Research Article - (2023) Volume 11, Issue 1
Phytochemical Screening and Antibacterial Effect of Stevia Rebaudiana (Bertoni) Alcoholic Leaves Extract on Streptococcus Oralis (Dental Plaqueâs Primary Colonizer)
Manar Ibrahim Ahmed* and Hadeel Mazin
*Correspondence: Manar Ibrahim Ahmed, Department of Periodontics, College of Dentistry, University of Baghdad, Baghdad, Iraq, Email:
Abstract
Background: Medicinal herbs have drawn the attention of many researchers due to their anti-microbial and health promoting properties, especially after the increase in the side effects of chemical mouthwashes. “Stevia raebaudiana bertoni”, a zero calorie sweetener native to South America, has been used by people for about 200 years to sweeten their drinks and treat some diseases. The anti-bacterial properties of stevia extracts were studied by many researchers, but none of them investigated the effect of stevia extract on Streptococcus oralis, a primary colonizer of dental plaque, which was addressed in this study.
Materials and methods: Supragingival plaque samples were cultured on MSA plate aerobically for 24 hours at 37°C. For identification and characterization of the isolated colonies, they were subjected to gram staining, hemolysis in Blood Agar Plate (BAP), catalase test, optochin sensitivity and Polymerase Chain Reaction (PCR) test. The alcoholic extract of stevia leaves was prepared by maceration with 70% ethanol in a ratio of 1:10 (gm plant/ml solvent). Four experiments were involved in this study, the first one concerning the sensitivity of S. oralis to the study extract starting from 16 mg/ml to 512 mg/ml, with 0.2% CHX as a positive control and distilled water as a negative one. While the second experiment involved the determination of MIC. The third one involved the determination of MBC, whereas the fourth experiment was done to determine some of the extract active ingredients by using High Performance Liquid Chromatography (HPLC) analysis.
Results: The diameter of inhibition zone for S. oralis increased with increasing the concentration of alcoholic extract of Stevia Rebaudiana bertoni and it is highest in chlorohexidine with significant difference (p<0.05). The MIC and MBC were 16 mg/ml and 32 mg/ml respectively. HPLC analysis revealed the presence of phytochemicals with antimicrobial activity including: Dihydroxy streptomycin: 0.86 ppm, rutin: 28.1 ppm, quercetin: 22.4 ppm and apigenin: 4.45 ppm.
Conclusions: The alcoholic extract of Stevia rebaudiana bertoni leaves had good antibacterial activity against S. oralis. 0.2% CHX demonstrated better antibacterial activity than the study extract, 512 mg/ml of stevia extract had comparable effect to 0.2% CHX. HPLC analysis of Stevia rebaudiana bertoni alcoholic leaves extract revealed the presence of many active antibacterial constituents.
Keywords
Stevia rebaudiana bertoni, Antibacterial effect, S. oralis, High Performance Liquid Chromatography (HPLC), Dihydroxy streptomycin
Introduction
The human oral cavity is one of the most dynamic habitats for many bacterial species, where they compete fiercely to form multispecies biofilm structures. Dental plaque is an adherent bacterial film that is the primary pathological agent in periodontal diseases and dental caries. Pioneer microorganisms adhere to the pellicle, proliferate and form colonies, following that, secondary colonizers interact physiologically and physically, resulting in maturation of the dental plaque [1]. Inter bacterial binding to streptococci has major implications for the development of periodontal diseases. Many co adhering organisms are potential periodontal pathogens e.g. (Porphyromons gingivalis, B. forsythus and Treponema denticola). The fimbriae of P. gingivalis are able to bind to several streptococcal species, including Streptococcus oralis, S. sanguinis, S. mitis and S. parasanguinis [2]. Furthermore, it has been shown to localize on streptococcal rich plaque when introduced into the mouths of human volunteers [3]. On the other hand, later researches discovered that the gram positive 'early oral colonizers,' such as Streptococcus oralis, S. gordonii, S. sanguinis and Actinomyces naeslundii, co-aggregate with F. nucleatum's arginine inhabitable adhesion RadD [4].
There has been a constant increase in the search of natural anti-microbial agent which can be obtaining from green field instead of the chemical substances. Chlorhexidine is regarded as the “gold standard” antiplaque treatment and is particularly effective against gingivitis and widely used as an adjunct treatment for periodontitis but it unfortunately has several side effects including tooth and some restorations staining, unpleasant taste, sloughing of oral mucosa and supra gingival calculus formation enhancement [5].
The bioactive properties of medicinal plants have been target of an increasing interest. Stevia rebaudiana bertoni is a South American plant belongs to Asteraceae family; it is a natural sweetener nearly 300 times sweeter than sucrose and with the advantage of being non caloric and avoiding high blood sugar levels [6]. Many chemical constituents present in Stevia exert prominent biological effects, among them antioxidant, anti-diabetic (antihyperglycemic, insulin tropic and glucagonostatic), antimicrobial, antiplatelet, anti-cariogenic and antitumor properties [7,8]. de slavutzy conclude in his study that Stevia can act as anti-cariogenic and anti-gingivitis herb. It has the ability to inhibit the growth of certain bacteria and this explains the traditional use in the treatment of wounds, sores and gum diseases [9]. Furthermore, it has anti-inflammatory and anti-plaque effect [10].
High Performance Liquid Chromatography (HPLC) is a popular separative and analytical method which is ideal for separating a variety of bio chemicals, foods, heavy industrial chemicals and pharmaceuticals; it has many advantages over traditional methods such as: Speed of analysis, ease of quantification, reliability and automation. Because lower temperatures can be used and there are two competing phases (mobile and stationary) in HPLC versus one phase (the stationary phase) in Gas Chromatography (GC), HPLC can frequently achieve separations that GC cannot. Furthermore, there are many selective detectors available for use in HPLC, so a complete separation on the column is not required and a detector can be chosen to monitor only the species of interest [11].
Studies have been reported the antimicrobial effect of Stevia extracts on fungi and various bacteria. Hardly no studies have been reported the antibacterial activity of Stevia rebaudiana bertoni extract on S. oralis.
Materials and Methods
Extraction of Stevia rebaurdiana bertoni leaves
Stevia leaves were obtained from and extracted in medical and aromatic plants research unit, college of agricultural engineering sciences, University of Baghdad. The alcoholic extract was prepared by maceration [12]. 100 gm powder of Stevia leaves were dissolved in 1000 ml of 70% ethanol concentration (conc.) with a ratio of 1:10 (gm plant/ml solvent). The filtered alcoholic extract was spray dried to obtain alcoholic extract powder using laboratory mini spray dryer. The required concentrations were accordingly and sterile distilled water was used as a solvent.
S. oralis isolation and identification
The study was approved by the medical ethics committee, college of dentistry/University of Baghdad. Supra gingival plaque samples were taken from people (males and females) with a sterilized gracy curette; their approval was obtained prior to taking samples. Inclusion criteria included: Patients should not use antibiotics or mouth rinse within at least one month before the study. Each sample was immediately transferred to a sterile eppendrof tube containing 1.5 ml brain heart infusion broth [13].
For the identification and characterization of the isolated colonies, they were subjected to gram stain, hemolysis in Blood Agar Plate (BAP), catalase test, optochin sensitivity and Polymerase Chain Reaction (PCR) [14-18].
Polymerase Chain Reaction (PCR)
Genomic DNA was isolated from bacterial growth according to the protocol of “ABIO pure TM genomic DNA”. Quantus fluorometer was used to detect the concentration of the extracted DNA. For 1 μl of DNA, 199 μl of diluted quantifluor dye was mixed. After 5 min incubation at room temperature, DNA concentration values were detected. Primers were supplied by macrogen company in a lyophilized form, S. oralis forward: 5`-TCCCGGTCAGCAAACTCCAGCC 3`, S. oralis reward: 5`-GCAACCTTTGGATTTGCAAC-3`. Annealing temperature: 66Ë?C, Product size (Pb): 374.
Lyophilized primers were dissolved in 300 μl of nuclease free water to give a final concentration of 100 pmol/μl as a stock solution. A working solution of the primer was prepared by adding 10 μl of primer stock solution (stored at freezer -20°C) to 90 μl of nuclease free water to obtain a working primer solution of 10 pmol/μl. For each sample, the following component were calculated according to manufacturer’s instructions and mixed in a tube to achieve a total volume of 20 μl: 10 μl master mix, 1 μl of 10 pmol/μl F primer, 1 μl of 10 pmol/μl R primer, 6 μl of nuclease free water and 2 μl of 22 ng/μl of sample DNA. According to manufacturer’s instructions, the calculated tubes of PCR components were applied in the PCR device for the amplification of the accused DNA sample, the reaction volume/run was 20, number of PCR cycles were 30. After DNA amplification, agarose gel electrophoresis was adopted to confirm the presence of S. oralis DNA. The ethidium bromide stained bands in gel were visualized using gel imaging system.
Prepartion of S. oralis suspension was done by direct colony suspension method according to cavalieri follows by an inoculating loop, isolated colonies were picked up from the agar plate (not older than 18-24 hours) and suspended in 1 ml Mueller Hinton broth (MHA) then vortexes and adjusted by absorbance micro plate reader to an absorbance of 0.600 at 600 nm which is corresponds to 0.5 McFarland standard (equivalent to 1 X 108 cells/ml) [19].
Testing the sensitivity of Streptococcus oralis to alcoholic Stevia rebaudiana bertoni leaves extract
Agar well diffusion method was applied to study the antibacterial effects of different concentrations of alcoholic Stevia extract (16 mg/ml, 32 mg/ml, 64 mg/ml, 128 mg/ml, 256 mg/ml and 512 mg/ml) on Mueller Hinton agar.
This test was done according to cavalieri follows two wells of equal depth and size (6 mm in diameter) were made in each agar plate using Pasteur pipette under aseptic conditions. Each well filled with 50 μl of each concentration. Non-alcoholic 0.2% chlorhexidine gluconate was used as positive control and sterile distilled water was the negative one. The plates were incubated aerobically at 37Ë?C for 24 hours. Using a ruler, the diameter of the inhibition zone across each well was measured in millimeters.
Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
The MIC experiment was done by two fold serial broth micro dilution. 100 μl of BHI broth was added into each nine wells of two rows, labeled W1 (well 1) to W9, in a 96 well micro titer plate. 100 μl of stevia extract with a concentration of 512 mg/ml was added into each W1 of the respective row and then two fold serial dilutions was done from W1 through W9. W10 of the first row contained 100 μl broth and 100 μl of 0.2% CHX while W11 and W12 contained 100 μl Mueller Hinton Broth (MHB) only, the entire first row was considered to be a blank row (without bacteria). All wells of the second row were inoculated with 100 μl of bacterial suspension. W10 of the second row was contained 100 μl of 0.2% CHX in broth and 100 μl bacterial suspension and it was considered the positive control, while W11, W12 was contained 100 μl MHB and 100 μl bacterial suspension and these wells were considered to be the negative control. The micro titer plate covered and incubated aerobically overnight at 37Ë?C. Following incubation, the wells were examined for turbidity and checked by an “absorbance micro plate reader” adjusted to (600 nm) wave length.
For the determination of MBC, an aliquots of 50 μl from the MIC well and from the well proceeded it were seeded on Brain Heart Agar (BHA) plates and incubated for 24 h at 37°C. Then the agar plates were observed for the presence or absence of bacteria [20].
High Performance Liquid Chromatography (HPLC) test for identifying and quantifying some of the active constituents in alcoholic Stevia rebaudiana leaves extract
This test was done in ministry of science and technology, department of material research according to Gini and Jeya Jothi with slight modification [21].
Stevia alcoholic extract was prepared for HPLC test to detect and quantitate each of: Streptomycin, rutin, quercetin and apigenin. The standard concentration for all of them was 5 ppm except for streptomycin it was 1 ppm. 1 gm of the sample (Stevia rebaudiana alcoholic extract) dissolved in 5 ml acetonitrile (HPLC grade) in a glass jar, mixed and closed by paraffin wax, the jar vortexed and putted in ultrasonic path at 35Ë?C for 15 min. The solution was filtered by 0.45 micro filter, 20 μl of the sample was injected in HPLC binary pump device at the following conditions: Mobile phases: A-acetonitrile: 0.5% formic acid=(70:30 v/v). B-acetonitrile: 0.5% formic acid=(30:70 v/v). Time (min): 0.01, 4, 8, 12, 15, 20. Gradient program (conc B): 80%, 80%, 90%, 90%, 80% stop. Flow rate: 0.5 ml/min. Detector: UV-VIS at 280 nm. Column stationary phase: ODS-Câ?â?? (150 x 4.6 I.D) mm, 5 μm particle size. Column temperature: 25Ë?C.
The concentration of the sample was calculated by the following formula:
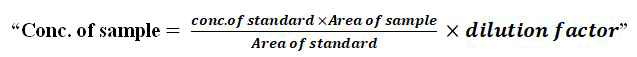
Statistical analysis
Data description, analysis and presentation were performed using minimum, maximum, mean, Standard Deviation (SD), Standard Error (SE), Shapiro Wilk test, Levene test and One Way Analysis of Variance (ANOVA) by using Statistical Package for social Science (SPSS version 22, Chicago, Illionis, USA). Not significant P>0.05, significant P<0.05.
Results
Identification of Streptococci
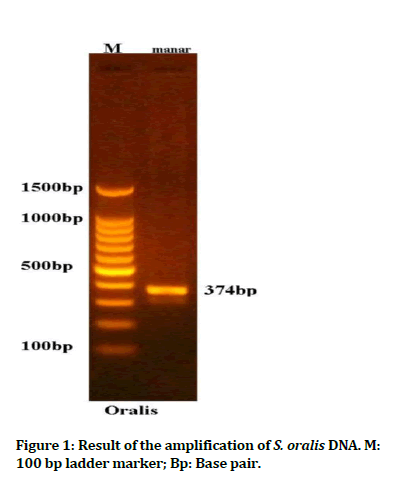
Colonies of S. oralis appeared spherical in shape with shiny dark blue color, smooth raised convex surface and easily removed from the agar surface. Biochemical results confirmed that the study bacterium is a gram positive, alpha hemolytic, optochin resistant and catalase negative streptococci. PCR result confirmed the identification of S. oralis (Figure 1).
Figure 1: Result of the amplification of S. oralis DNA. M: 100 bp ladder marker; Bp: Base pair.
Results of the experiments
Findings from the statistical analysis show that the diameter of inhibition zone for S. oralis increased with increasing the concentration of alcoholic extract of Stevia rebaudiana bertoni and it is highest in chlorohexidine with significant difference (p<0.05), further more using multiple comparisons between groups by Tukey's HSD each group when compared against other group and with chlorohexidine, the result showed significance difference (p<0.05). The results are shown in Tables 1-3. The MIC and MBC of the alcoholic Stevia rebaudiana bertoni extract against S. oralis were 16 mg/ml and 32 mg/ml respectively, 0.2% CHX (0.1% in broth) revealed bacteriostatic effect.
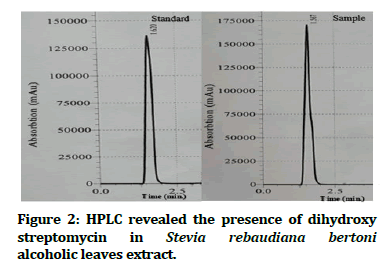
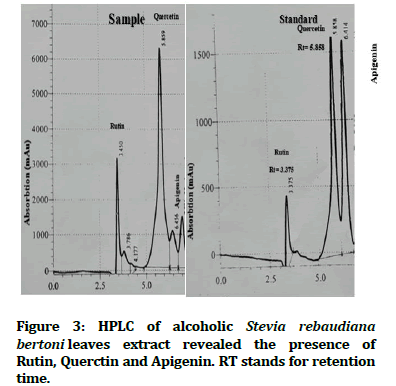
High performance liquid chromatography revealed the presence of active antimicrobial phytochemicals with the following concentrations: Dihydroxy streptomycin 0.86 ppm (Figure 2); rutin 28.1 ppm, quercetin 22.4 ppm and apigenin 4.45 ppm (Figure 3).
Figure 2:HPLC revealed the presence of dihydroxy streptomycin in Stevia rebaudiana bertoni alcoholic leaves extract.
Figure 3:HPLC of alcoholic Stevia rebaudiana bertoni leaves extract revealed the presence of Rutin, Querctin and Apigenin. RT stands for retention time.
| Groups (mg/ml) | Mean | ± SD | ± SE | Minimum | Maximum |
|---|---|---|---|---|---|
| 16 | 11.3 | 0.447 | 0.2 | 11 | 12 |
| 32 | 13.7 | 0.447 | 0.2 | 13 | 14 |
| 64 | 16.3 | 0.447 | 0.2 | 16 | 17 |
| 128 | 18.5 | 0.5 | 0.224 | 18 | 19 |
| 256 | 20.9 | 0.652 | 0.292 | 20 | 21.5 |
| 512 | 24.6 | 0.418 | 0.187 | 24 | 25 |
| CHX | 26.7 | 0.447 | 0.2 | 26 | 27 |
| Levene test=0.437, p value=0.848 NS | |||||
Table 1: Descriptive statistics of diameter of inhibition zone for S. oralis among groups.
| ANOVA | |||||
|---|---|---|---|---|---|
| Sum of squares | Df | Mean square | F | P value | |
| Between groups | 945.186 | 6 | 157.531 | 668.313 | 0.000 Sig |
| Within groups | 6.6 | 28 | 0.236 | ||
| Total | 951.786 | 34 | |||
| Sig=Significant at p<0.05. | |||||
Table 2: Statistical test of diameter of inhibition zone for S. oralis among groups using One Way Analysis of Variance (ANOVA).
| Groups (mg/mL) | Mean difference | P value | ||
|---|---|---|---|---|
| 16 | 32 | -2.4 | 0.00000 | Sig |
| 64 | -5 | 0.00000 | ||
| 128 | -7.2 | 0.00000 | ||
| 256 | -9.6 | 0.00000 | ||
| 512 | -13.3 | 0.00000 | ||
| CHX | -15.4 | 0.00000 | ||
| 32 | 64 | -2.6 | 0.00000 | |
| 128 | -4.8 | 0.00000 | ||
| 256 | -7.2 | 0.00000 | ||
| 512 | -10.9 | 0.00000 | ||
| CHX | -13 | 0.00000 | ||
| 64 | 128 | -2.2 | 0.00000 | |
| 256 | -4.6 | 0.00000 | ||
| 512 | -8.3 | 0.00000 | ||
| CHX | -10.4 | 0.00000 | ||
| 128 | 256 | -2.4 | 0.00000 | |
| 512 | -6.1 | 0.00000 | ||
| CHX | -8.2 | 0.00000 | ||
| 256 | 512 | -3.7 | 0.00000 | |
| CHX | -5.8 | 0.00000 | ||
| 512 | CHX | -2.1 | 0.00000 | |
| Sig=Significant at p<0.05. | ||||
Table 3: Multiple comparisons of diameter of inhibition zone for S. Oralis between groups using tukey HSD.
Discussion
Medicinal herbs have been used for centuries to treat human diseases due to their health promoting properties. It has been estimated that 80% of the population still use them as complementary and alternative medicines because of its safety and effectively, as a result, scientists began to look into the biological activities of medicinal plants especially after the emergence of microbial resistance and side effects of the chemical drugs [22,23].
Stevia rebaudiana bertoni has many nutritional and therapeutic properties and it is considered a potential drug candidates. Antimicrobial activity screening studies of Stevia extracts showed enough activity to inhibit the growth of pathogenic bacteria like E. coli, Salmonella typhi, Enterococcus faecalis, Bacillus subtilis, Pseudomonas aeruginosa, Proteus mirabilis, Vibrio cholerae, Staphylococcus aureus, Aeromonas hydrophila, Streptococcus mutuns and Lactobacillus. Also it has anticariogenic, anti-gingivitis, anti-plaque, anti-inflammatory and healing properties [24]. For the previously mentioned properties and because there is no study on the antibacterial activity of Stevia on S. oralis in comparison to 0.2% CHX, the present study was conducted and may be considered as the first report.
The present study found a concentration dependent antibacterial activity of the alcoholic Stevia extract against S. oralis in that the diameter of inhibition zone increases as the concentration of the extract increases, this could be attributed to the increase in the amount of the dissolved active ingredients of the extract.
The alcoholic Stevia extract showed promising results; the mean value of Inhibition zone of the alcoholic Stevia extract at 512 mg/ml against S. oralis (24.6 mm) was somewhat comparable to that of 0.2% CHX against S. oralis (26.7 mm), it could be considered a satisfactory result when we weight the benefits of Stevia against the side effects of CHX. The MIC and MBC of alcoholic Stevia extract against the study bacteria were found to be 16 mg/ml and 32 mg/ml respectively, these results indicate that Stevia extract exert bactericidal action according to levison as mentioned in mogana in which stated that the agent exhibit bactericidal action if the mbc/mic ratio ≤ 4 otherwise it is considered a bacteriostatic drug [25].
The precise antimicrobial action of Stevia is unknown, but it could be linked to the presence of many antimicrobial components such as: Diterpene glycosides, flavonoids, tannins, saponins, dihydrodeoxy streptomycin and polyphenols [26-30]. And the synergism between the phytochemical compounds in the extract. It is more difficult to develop bacterial resistance through genetic mutations triggered by external stimuli because of the association of numerous different molecules in plant extracts [31].
High performance liquid chromatography analysis of alcoholic Stevia extract revealed the presence of many active constituents including: Dihydroxy streptomycin, apigenin, quercetin and rutin. Dihydroxy streptomycin found with a concentration of (0.86 ppm) in the study extract. It exerts its antibacterial action by inhibiting the bacterial proteins biosynthesis [32]. While rutin occur at a relatively high concentration (28.1 ppm), which exhibits a good antibacterial property, it is thought to alter glucose and amino acid metabolism, protein synthesis and cell wall integrity [33]. It has been reported that rutin exhibits antibacterial action against Staphylococcus aureus, Eschericia coli and Staphylococcus glurance [34]. Also against Klebsiella pneumonia and Pseudomonas aeruginosa [35].
Quercetin is a flavonoid with anti-bacterial properties, it is found in the study extract at a concentration of (22.4 ppm). According to Siriwong quercetin has a synergistic impact with amoxicillin against Amoxicillin Resistant Staphylococcus Epidermidis (ARSE) through four mechanisms: Inhibiting peptidoglycan production and β- lactamases activity, increasing cell membrane permeability and protein amide I and II and decreasing fatty acids in bacterial cells. It also demonstrated strong antibacterial efficacy against a broad spectrum pathogens responsible for hospital and community acquired infections by inhibiting bacterial DNA gyrase and topoisomerase IV [37].
Apigenin is a flavone occurs naturally in the medicinal plants, it is found in the study extract at a concentration of (4.45 ppm). Kim implies that apigenin causes bacterial death via activating cellular oxidative pathways that are reliant on Reactive Nitrogen Species (RNS) and Reactive Oxygen Species (ROS) generation and accumulation [38].
Conclusion
The ethanolic Stevia rebaudiana bertoni leaves extract has good antibacterial effect against S. oralis starting from 16 mg/ml to 512 mg/ml but 0.2% CHX demonstrated better antibacterial activity. HPLC analysis revealed the presence of many active antibacterial constituents. Further studies concerning its antimicrobial effect on periodontal pathogens are required to validate its usage as supportive oral health measure.
Conflict of Interest
No conflicts of the interest
Acknowledgements
Thanks to everyone who supported me to complete this research.
References
- Rosan B, Lamont RJ. Dental plaque formation. Microbes Infect 2000; 2:1599–1607. [Crossref][Googlescholar][Indexed]
- Nagata H, Murakami Y, Inoshita E, et al. Inhibitory effect of human plasma and saliva on coaggregation between Bacteroides gingivalis and Streptococcus mitis. J Dent Res 1990; 69:1476-1479. [Crossref][Googlescholar][Indexed]
- Slots J, Gibbons RJ. Attachment of Bacteroides meianinogenicus subsp. asaccliarolylicus to oral surfaces and its possible role in the colonization of the tooth and of periodontal pockets. Infect Immnun 1978; 19:254-264. [Crossref][Indexed]
- Kaplan CW, Lux R, Haake SK, et al. The Fusobacterium nucleatum outer membrane protein RadD is an arginine inhabitable adhesion required for inter species adherence and the structured architecture of multispecies biofilm. Mol Microbiol 2009; 71:35–47. [Crossref][Googlescholar][Indexed]
- Addy M, Moran J. Mechanisms of stain formation on teeth, in particular associated with metal ions and antiseptics. Adv Dent Res 1995; 9:450-456. [Crossref][Googlescholar][Indexed]
- Lemus Mondaca R, Vega Galvez A, Rojas P, et al. Antioxidant, antimicrobial and anti-inflammatory potential of Stevia rebaudiana leaves: Effect of different drying methods. J Appl Res Med Aromat Plants 2018; 11:37-46. [Crossref][Googlescholar][Indexed]
- Reshu G, Vidushi Y, Manvi R. A review on importance of natural sweetener, a zero caloric plant stevia having medicinal and commercial importance. Int J Food Nutr Sci 2014; 3:90-94.
- Salehi B, Lopez MD, Martinez Lopez S. Stevia rebaudiana bertoni bioactive effects: From in vivo to clinical trials towards future therapeutic approaches. Phytother Res 2019; 33:2904-2917. [Crossref][Googlescholar][Indexed]
- de Slavutzky SMB. Stevia and sucrose effect on plaque formation. J Fur Verbraucherschutz Leb 2010; 5:213-216. [Crossref][Googlescholar][Indexed]
- Boonkaewwan C, Toskulkao C, Vongsakul M. Anti-inflammatory and immunomodulatory activities of stevio side and its metabolite steviol on THP-1 cells. J Agric Food Chem 2006; 54:785-789. [Crossref][Googlescholar][Indexed]
- Lewis NG. High Performance Liquid Chromatography (HPLC). In methods in lignin chemistry. Springer, Berlin, Heidelberg, 1992; 549-567.
- Seidel V. Initial and bulk extraction of natural products isolation. Methods Mol Biol 2012; 864:27-41. [Crossref][Googlescholar][Indexed]
- Abd Noor HJ. Potential effect of gold nanoparticles against Streptococcus mitis and Streptococcus oralis (primary periodontal colonizers). Doctoral dissertation, University of Baghdad, 2020.
- Koneman EW, Allen SD, Janda WM, et al. Diagnostic microbiology. The non-fermentative gram negative bacilli, philedelphia: Lippincott Raven Publishers, 4th Edition, USA, 1997. [Googlescholar][Indexed]
- Buxton R. Blood agar plates and hemolysis protocols. ASM Micro Library, 2013. [Googlescholar]
- Reiner K. Catalases test protocol. ASM Micro Library, 2013. [Googlescholar]
- Cavalieri S, Harbeck R, Mccarter Y, et al. Manual of antimicrobial susceptibility testing. American society for microbiology. Pan American Health Organization: Washington, DC, USA, 2005.
- Hoshino T, Kawaguchi M, Shimizu N, et al. PCR detection and identification of oral streptococci in saliva samples using GTF genes. Diagn Microbiol Infect Dis 2004; 48:195-199. [Crossref][Googlescholar][Indexed]
- Abdulbaqia HR, Himratul Aznita WH, Baharuddin NA. Anti-plaque effect of a synergistic combination of green tea and Salvadora persica L. against primary colonizers of dental plaque. Arch oral biol 2016; 70:117-124. [Crossref][Googlescholar][Indexed]
- Parvekar P, Palaskar J, Metgud S, et al. The Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater Investig Dent 2020; 7:105-109. [Crossref][Googlescholar][Indexed]
- Gini TG, Jeya Jothi G. Column chromatography and HPLC analysis of phenolic compounds in the fractions of Salvinia molesta Mitchell. Egypt J Basic Appl Sci 2018; 5:197-203. [Crossref][Googlescholar][Indexed]
- Alotaibi G, Irfan UM, Ali S. An in vitro study to test antimicrobial effects of commiphora myrrha in comparison to biocides. Eur J Pharm Med Res 2017; 4:449-453.
- Wendakoon C, Calderon P, Gagnon D. Evaluation of selected medicinal plants extracted in different ethanol concentrations for antibacterial activity against human pathogens. J Med Active Plants 2012; 1:60-68. [Crossref][Googlescholar][Indexed]
- Contreras M. Anti-cariogenic properties and effects on periodontal structures of Stevia rebaudiana bertoni: Narrative review. J Oral Res 2013; 2:158-166. [Googlescholar][Indexed]
- Mogana R, Adhikari A, Tzar MN, et al. Antibacterial activities of the extracts, fractions and isolated compounds from Canarium patentinervium Miq. against bacterial clinical isolates. BMC Complement Med Ther 2020; 20:55. [Crossref][Googlescholar][Indexed]
- Brambilla E, Cagetti MG, Ionescu A, et al. An in vitro and in vivo comparison of the effect of Stevia rebaudiana extracts on different caries related variables: A randomized controlled trial pilot study. Caries Res 2014; 48:19-23. [Crossref][Googlescholar][Indexed]
- Fasiha A, Shahid B, Faiz-Ul-Hassan S. Nutritional and medicinal properties of Stevia rebaudiana. Curre Res Diabetes Obes J 2020; 13:555867. [Crossref]
- Shakya AK. Medicinal plants: Future source of new drugs. Int J Herbal Med 2016; 4:59-64. [Googlescholar]
- Raut D, Aruna K. Antimicrobial activity of Stevia rebaudiana against antibiotic resistant ESBL producing uropathogens and evaluation of its antioxidant activity. Int J Adv Res Biol Sci 2017; 4:110-118. [Crossref]
- Lemus-Mondaca R, Vega Galvez A, Rojas P, et al. Antioxidant, antimicrobial and anti-inflammatory potential of Stevia rebaudiana leaves: Effect of different drying methods. J Appl Res Med Aromat Plants 2018; 11:37-46. [Crossref][Googlescholar][Indexed]
- Moussaoui F, Alaoui T. Evaluation of antibacterial activity and synergistic effect between antibiotic and the essential oils of some medicinal plants. Asian Pac J Trop Biomed 2016; 6:32-37. [Crossref][Googlescholar][Indexed]
- Casciano DA. Study of the mode of action of streptomycin and dihydro streptomycin on mammalian cells. Purdue University, 1971. [Googlescholar][Indexed]
- Mazzeo MF, Lippolis R, Sorrentino A, et al. Lactobacillus acidophilus rutin interplay investigated by proteomics. Plos one 2015; 10:e0142376. [Crossref][Googlescholar][Indexed]
- Himesh S, Jitender M, Singhai A, et al. Antimicrobial and anti-inflammatory activity of the hydrogels containing rutin deliveryn. Asian J Chem 2013; 25:8371-8373.
- Singh M, Govindarajan R, Rawat AKS, et al. Antimicrobial flavonoid rutin from Pteris vittata L. against pathogenic gastrointestinal micro flora. Am Fern J 2008; 98:98–103.[Googlescholar][Indexed]
- Siriwong S, Teethaisong Y, Thamanu K, et al. 2016. The synergy and mode of action of quercetin plus amoxicillin against amoxicillin resistant Staphylococcus epidermidis. BMC Pharmacol Toxicol 2016; 17:1-14. [Crossref][Googlescholar][Indexed]
- Hossion AM, Zamami Y, Kandahary RK, et al. Quercetin diacylglycoside analogues showing dual inhibition of DNA gyrase and topoisomerase IV as novel antibacterial agents. J Med Chem 2011; 54:3686-3703. [Crossref][Googlescholar][Indexed]
- Kim S, Woo ER, Lee DG. Apigenin promotes antibacterial activity via regulation of nitric oxide and superoxide anion production. J Basic Microbiol 2000; 60:862-872. [Crossref][Googlescholar][Indexed]
Author Info
Manar Ibrahim Ahmed* and Hadeel Mazin
Department of Periodontics, College of Dentistry, University of Baghdad, Baghdad, IraqCitation: Manar Ibrahim Ahmed, Hadeel Mazin, Phytochemical Screening and Antibacterial Effect of Stevia rebaudiana (Bertoni) Alcoholic Leaves Extract on Streptococcus oralis (Dental Plaque’s Primary Colonizer, J Res Med Dent Sci, 2023, 11 (01):095-102
Received: 07-Nov-2022, Manuscript No. JRMDS-22-65196; , Pre QC No. JRMDS-22-65196 (PQ); Editor assigned: 11-Nov-2022, Pre QC No. JRMDS-22-65196 (PQ); Reviewed: 24-Nov-2022, QC No. JRMDS-22-65196; Revised: 06-Jan-2023, Manuscript No. JRMDS-22-65196 (R); Published: 13-Jan-2023