Research - (2019) Volume 7, Issue 6
Potential Effect of Gold Nanoparticles against Streptococcus mitis (Primary Periodontal Colonizer)
Hussein Jameel Abd Noor* and Basima GH Ali
*Correspondence: Hussein Jameel Abd Noor, Department of periodontics, College of Dentistry, University of Baghdad, Iraq, Email:
Abstract
Background: Periodontal diseases are an infection caused by many factors include different bacterial pattern. Biofilm is the main reason for periodontal disease that consists of many microorganisms. Streptococci of Mitis group such as Streptococcus mitis, Streptococcus oralis, are early colonizers within development of dental biofilms, which is the cause of periodontal disease. The periodontal diseases treatment focuses primarily on the reduction of the microorganism count thus antimicrobials agents has been used as an adjunct to conventional therapy. Multi-drug resistance within the treatment of infectious diseases is a growing problem and also the widespread use of broad-spectrum antibiotics has created antibiotic resistance for several human microorganisms. Recently, nanoparticles have significant attention for medical applications because of their antimicrobial effectiveness. Gold nanoparticles (Au Nps) were considered as potential agent to control oral bacterial infection because of its optimal antibacterial activity and non-acute cytotoxic effects on human cells. In this study, gold Nps with average particles size (43 nm) will be use. The objective of this study is to investigate the antibacterial effect of gold nanoparticles against Streptococcus mitis in dental plaque.
Materials and Methods: The first step of present study was tacking aerobic and anaerobic plaque samples from 10 patients with periodontal disease. Second step was morphological and microscopicale examination in addition to the biochemical tests and vatic 2 machines were used to confirm identification of Streptococcus mitis. The next steps were synthesis of gold nanoparticles by chemical method (seed growth method) and testing its characterization. Finally test the potential antimicrobial effect of gold nanoparticles on Streptococcus mitis. The data were analyzed by using descriptive statistics including mean and standard deviation, LSD were done for the analysis of MIC of (gold nanoparticles) against (Streptococcus mitis Bacteria). Significance of all the statistical tests were determined at p<0.05 using SPSS (Statistical Package for SocialScience) version 22 software.
Results: The gold NP in (100 ppm) concentration has the same antimicrobial effect of chlorhexidine against Streptococcus mitis, and there is no significant difference between them, (p>0.05).
Conclusions: Gold nanoparticles were effective against Streptococcus mitis similarly to chlorhexidine when it was used in high concentration as antimicrobial agent.
Keywords
Antibacterial, Chlorhexidine, Streptococcus mitis, Gold nanoparticles
Introduction
Periodontal disease which is one of the most widespread diseases affecting mankind, involve the adherence of bacteria and development of biofilms on both the natural and restored tooth surface [1]. Dental plaque is a biofilm which is pale yellow in color that develops naturally on the teeth. A dental plaque is formed by colonizing bacteria trying to attach themselves to the tooth surface. Dental biofilm, more commonly referred to as dental plaque is composed of about thousand species of bacteria that take part in the complex ecosystems of the mouth [2]. Dental plaque starts when bacteria that are present in the mouth attach to teeth and begin multiplying. Plaque can form on teeth both supra gingival plaque, or sub gingival plaque [3]. The plaque is made up of colonies of microorganisms such as bacteria, yeast dumped together in a gel like organic material composed of bacterial byproducts including sugar, food debris and body tissue [4]. The predominant initial colonizers of teeth are Gram-positive facultative anaerobic cocci and rods, including Streptococcus and Actinomyces species. These initial colonizers provide a foundation for further development of dental biofilm [5]. Streptococcus mitis, Streptococcus oralis, and Streptococcus sanguinis of the Mitis group streptococci [6,7] are close relatives to Streptococcus pneumonia which is the important human pathogen. They are part of human normal oral flora, but occasionally can cause acute or chronic disease as opportunistic human pathogens in contrast to S. pneumonia. They are associated with gingival disease and caries and occasionally cause sub-acute infective endocarditis [8]. As they are the early colonizers in the development of dental biofilms [9]. They are able to develop natural competence for genetic transformation as S. pneumonia [10] and have horizontal gene transfer, such as virulence genes, that has been documented between these species [6,11-13]. The emergence of multi-drug-resistant (MDR) microorganism has become a danger to public health [14]. Antibiotic resistant bacteria cause millions of infections and thousands of deaths every year in the U.S. according to a report published by the U.S. Centers for Disease Control and Prevention, addition to that, the continuous and significant reduction in the number of approved antibiotics in the past decade has a role factor in the increasing threatening situation [15] that lead to an urgent need for the invention of novel antibacterial and treatment strategies [16]. Nanoparticles (NPs) give versatile platforms for medical therapy depend on their physical properties [17,18]. NPs have significant interest in their development as potential antimicrobial agent [19]. Among NPs, gold nanoparticles are wide used as a catalyst for diagnostic, biological purposes, medical therapy and gene therapy [20,21]. The gold NPs can synthesize easily by chemical technique and they have a less toxic effect compared to other nanomaterial’s [22-24]. However we need to measure the antimicrobial activity of gold NPs against Streptococcus mitis bacteria in dental plaque.
Materials and Methods
The first step was tacking plaque samples from 10 patients with periodontal disease. Two aerobic and anaerobic samples will be taken from each patient. Aerobic bacterial sample was obtained from supragingival area and anaerobic sample was obtain from subgingival pocket of all patients by sterile curate.
Bacterial isolation
Collected samples were put in brain heart infusion broth (BHIB) (Figure 1), incubated for 24 hours, 37°C under aerobic and CO2 incubator then inoculate the bacteria from BHI to brain heart infusion agar (BHIA) and blood agar and incubated for 24 hours, 37°C under aerobic and CO2 incubator to differentiate it from other microorganisms (Figure 2) then subculture was done. To suspected colony until we have a pure colony [25].
Figure 1. Bacterial samples in (BHIB).
Figure 2. Bacterial culture on (BHIA) and blood agar.
Second step was morphological and microscopicale examination in addition to the biochemical tests and vatic 2 machines were used to confirm identification of Streptococcus mitis.
Gram’s stains
Single pure colony was picked up from blood gar plate under sterilized condition and subjected to Grams stain in order to identify their response to this stain microscopically under oil immersion with magnification power 100X [26].
Blood Hemolysis test this test used to determine the ability of Streptococcus mitis to bleach the heme iron by hydrogen peroxide (H2O2), leading to greenish color on blood agar [27].
Catalase production test
This test was conducted on cells of bacteria colonies. Hydrogen peroxide 3% (H2O2) had been used to determine the ability to produce catalase enzyme by bacteria [28].
Vitek 2 machine
Vitek 2 for identification the specific bacterial type (Figure 3) [29].

Figure 3. Vitek 2 machine.
Third step was synthesis of gold nanoparticles
Sample Preparation: Chlorauic acid, Trisodium citrate, Sodium borohydride, Ascorbic acid, and Cetyl Trimethylammonium Bromide (CTAB) were used. Ultrapure de ionised water was used as the medium during the preparation of all suspensions. The gold seeds and growth solution were prepared independently prior to the seeding growth technique [30]. Characterization of gold nano particles was analyzed using the ultraviolet (UV)-visible spectroscopy (Figure 4), Scanning electron microscope (SEM) (Figure 5), Atomic force microscope (AFM) and X-ray diffraction (XRD). It has spherical shape and average diameter 43.36 nm.
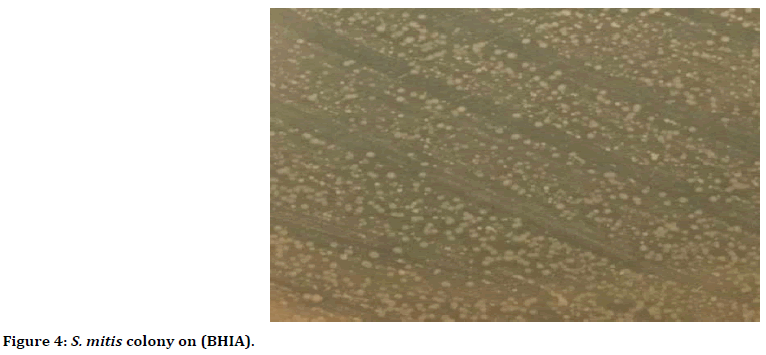
Figure 4. S. mitis colony on (BHIA).
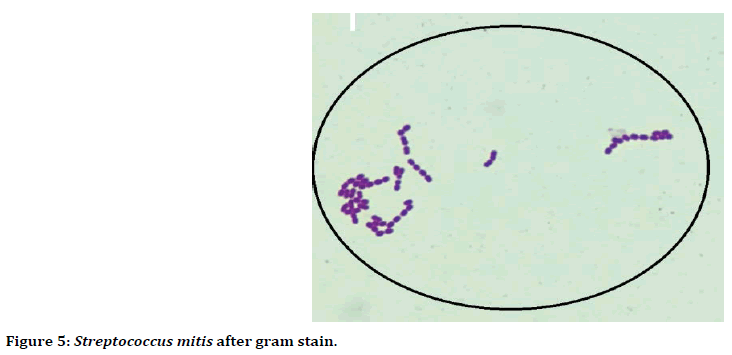
Figure 5. Streptococcus mitis after gram stain.
Chlorhexidine concentrations
Chlorhexidine gluconate was used in a concentration of 0.2% all over the experiment as a positive control material.
Activation of inoculums
Inoculums of bacteria were activated by the addition of 0.1 ml of bacterial culture to 10 ml of BHI-B followed by incubation for 24 hrs at 37°C [31].
Final step
Final step was Determination of antibacterial activity of gold nanoparticles on Streptococcus mitis bacteria. In this experiment, agar well diffusion method was applied to study the antimicrobial effect of the gold NPs on Streptococcus mitis bacteria were used) [32]. MHA agar media was poured separately into sterile petri dishes, 0.1 ml of activated S. mitis were spread in duplicate on MHA agar plates, then wells of equal size and depth made with sterile stainless steel Cork borer in the MH Aagar 4 mm in diameter were prepared in the agar. Each well was filled with gold NPs in different concentrations (100 ppm, 50, 25, 12.5, 6.25, 3.125, 1.562, 0.781, 0.391, 0.195, 0.097) and one well for chlorhexidine 2% as positive control and last well for deionized water as negative control, then incubated aerobically for 24 hrs at 37°C. The diameter of inhibition zone containing the test materials were measured and recorded after the incubation under aseptic condition.
Results
Morphological and microscopical properties of Streptococcus mitis
Morphologically they were characterized through observing their small, round, convex, smooth, glistening colonies and without pigments (Figure 4).
Microscopic examination
Microscopically, after stained with gram stain Streptococcus mitis bacteria appeared Gram positive, cocci shaped, arranged in long chain and short chains (Figure 5).
Catalase test
Streptococcus mitis is catalase negative due to absence of gas bubbles indicates the absence of catalase enzyme.
Hemolysis test
Streptococcus mitis is α-hemolysis, due to bleach of heme iron, resulting in a greenish color on blood agar (Figure 6).
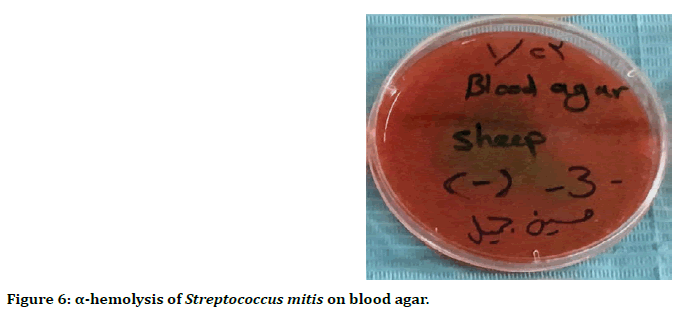
Figure 6. α-hemolysis of Streptococcus mitis on blood agar.
Result of Vitek
The vitek 2 system showed the presence of Streptococcus mitis with 90% probability and good identification (Figure 7).

Figure 7. Identification of S. mitis by vitec 2 machine.
Synthesis of gold nanoparticles
The resultant gold nanoparticles have red color with spherical shape and average diameter 43.36 nm (Figure 8).

Figure 8. Gold nanoparticles suspension.
Characterization of gold nanoparticle
The ultraviolet (UV)-visible spectroscopy: UVVis absorption spectroscopy was used to study the optical and structural properties of the prepared gold nanoparticles. Figure 9 shows the absorption band characteristics with plasmon peak placed at around 538 nm for AuNPs and this related to Surface Plasmon Resonance (SPR). The presence of single SPR indicates sensible dispersion of nanoparticles within the solution and this property associated with AuNPs.
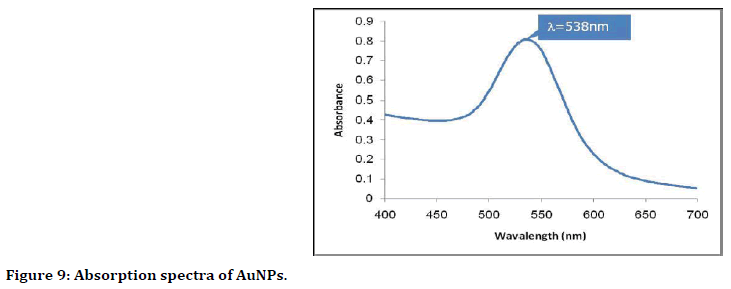
Figure 9. Absorption spectra of AuNPs.
Scanning electron microscope SEM analysis: The results showed well-dispersed nanoparticles with variable shapes most of them present in spherical form (Figure 10).
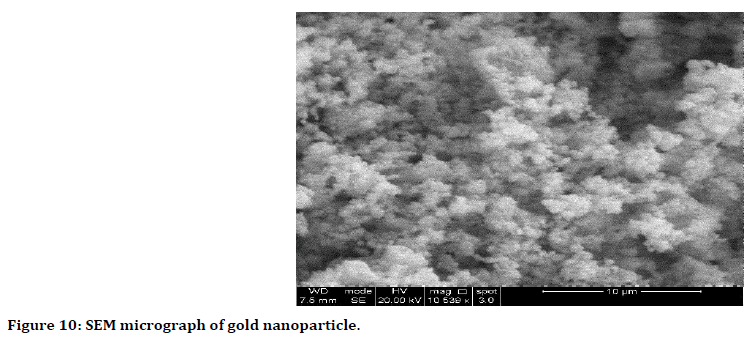
Figure 10. SEM micrograph of gold nanoparticle.
Atomic force microscope (AFM): Two dimentions and three dimentions imsages of AFM are in Figure 11, it demonstrate the average grain size of gold NPs is 43.36 nm and it distributed uniformly and homogenously.
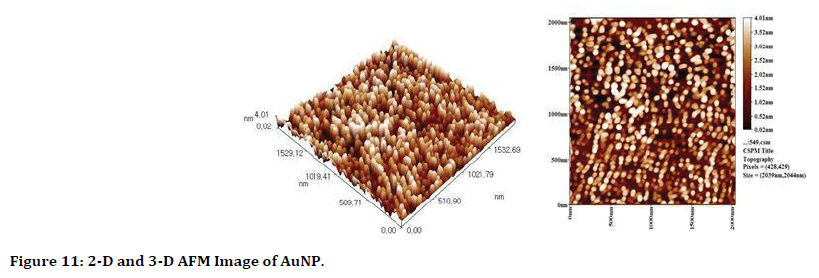
Figure 11. 2-D and 3-D AFM Image of AuNP.
Antimicrobial Activity of Gold Nanoparticles: The gold NPs exhibit excellent antibacterial activity against Streptococcus mitis Table 1.
| S. mitis | N | Mean | Std. Deviation | Std. Error | 95% Confidence Interval for Mean | Minimum | Maximum | Between- Component Variance | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||||
| 100 | 3 | 19 | 0.732 | 1 | 13.7 | 22.3 | 16 | 19 | 51.623 | |
| 50 | 3 | 17.5 | 0.625 | 1 | 12.7 | 21.3 | 15 | 18 | ||
| 25 | 3 | 17 | 0.22 | 0 | 17 | 17 | 17 | 17 | ||
| 12.5 | 3 | 15 | 0.577 | 0.333 | 14.23 | 17.1 | 15 | 16 | ||
| 6.25 | 3 | 15 | 0.21 | 0 | 15 | 15 | 15 | 15 | ||
| 3.125 | 3 | 12 | 0.511 | 0.333 | 11.23 | 14.1 | 12 | 13 | ||
| 1.562 | 3 | 11 | 0.021 | 0 | 11 | 11 | 11 | 11 | ||
| 0.781 | 3 | 8 | 0.24 | 0 | 8 | 8 | 8 | 8 | ||
| 0.391 | 3 | 6 | 0.011 | 0 | 6 | 6 | 6 | 6 | ||
| 0.195 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 0.097 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| chx | 3 | 18 | 0.121 | 0 | 19 | 19 | 19 | 19 | ||
| Deionized water | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Total | 39 | 10.72 | 7.03 | 1.126 | 8.44 | 13 | 0 | 19 | ||
| Model | Fixed Effects | - | 0.716 | 0.115 | 10.48 | 10.95 | - | - | ||
| Random Effects | - | - | 1.996 | 6.37 | 15.07 | - | - | |||
Table 1: Descriptive statistic of antibacterial activity of AuNPs against S. mitis using well diffusion test.
The wells diffusion method result showed, the highest antibacterial activity when used 100 ppm of gold nanoparticles against Streptococcus mitis with inhibition zone reached to 19 mm (Figure 12). While the lowest inhibition zone was 6 mm, when used 0.391 ppm concentration of gold nanoparticles (Figure 13).
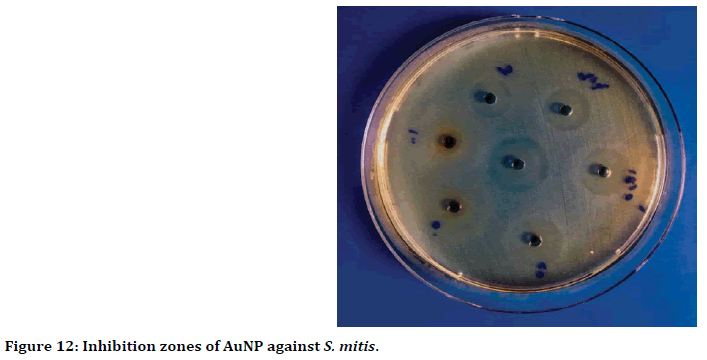
Figure 12. Inhibition zones of AuNP against S. mitis.
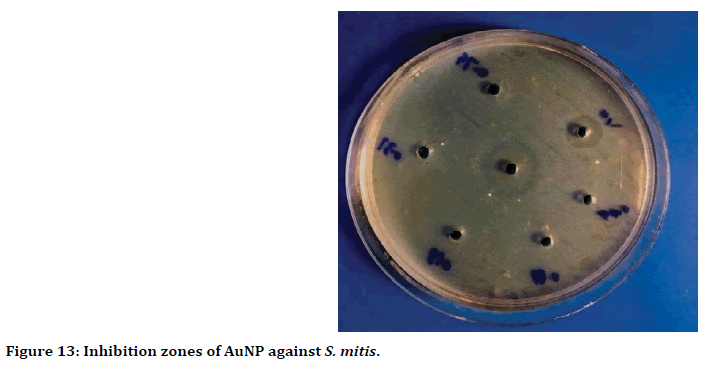
Figure 13. Inhibition zones of AuNP against S. mitis.
Antimicrobial Activity of Gold Nanoparticles: The gold NPs exhibit excellent antibacterial activity against Streptococcus mitis Table 1.
Discussion
The gold nanoparticles exhibit optimal antimicrobial activity against Streptococcus mitis. This could as a result of the gold is that the nanomaterial’s revealed effective treatment to the microbial infections. Gold nanoparticles show no toxic effects and are therefore biocompatible and have incorporated into a varity of industrial and biomedical processes [33]. In the food industry, Au NPs has been extremely effective as antimicrobial agent [34]. Moreover, they have good activity on microorganism by many ways and have different mode of action [35,36]. Basically, Au NPs act on microbes by inhibit the process of DNA transcription by preventing the uncoiling of DNA by bind to the DNA which lead to death of cell [37,38]. Also, the highly reactive oxygen species (ROS), is a potential mechanisms for the antibacterial action which can be formed in the presence of metallic nanoclusters may lead to cell membranes dysfunction and DNA damage in bacteria [39,40]. The results of present study are in agreement with studies done by Xiaoning et al. [41] was mentioned that gold nanoparticles have antibacterial activity by exposure to pathogenic bacteria when use MICs of gold nanoparticles, Senthilkumar et al. [42] that demonstrate the antibacterial activity of different concentration of AuNPs solutions were inhibit both gram positive and gram negative bacteria and Ghanavati et al. [43] That demonstrate the gold nanoparticles have the most MIC (Minimum Inhibitory Concentration at 100 ppm concentration that 6 strains of Salmonella were inhibited by this concentration.
Conclusion
Gold nanoparticles are effective as same as chlorhexidine against Streptococcus mitis at concentration of 100 ppm, so it can be used as an alternative to chx. further in vitro and vivo studies are essential to validate the use of gold nanoparticle as antimicrobial agent against primary colonizer microorganism.
References
- Marsh PD, Martin MV. Oral Microbiology. 5th Edn, Churchill-Livingston Publisher, 2009.
- Becker MR, Paster BJ, Leys EJ, et al. Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol 2002; 40:1001-1009.
- Beer DD, Stoodley P. Microbial biofilms. Prokaryotes 2006; 1:904-937.
- Cury JA, Rebelo MAB, Cury AADB, et al. Biochemical composition and cario geneicity of dental plaque formed in the presence of sucrose or glucose and fructose. Caries Res 2000; 34:491-497.
- Okahashi N, Nakata M, Terao Y, et al. Pili of oral Streptococcus sanguinis bind to salivary amylase and promote the biofilm formation. Microb Pathog 2011; 50:148–154.
- Whatmore AM, Efstratiou A, Pickerill AP, et al. Genetic relationships between clinical isolates of Streptococcus pneumoniae, Streptococcus oralis, and Streptococcus mitis: Characterization of ‘‘Atypical’’ pneumococci and organisms allied to S. mitis harboring S. pneumoniae virulence factor encoding genes. Infect Immun 2000; 68:1374–1382.
- Kilian M. Streptococcus and Lactobacillus. In: Topely and Wilson’s microbiology and microbial infections, John Wiley & Sons, Ltd 2010.
- Doern CD, Burnham CA. It’s not easy being green: The viridans group streptococci, with a focus on pediatric clinical manifestations. J Clin Microbiol 2010; 48: 3829–3835.
- Kolenbrander PE. Oral microbial communities: Biofilms, interactions, and genetic systems. Annu Rev Microbiol 2000; 54:413–437.
- Ronda C, Garcia JL, Lopez R. Characterization of genetic transformation in Streptococcus oralis NCTC 11427: Expression of the pneumococcal amidase in S. oralis using a new shuttle vector. Mol Gen Genet 1988; 215: 53–57.
- Kilian M, Poulsen K, Blomqvist T, et al. Evolution of Streptococcus pneumoniae and its close commensal relatives. PLoS One 2008; 3: 2683.
- Donati C, Hiller NL, Tettelin H, et al. Structure and dynamics of the pan-genome of Streptococcus pneumoniae and closely related species. Genome Biology 2010; 11:107.
- Johnston C, Hinds J, Smith A, et al. Detection of large numbers of pneumococcal virulence genes in streptococci of the mitis group. J Clin Microbiol 2010; 48:2762–2769.
- Neu HC. The crisis in antibiotic-resistance. Science 1992; 257:1064–1073.
- Spellberg B, Powers JH, Brass EP, et al. Trends in antimicrobial drug development: Implications for the tuture. Clin Infect Dis 2004; 38:1279–1286.
- Alanis AJ. Resistance to antibiotics: Are we in the post antibiotic era? Arch Med Res 2005; 36:697–705.
- Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat Rev Drug Discovery 2008; 7:771–782.
- De M, Ghosh PS, Rotello VM. Applications of nanoparticles in biology. Adv Mater 2008; 20:4225–4241.
- Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for gram-negative bacteria. J Colloid Interface Sci 2004; 275:177-182.
- Giasuddin ASM, Jhuma KA, Haq AMM. Use of gold nanoparticles in diagnostics, surgery and medicine: A review. Bangladesh J Med Biochem 2012; 5:56–60.
- Misbahi A. A review on gold nanoparticles radio sensitization effect in radiation therapy of cancer. Rep Pract Oncol Radiother 2010; 15:176–180.
- Yugang SUN, Changhua AN. Shaped gold and silver nanoparticles. Front Mater Sci. 2011; 5:1–24.
- Zhang Z, Ross RD, Roeder RK. Preparation of functionalized gold nanoparticles as a targeted X-ray contrast agent for damaged bone tissue. Nanoscale 2010; 2:582–586.
- Capek I. Preparation and functionalization of gold nanoparticles. J Surf Sci Technol 2013; 29:1–18.
- Mahmoudpour A, Rahimi S, Sina M, et al. Isolation and identification of Enterococcus faecalis from necrotic root canals using multiplex PCR. J Oral Sci 2007; 49:221-227.
- Holt JG, Krieg NR, Sneath PHA. Bergy’s manual of determinative bacteriology. 9th Edn Williams &Wilkins 1994; 532-551.
- Barnard JP, Stinson MW. The alpha-hemolysin of Streptococcus gordonii is hydrogen peroxide. Infect Immun 1996; 64: 3853–3857.
- Harley JP, Prescott LM. Laboratory exercises in microbiology. 5th Edn The McGraw-Hill Companies 2000.
- Garcia-Garrote F, Cercenado E, Bouza E. Evaluation of a new system, VITEK 2, for Identification and antimicrobial susceptibility testing of Enterococci. J Clin Microbiol 2000; 38:2108–2111.
- Tan KH, Tajuddin HA, Johan MR. Feasibility study on the use of the seeding growth technique in producing a highly stable gold nanoparticle colloidal system. J Nanomat 2015; 2015:1-8.
- Chaudhari PR, Masurkar SA, Shidore VB, et al. Antimicrobial activity of extracellularly synthesized silver nanoparticles using Lactobacillus species obtained from vizylac capsule. J Appl Pharma Sci 2012; 2:25-29.
- Rajeshkumar S, Malarkodi C. In Vitro antibacterial activity and mechanism of silver nanoparticles against foodborne pathogens. Bioin Chem App 2014; 2014:1-10.
- Huang WC, Tsai PJ, Chen YC. Multifunctional Fe3O4 Au nanoeggs as photothermal agents for selective killing of nosocomial and antibiotic-resistant bacteria. Small 2009; 5:51-56.
- Liu Y, He L, Mustapha A, et al. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157: H7. J App Microbiol 2009; 107: 1193-1201.
- Nam JM, Thaxton CS, Mirkin CA. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science 2003; 301:1884–1886.
- Osterfeld SJ, Yub H, Gaster RS, et al. Nanoparticles: Alternatives against drug-resistant pathogenic microbes. Molecules 2016; 21:836.
- Gil-Tomas J. Lethal photosensitisation of Staphylococcus aureus using a toluidine blue Otio pronin-gold nanoparticles conjugate. J Mater Chem 2007; 17:3739–3746.
- Rai A, Prabhune A, Perry CC. Antibiotic mediated synthesis of gold nanoparticles with potent antimicrobial activity and their application in antimicrobial coatings. J Mater Chem 2010; 20:6789–6798.
- Yamanaka M, Hara K, Kudo J. 2005. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl Environ Microbiol 2005; 71:7589-7593.
- http://ljzx.cqrmhospital.com/upfiles/201601/20160112155335884.pdf
- Xiaoning Li, Robinson SM, Gupta A, et al. (2014) Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria ACS Nano 2014; 8:10682–10686.
- Senthilkumar S, Kashinath L, Ashok M, et al. Antibacterial properties and mechanism of gold nanoparticles obtained from pergularia daemia leaf extract. J Nanomed Res 2017; 6:146.
- Ghanavati Behbahan F, Salari M, Mousavi SR, et al. Antimicrobial activities of Gold nanoparticles against Salmonella typhimurium. Adv Herb Med 2016; 2:26-30.
Author Info
Hussein Jameel Abd Noor* and Basima GH Ali
Department of periodontics, College of Dentistry, University of Baghdad, IraqCitation: Hussein Jameel Abd Noor, Basima GH Ali, Potential Effect of Gold Nanoparticles against Streptococcus mitis (Primary Periodontal Colonizer), J Res Med Dent Sci, 2019, 7(6): 07-15.
Received: 03-Oct-2019 Accepted: 30-Oct-2019
