus sanguinis.
Sensitivity of S. mitis and S. sanguinis to different concentrations of aqueous suspension of manuka EO by the agar well diffusion method has been tested in this study.
In this study, it was decided to use commercially available product (East Cape Te Araroa New Zealand, naturals New Zealand store) because it is difficult to get in season raw plant material. Solvent selection between DMSO and alcohols (i.e. ethanol and methanol) and water for oil dilution, was a challenge. Oils separate when mixed with water, thus, water was ruled out, as oil separation reduces the EO surface contact with the broth medium diminishing its effects. Adding to this, studying antibacterial effects of the extract in vitro was with the goal of introducing a natural agent employed in chemical plaque management in vivo, thus it was best to avoid the added antimicrobial as well as the side effects of alcohol even if alcoholic extract can give better anti-microbial effects [21]. 4% DMSO works by emulsifying the oil in a liquid medium without interfering with EO action [22].
Results showed that manuka oil aqueous suspension was able to inhibit the growth of S. mitis and S. sanguinis. The diameters of inhibition zones were found to increase with increasing concentration, this may be due to the amount of the dissolved active constituents of the extract will be more abundant as the concentrations increase, causing increased antimicrobial activity of the extract.
S. mitis was notably more sensitive to manuka oil suspension than S. sanguinis, as 20% v/v produced similar inhibition zone to the positive control agent. While S. sanguinis displayed smaller inhibition zones on muller hinton agar, the difference was not statistically different than the positive control agent (p>0.05).
This present study comes in partial agreement with some previous studies. Takarda, et al. reported that manuka EO was more effective than tea tree oil, euclyptus oil, lavandula oil and romarinus oil [23]. With a concentration of 0.2% manuka EO is sufficient to kill periodontopaththic bacteria tested.
Chen further confirmed manuka EO antibacterial potential when testing it against S. aureus, S. mutans, S. sobrinus and E. coli [24]. Even at a low concentration (10%), it had the ability to inhibit bacterial growth of gram positive as well as gram negative bacteria.
These findings were found consistent with results which showed 100% inhibition of Staphylococcus sobrinus and Staphylococcus mutans by a solution of 10% manuka EO dissolved in DMSO [25].
Gram positive bacteria are more vulnerable to manuka oil's antimicrobial actions than gram negative bacteria, with the latter needing higher concentrations of the oil to inhibit growth, because the lipopolysaccharide cell wall serves as a barrier encapsulating the bacterial cytoplasmic membrane. Triketones mainly act by disrupting the cytoplasmic membrane, inhibiting important energy generating systems that are located in it (i.e. electron transport chain, F1 F0 ATP synthase) due to their hydrophobic nature [26].
Takarda reported MIC for gram positive cariogenic bacteria S. sorbinus and S. mutans 0.25% v/v, while Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum and Porphyromonas gingivalis had MIC values of 0.03% v/v. However, did not specify the origin of the oil, which has a direct effect on its β-Triketone content. That might explain the higher concentration needed to obtain MIC values. The ratio of tri ketone to other components such as pinene and sesquiterpin is what differentiates the oils with higher antibacterial activity from others. With East cape and marlbo rough sounds plants being especially high in tri ketone content (over 30%) [27,28].
The minimum bactericidal concentration results in my study partially agree with that of Porter, et al. that found a concentration of 0.1% (w/w) was required to kill S. mitis and S. sanguinis bacteria after 4 hour of incubation under anaerobic conditions, which is comparable to 0.125%, 0.0313% (v/v) required a to kill S. mitis and S. sanguinis respectively after 24 hours of incubation under aerobic conditions.
There is limited clinical data regarding the use of manuka EO in vivo, however, lauten, et al. tested clinically a formulation 0.67% (v/v) melaleuca EO, 0.33% (v/v) manuka EO, 1% (v/v) Calendula flower extract and 0.5% (w/v) green tea mouth rinse on seventeen subjects in a double blind placebo controlled study and did not demonstrate a significant reduction in mean periodontal index or mean gingival index as compared to placebo group [29]. This may be due to the fact that the ingredients at the chosen concentrations have synergistic or inactivating effects upon each other. In addition, the high concentration of the tea tree oils imparts a pungent flavor and odor that could diminish compliance. Lastly, the solubility is problematic and therefore this formulation would not likely stay completely in solution for extended periods of time, such as with commercial manufacturing and marketing practices.
Conclusion
Manuka EO clearly demonstrates a strong antibacterial effect on oral bacteria in vitro. This also opens up the door to further research in an active safe formulation to be used in vivo.
References
- Falco M. The lifetime impact of sugar excess and nutrient depletion on oral health. Gen Dent 2001; 49:591-595. [Googlescholar][Indexed]
- Marsh PD, Martin MV, Lewis MA, et al. Oral microbiology e-book. 5th edition, Elsevier Health Sciences, Netherlands, 2009. [Googlescholar]
- Wilson M. Microbial inhabitants of humans: Their ecology and role in health and disease. Cambridge University press, United Kingdom, 2005. [Googlescholar]
- Huang R, Li M, Gregory RL. Bacterial interactions in dental biofilm. Virulence 2011; 2:435-444. [Crossref][Googlescholar][Indexed]
- Nobbs AH, Lamont RJ, Jenkinson HF. Streptococcus adherence and colonization. Microbiol Mol Biol Rev 2009; 73:407-450. [Crossref][Googlescholar][Indexed]
- Dige I, Raarup M, Nyengaard J, et al. Actinomyces naeslundi in initial dental biofilm formation. Microbiology 2009; 155:2116-2126. [Crossref][Googlescholar][Indexed]
- Lang NP, Lindhe J. Clinical periodontology and implant dentistry. 5th Edition, John Wiley and Sons, United States, 2015; 2. [Googlescholar]
- Hannig C, Hannig M, Attin T. Enzymes in the acquired enamel pellicle. Eur J Oral Sci 2005; 113:2-13. [Crossref][Googlescholar][Indexed]
- Hannig C, Hannig M. The oral cavity: A key system to understand substratum dependent bioadhesion on solid surfaces in man. Clin Oral Investig 2009; 13:123-139. [Crossref][Googlescholar][Indexed]
- Flotra L, Gjermo P, Rolla G, et al. Side effects of chlorhexidine mouth washes. Scand J Dent Res 1971; 79:119-125. [Crossref][Googlescholar][Indexed]
- Brooker SG, Cambie RC, Cooper RC. New Zealand medicinal plants. Heinemann, New Zealand, 1987.
- Mathew C, Tesfaye W, Rasmussen P, et al. Manuka oil: A review of antimicrobial and other medicinal properties. Pharmaceuticals 2020; 13:343. [Crossref][Googlescholar][Indexed]
- Holbrook W, Beighton D. Streptococcus mutans levels in saliva and distribution of serotypes among 9 year old Icelandic children. Scand J Dent Res 1987; 95:37-42. [Crossref][Googlescholar][Indexed]
- Banas JA, Zhu M, Dawson DV, et al. PCR based identification of oral streptococcal species. Int J Dent 2016; 2016. [Crossref][Googlescholar][Indexed]
- Li Y, Pan Y, Qi F, et al. Identification of Streptococcus sanguinis with a PCR generated species specific DNA probe. J Clin Microbiol 2003; 41:3481-3486. [Crossref][Googlescholar][Indexed]
- Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal 2016; 6:71-79. [Crossref][Googlescholar][Indexed]
- Cavalieri S, Harbeck R, McCarter Y, et al. Manual of antimicrobial susceptibility testing. American Society for Microbiology. Pan American Health Organization: Washington, DC, USA, 2005.
- Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother 2001; 48:5-16. [Crossref][Googlescholar][Indexed]
- Li J, Helmerhorst E, Leone CW, et al. Identification of early microbial colonizers in human dental biofilm. J Appl Microbiol 2004; 97:1311-1318. [Crossref][Googlescholar][Indexed]
- Porter GC, Safii SH, Medlicott NJ, et al. Formulation of a semisolid emulsion containing Leptospermum scoparium essential oil and evaluation of in vitro antimicrobial and anti-biofilm efficacy. Planta Med 2021; 87:253-266. [Crossref][Googlescholar][Indexed]
- Das K, Tiwari R, Shrivastava D. Techniques for evaluation of medicinal plant products as antimicrobial agents: Current methods and future trends. J Med Plant Res 2010; 4:104-111. [Crossref][Googlescholar][Indexed]
- Hili P, Evans C, Veness R. Antimicrobial action of essential oils: The effect of dimethylsulphoxide on the activity of cinnamon oil. Lett Appl Microbiol 1997; 24:269-275. [Crossref][Googlescholar][Indexed]
- Takarada K, Kimizuka R, Takahashi N, et al. A comparison of the antibacterial efficacies of essential oils against oral pathogens. Oral Microbiol Immunol 2004; 19:61-64. [Crossref][Googlescholar][Indexed]
- Chen CC, Yan SH, Yen MY, et al. Investigations of kanuka and manuka essential oils for in vitro treatment of disease and cellular inflammation caused by infectious microorganisms. J Microbiol Immunol Infect 2016; 49:104-111. [Crossref][Googlescholar][Indexed]
- Fratini F, Mancini S, Turchi B, et al. A novel interpretation of the fractional inhibitory concentration index: The case Origanum vulgare L. and Leptospermum scoparium JR et G Forst essential oils against Staphylococcus aureus strains. Microbiol Research 2017; 195:11-17. [Crossref][Googlescholar][Indexed]
- van Klink JW, Larsen L, Perry NB, et al. Triketones active against antibiotic resistant bacteria: Synthesis, structure activity relationships, and mode of action. Bioorg Med Chem 2005; 13:6651-6662. [Crossref][Googlescholar][Indexed]
- Douglas M, Anderson R, Van Klink J, et al. Defining North Island manuka chemotype resources. Crop Food Res Rep 2001; 447:1-13.
- Douglas MH, van Klink JW, Smallfield BM, et al. Essential oils from New Zealand manuka: Triketone and other chemotypes of Leptospermum scoparium. Phytochemistry 2004; 65:1255-1264. [Crossref][Googlescholar][Indexed]
- Lauten JD, Boyd L, Hanson MB, et al. A clinical study: Melaleuca, manuka, calendula and green tea mouth rinse. Phytother Rese 2005; 19:951-957. [Crossref][Googlescholar][Indexed]
Author Info
Abbas S Alkurwi* and Alaa Omran Ali Almosawi
Department of Oral and Maxillofacial Surgery, College of Dentistry, University of Baghdad, Baghdad, IndiaCitation: Abbas S Alkurwi, Alaa Omran Ali Almosawi, Potential Effect of Manuka Oil Extract (Leptospermum scoparium) against Streptococcus mitis and Streptococcus sanguinis , J Res Med Dent Sci, 2023, 11 (01):168-175.
Received: 25-Oct-2022, Manuscript No. JRMDS-22-65198; , Pre QC No. JRMDS-22-65198 (PQ); Editor assigned: 28-Oct-2022, Pre QC No. JRMDS-22-65198 (PQ); Reviewed: 11-Nov-2022, QC No. JRMDS-22-65198; Revised: 26-Dec-2022, Manuscript No. JRMDS-22-65198 (R); Published: 12-Jan-2023