Case Report - (2022) Volume 10, Issue 9
Polymethylmethacrylate Skull Prosthesis: A Case Report
Shivani Sunil Mitta*, Sweta Kale Pisulkar and Chinmayee Dahihandekar
*Correspondence: Shivani Sunil Mitta, Department of Prosthodontics, Sharad Pawar Dental College and Hospital, Datta Meghe Institute of Medical Sciences (Deemed to be university) Sawangi (Meghe) Wardha, Maharashtra, India, Email:
Abstract
A successful surgical operation improves the patient's functional result as well as their survival. Cranioplasty is a procedure that improves the brain's underlying physiology, such as cerebral hemodynamics and metabolism, as well as its anatomical structure. This approach, however, does not allow for the replacement of the skull bone during surgery, leading in a significant skull deformity that may necessitate further cranioplasty. The following case report covers the use of a PMMA transplant to treat a cranial deformity. In cranial surgery, Polymethylmethacrylate (PMMA) is the most commonly used synthetic material. PMMA is a most biocompatible alloplastic and a flexible acrylic resin that has the strength and durability of actual bone tissue. The fabrication of the cranial prosthesis was divided into three stages: digital, prosthetic, and surgical.
Keywords
Cranioplasty, Polymethylmethacrylate, Synthetic material, Cranial prosthesisIntroduction
Trauma, infection, congenital deformities, disease, and tumors can all cause skull abnormalities and defects in individuals of all ages, and their surgical treatment can all lead to anomalies and malformations in the skull. Small defects concealed by dense soft tissue may not require surgery. Small defects concealed by dense soft tissue may not require surgery. Secondary reconstruction is required for other cranial deformities [1].
A successful surgical operation improves the patient's functional result as well as their survival. This approach, however, does not allow for the replacement of the skull bone during surgery, leading in a significant skull deformity that may necessitate further cranioplasty. Cranioplasty is commonly performed for aesthetic reasons or to protect brain tissue. A successful surgical operation improves the patient's functional result as well as their survival. This approach, however, does not allow for the replacement of the skull bone during surgery, leading in a significant skull deformity that may necessitate further cranioplasty. Cranioplasty is routinely performed for cosmetic reasons or to maintain the brain tissue under the surface. Cranioplasty is an operation that optimizes the underlying physiology of the brain, including cerebral hemodynamic and metabolism, as well as the anatomical reconstruction [2].
Biological and synthetic materials are the two primary kinds of materials used in cranial surgery. Autologous grafts, allografts, and xenografts are the different types of biological materials.
Synthetic bone transplants became popular because they had fewer infection, resorption, and reoperation risks; than bone auto grafts. Furthermore, the use of synthetic bone grafts has decreased operating times and resulted in greater aesthetic results because to the introduction of computer-based customization and three-dimensional printing. Metals, Polymethyl Methacrylate (PMMA), Hydroxyapatite, Titanium mesh, Alumina ceramics, and Polyetheretherketone (PEEK) are examples of synthetic materials [3].
PMMA is the most often utilized substance among them. The following case report covers the use of a PMMA transplant to treat a cranial deformity.
Case Presentation
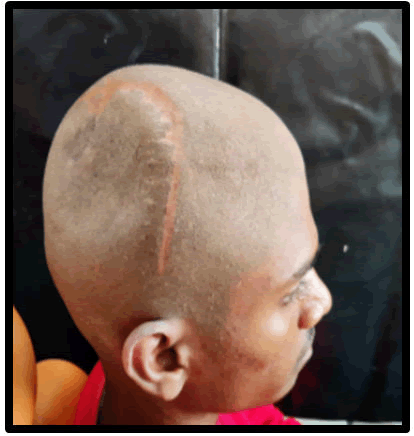
A 20 years old boy had a history of previous trauma, including an automobile accident that resulted in many fractures, along with the skull. The patient was sent to the department of prosthodontics for cranial prosthesis construction, with the primary complaint being a postoperative calvarial defect. Parts of the right parietal and temporal bones were affected. The defect was ten centimeter by six centimeter by six centimeters in dimension.
Figure 1: Postero-lateral view of defect area before reconstruction.
The fabrication of the cranial prosthesis was divided into three stages: digital, prosthetic, and surgical. The materials and techniques employed are depicted in the following phases:
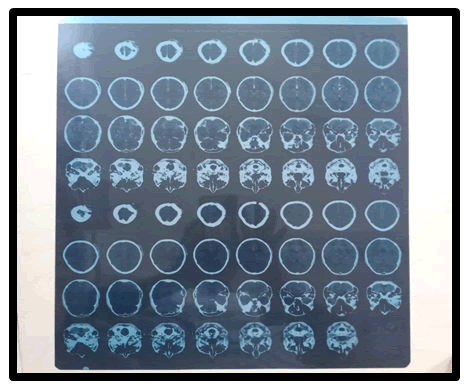
Digital phase: The procedure's feasibility is normally determined by a supine CT scan, however it might be difficult to evaluate if the surgery can be conducted safely. Although nine patients had a decompressive craniectomy during the research period, only six were able to get a brain CT before cranioplasty (first in a supine position, then in a lateral decubitus position with the operative side upward). The distance from the midline to the brain surface on CT scans was measured on the picture with the most brain bulging, and the bulging was estimated by comparing the image to the distance recorded on the contralateral side [8].
Figure 2: CT scan of the patient before operation.
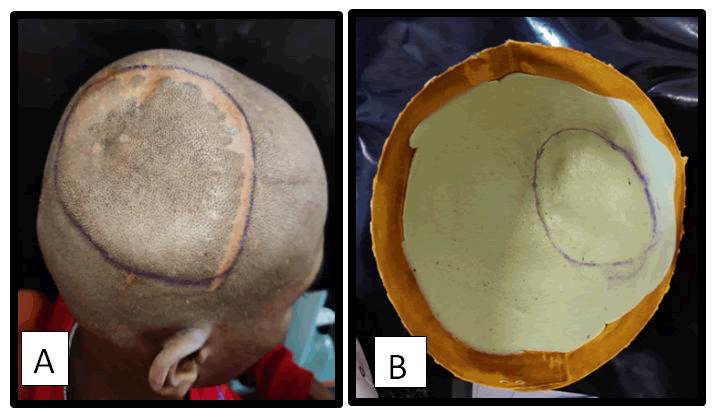
Prosthetic phase: Defect is delineated using haematoxylin pencil and Impressions recorded using irreversible hydrocolloid (i.e., Alginate).
Figure 3: A) Haematoxylin line delineate the defect; B) Alginate impression.
Figure 4: Fabrication of wax pattern-superior view.
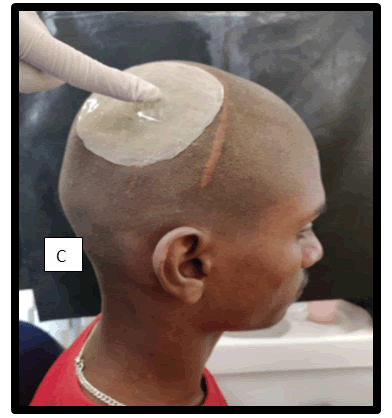
Material used for prosthesis: Heat cured Poly-Methyl Methacrylate (PPMMA) in conjunction with Bone cement layer.
Figure 5: Fabrication of prosthesis-view from the side.
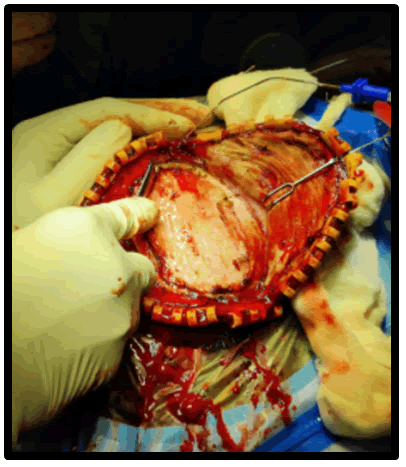
Surgical phase: The defect was delineated Flaps are raised.
Figure 6: Reflection of flap and placement of the prosthesis.
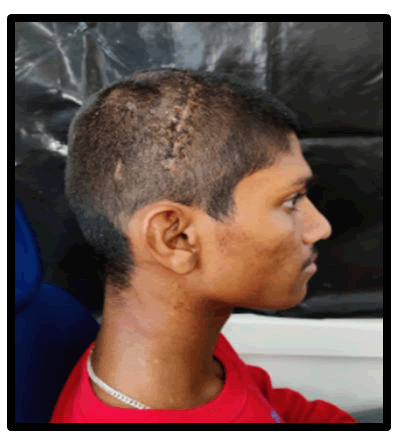
After one week after surgery, the patient was evaluated in the prosthodontics department, and the shape of the skull had significantly improved. The patient was delighted with the alleviation from psychological trauma and terrible headaches, as well as the fantastic visual results. After four months, the patient was re-evaluated and was found to be living a regular social life.
Figure 7: Post-operative of the patient lateral view.
Discussion
Cranioplasty, or the surgical repair of a skull defect, is one of the oldest known neurosurgical procedures, dating back to 3000 BC when the Paracas Indians of Peru used it to treat large cranial defects. Because of the high number of cranial injuries that occur in our contemporary age, the number of patients requiring cranioplasty has grown significantly in recent years [1]. The most widely approved therapy for a rise in intracranial pressure caused by severe injuries, cerebral infarcts or haemorrhage, tumors, and other causes is cranial decompression. Apart from that, the repair of these abnormalities improves the patient's hemodynamic, neurological, and psychosocial state of patient. A wide range of rehabilitating materials, including resin based, ceramics, and other noble metals, are available with metal or non-metal bases [6].
PMMA is a polymerized acrylic acid ester. In 1940, Zander became the first to use methyl methacrylate as a cranioplasty material. PMMA is one of the most bio compatible alloplastic polymers on the market today. Polymethylmethacrylate is an acrylic resin that can be moulded and has the same strength and durability as real bone tissue. According to testing, it's durable, radiolucent, non-irritating, and non-conductive [5]. PMMA implants cannot be punctured by developing bone tissue due to their lack of porosity; they interfere with osteoconduction and vascularization, do not interact with the surrounding tissue, and may be more prone to infection than autogenous/autoclaved bone cranioplasty. In terms of resistance and compression, it outperforms hydroxyapatite [6]. PMMA has been used as bone cement and has been demonstrated to be effective space filler with no resorption and to be stronger in compression and torsion tests than the neighbouring skull bone. There are two elements to PMMA: a powder polymer and a liquid monomer. The toxicity is proportional to the quantity of free monomer present in the chemical process, which is exothermic [7]. PMMA plates are also not suggested for children since they interfere with the development of the skull structure [4].
Conclusion
The size and location of the bone defect, as well as surgical expertise and the quality of nearby soft tissues, are all considered to influence the success of cranial bone restoration [4]. In the domain of craniofacial aesthetic surgery, plastic surgeons frequently do reconstructive procedures. The use of polymethylmethacrylate in adult craniofacial reconstructions has continued with positive results [5].
Authors Contribution
The authors recognize the invaluable assistance provided by the academics whose publications are mentioned and included in the manuscript's references. The authors also like to thank the authors, editors, and publishers of all of the articles, journals, and books that were utilized to review and discuss the literature for this study.
References
- Sharma A. Neuroprosthetic Rehabilitation of Acquired Skull Defect Int J Pros Res Den 2011; 1:65-70.
- Cheung LS, Tsai WC, Tseng LS, et al. Cranioplasty using polymethyl methacrylate prostheses. J Clin Neuro sci 2009; 16:56-63.
- Alkhaibary A, Alharbi A, Alnefaie N, et al. Cranioplasty: A Comprehensive Review of the History, Materials, Surgical Aspects and Complications. World Neurosurg 2020.
- Bruno Z, Nicola Z, Angela V, et al. Cranioplasty: Review of Material J Craniofacial Surg 2016; 27:2061–2072.
- Alkhaibary A, Alharbi A, Alnefaie N, et al. Cranioplasty: A Comprehensive Review of the History, Materials, Surgical Aspects and Complications. World Neurosurg 2020.
- Sweta P, Hetal P, Rohit M, et al. Encasing the Encephalon: Enhancing Psychosocial Rehabilitation. Int J Curr Res Rev 2020; 12:14.
- Rah DK. Art of replacing cranial bone defect. Yonsei Med J 2000; 41:6.
- Neuroprosthetic Rehabilitation of Acquired Skull Defect
Author Info
Shivani Sunil Mitta*, Sweta Kale Pisulkar and Chinmayee Dahihandekar
Department of Prosthodontics, Sharad Pawar Dental College and Hospital, Datta Meghe Institute of Medical Sciences (Deemed to be university) Sawangi (Meghe) Wardha, Maharashtra, IndiaReceived: 15-Jul-2022, Manuscript No. JRMDS-22-49607; , Pre QC No. JRMDS-22-49607; Editor assigned: 19-Jul-2022, Pre QC No. JRMDS-22-49607; Reviewed: 01-Aug-2022, QC No. JRMDS-22-49607; Revised: 15-Sep-2022, Manuscript No. JRMDS-22-49607; Published: 22-Sep-2022