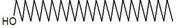Research Article - (2023) Volume 11, Issue 1
Phytochemical Investigation and Pharmacological Activity of Petroleum Ether Fraction of Solidago canadensis L. against COVID-19 Activity
Hayder T Hasan* and Enas J Kadhim
*Correspondence: Hayder T Hasan, Department of Pharmacognosy and Medicinal Plants, College of Pharmacy, University of Baghdad, Baghdad, Iraq, Email:
Abstract
Coronavirus COVID-19 is a dangerous viral disease caused by a new Coronavirus (SARS-CoV-2), medicinal plants have been used in the treatment of a large number of diseases since ancient times, Solidago canadensis L. belongs to the asteraceae family, which includes approximately one hundred species, widely distributed in North American wildflowers and greater than a dozen species inhabiting South America, Europe and Asia. It has been cultivated in Iraq in the provinces of Babylon and Diyala recently. 750 gm has been extracted with ethanol 85% then fractionated after drying with petroleum ether, then GC/MS analysis by Shimadzu GCMS-QP2020 show the detection of 60 compounds. The results showed the presence of a variety of compounds; fatty acids like palmitic acid and linoleic acid methyl ester. Terpenes and terpenoids like α-pinene, bornyl acetate, myrcene, verbenol, terpinolene, germacrene-D, andrographolide, caryophyllene oxide, as well as vitamin E.
High throughput Cytopathic Effect (CPE) inhibitory tests for the COVID-19 virus on vero cells were developed in investigating new potential antiviral agents. A crystal violet uptake assay was used to measure the cytotoxic and antiviral effects.
The identified compounds in the petroleum ether fraction showed high antiviral activities against COVID-19 with Selective Index (SI)=Estimated CC50/estimated IC50=2,283.9, consequently, the tested samples are good candidates for further experiments as anti-COVID-19.
Keywords
Solidago canadensis L, Terpenes, Terpenoids, Germacrene, Garyophyllene, Anti-COVID-19
Introduction
Coronaviruses are cytoplasmic positive stranded RNA viruses with an envelope [1]. When the Severe Acute Respiratory Syndrome (SARS) outbreak shocked the world in 2002–2003, Coronaviruses garnered a lot of attention, it infect a wide range of animals and birds, producing respiratory and gastrointestinal infections, as well as hepatitis and neurologic disease in certain rare cases [2].
Human Coronaviruses (HCoVs) have been found in seven different strains; HCoV-229E, HCoV-OC43, HCoVNL63 and HCoV-HKU1 are four non-zoonotic viruses that produce widespread epidemics of upper respiratory tract illnesses, mostly in the winter [3].
According to WHO, there have been 258,164,425 confirmed cases of COVID-19 worldwide, with 5,166,192 fatalities as of November 21, 2021 [4].
Natural compounds have always piqued scientists' interest when it comes to drug development; traditional healers in many civilizations believe medicinal plants to be extremely effective in preventing and treating a variety of illnesses and disorders. Various traditional herbal treatments have been employed since the COVID-19 pandemic outbreak and have resulted in desirable health results among COVID-19 patients [5].
Medicinal plants have been used in the treatment of a large number of diseases since ancient times [6]. The human use of plants as medications has been dated at least in the middle paleolithic from a thousand years ago [7].
The potential for phytochemicals like terpenes to be used as effective antiviral medicines has recently gotten a lot of attention, especially because these compounds are plentiful and have minimal toxicity and cost [8].
The asteraceae family consists of about 25,000 species, of about 1,500 genres, they are incredibly very diverse subordinate plants, herbs, sub-shrubs or trees and 98% are composed of small plants. The most frequently studied species are Solidago virgaurea L, Solidago gigantea L, Solidago canadensis L. and Solidago chilensis [9].
Genus Solidago L. belongs to the Asteraceae family, which includes approximately one hundred species; it is widely distributed in North American wildflowers and greater than a dozen species inhabiting South America, Europe and Asia [10].
Solidago canadensis has been cultivated in Iraq in the provinces of Babylon and Diyala recently.
S. canadensis (S. altissima), tall goldenrod, it is an erect plant 1 m–2.5 m with a range of about 1 m.
The stems are pubescent all through with lanceolate, sharply pointed, three veined leaves, roughish above, pubescent below. Broadly plumose heads of yellow flowers form a big pyramidal panicle from August to November making for an appealing autumn border plant [11-13].
The genus Solidago possesses variant important secondary metabolites like saponins like canadensis saponin, polysaccharides, flavonoids glucosides like quercetin, rutin, phenolic acids like caffeic acid, chlorogenic acid, alkaloids like dictamnine-7-b-dmannopyranoside and 8-methoxydictamnine-7-b-dmannopyranoside [14-18].
Terpenes or terpenoids are a class of chemicals that are made up of multiple isoprene structural units; hence they are also called isoprenoids [19].
Terpenoids are usually found in the form of various oxygen containing derivatives, such as alcohols, aldehydes, carboxylic acids, ketones, esters and glycosides, in addition to terpene hydrocarbons [20].
Several monoterpenes like α-pinene and myrcene, monoterpenoids like verbenol and bornyl acetate, sesquiterpenes like germacrene D, β-elemene and β- caryophyllene, copaene and ylangene, sesquiterpenoids like viridoflorol have been detected in S. Canadensis [21-29].
S. canadenesis and other Solidago species exhibited variant pharmacological activities like anticancer activity, antimutagenic, diuretic, antibacterial, anti-inflammatory, antioxidant, gastroprotective, hypolipidimic, anti-tumor, hypoglycemic, antimalarial and antidiabetic [30-37].
Terpenes have a wide range of pharmacological activities in both animals and humans like analgesic, antiinflammatory, antiviral and antibacterial properties [38,39].
Terpenes potential for usage against a wide spectrum of viruses has been established in several in vitro experiments like Herpes simplex virus, Anti-Infectious Bronchitis Virus (IBV), West nile virus and Human Immunodeficiency Virus 1 (HIV-1) [40-43].
The main objective of this study is to evaluate the antiviral activities of the petroleum ether fraction on the HCoV-229E strain.
Depending on these facts our research will focus on the ability to use the petroleum ether fraction to evaluate the antiviral activity of the terpenes in the plant.
Materials and Methods
Collection of plant materials
S. canadensis plant (aerial part) was collected during flowering in October; the plant was washed thoroughly, dried under shade and grinded in a mechanical grinder to a fine powder.
Equipment and chemicals
The instruments used were a rotary evaporator (BUCHI Rotavapor R-205, Swiss), sonicator (Branson Sonifier, USA), all chemicals and solvents used were of analytical grade and obtained from Riedel-de Haen, Germany.
Experimental work
The experimental work is divided into:
Extraction of plant materials (aerial part): 750 grams of the shade dried pulverized S. canadensis plant materials were defatted through soaking the pulverized material in a glass beaker with 1000 ml of hexane at room temperature, the mixture was occasionally shaken by magnetic stirrer for 3 days. The extract was filtered and the residue obtained from the method i was extracted by soxhlet using aqueous ethanol 85% as a solvent for 24 h. The extract was filtered and the solvent was evaporated under reduced pressure using a rotary evaporator to get a dry extract, The residue about 27 gm was suspended in 400 ml water and partitioned with petroleum ether (BP 30–60), (3 × 300 ml). Then the petroleum ether layer filtered and evaporated to dryness [44,45].
Preliminary qualitative phytochemical analysis of crude extracts: Phytochemical analysis for the screening and identification of bioactive chemical constituents in the medicinal plants under study was carried out on crude extracts, as well as powder specimens using the standard procedures as described by Harborne [46].
Test for terpenoids (Salkowski test): 5 ml of each plant extract were mixed in 2 ml of chloroform followed by the careful addition of 3 ml concentrated (H2SO4). A layer of the reddish brown coloration was formed at the interface thus indicating a positive result for the presence of terpenoids.
GC-MS analysis for essential oils: GC/MS analysis was done using Shimadzu GCMS-QP2020 (Tokyo, Japan). The GC was equipped with Rtx-1 MS fused bonded column (30 m × 0.25 mm ID × 0.25 μm film thickness) (Restek, USA) and a split split less injector. The initial column temperature was kept at 45°C for 2 min (isothermal) and programmed to 300°C at a rate of 5°C/min and kept constant at 300°C for 5 min (isothermal). The injector temperature was 250°C. Helium carrier gas flow rate was 1.41 ml/min. All the mass spectra were recorded applying the following condition: (Equipment current) filament emission current, 60 mA; ionization voltage, 70 eV; ion source, 200°C. Diluted samples (1% v/v) were injected with split mode (split ratio 1:15).
The gas chromatography mass analysis of petroleum ether fraction was performed on a Shimadzu QP2010 quadrupole. Gas Chromatography-Mass Spectrometer (GC-MS) instrument equipped with a carbowax (30 m × 0.25 mm ID; 0.25 mm film thickness) capillary column (intercut DB5MS, Japan). One microliter of the sample was injected into the column.
Helium was used as the carrier gas. Injector and detector temperatures were set at 280°C. The injection was performed in split mode (1:30). The column temperature was programmed initially at 50°C and then to increase at a rate shown in Table 1 to a final temperature of 280°C.
The compounds were separated at constant pressure (100 kPa) and peaks were identified by comparing the mass spectra with the mass spectral database. The identification of compounds was based on the comparisons of their mass spectra with the NIST library [47].
| Rate | Temperature (°C) | Hold time (min) |
|---|---|---|
| - | 50 | 5 |
| 50 | 100 | 2 |
| 9 | 280 | 2 |
Table 1: The GC mass oven temperature program.
Investigate the antiviral activity of the petroleum ether fraction isolated from S. Canadensis
The antiviral activity of petroleum ether fraction against human Coronavirus 229E was identified using the high throughput cytopathic effect CPE inhibition assay. The assay was developed to evaluate the range of efficacy; i.e. the 50% Inhibitory concentration (IC50) and cytotoxicity for the chosen antiviral (CC50), in cell culture systems, this assay is crucial for evaluating antiviral efficacy.
Cell line and culture: The 229E virus and Vero E6 cells (African green monkey kidney) were obtained from Nawah-Scientific, Egypt. Vero E6 cells were grown in DMEM medium (Dulbecco’s Modified Eagle’s Medium) supplemented with 10% fetal bovine serum and 0.1% antibiotic/antimycotic solution. The antibiotic and antimycotic solution was obtained from Gibco BRL, trypsin EDTA, fetal bovine serum and DMEM medium (Grand Island, NY, USA) [48].
Antiviral activity test: The antiviral activity and cytotoxicity evaluation was performed using the crystal violet assay [49]. The Vero E6 cells were seeded into a 96 well culture plate at a density of 2 × 104 cells/well one day before infection. The culture medium was removed the next day and the cells were washed with phosphate buffered saline. The infectivity of Coronavirus 229E was determined using the crystal violet method, which monitored CPE and allowed the percentage of cell viability to be calculated. 0.1 mL of diluted virus suspension of 229E virus containing CCID50 (50 percent cell culture infective dose) of virus stock was added to mammalian cells. This dose was selected to produce the desired CPEs. For compound treatments, 0.01 mL of medium containing the desired compound concentration was added to the cells. Each test sample's antiviral activity was determined using a tenfold diluted concentration range of 0.1 μg/mL-100 μg/mL. The virus controls (virus infected, nondrug treated cells) and cell controls (non-infected, nondrug treated cells). For 72 hrs, culture plates were incubated at 37°C in 5% CO2.
The development of the cytopathic effect was monitored by light microscopy. Following a PBS wash, the cell monolayers were fixed and stained with a 0.03% crystal violet solution in 2% ethanol and 10% formalin.
After washing and drying the optical density of individual wells was quantified spectrophotometrically at 540 nm/630 nm. The percentage of antiviral activities of the tested compounds was calculated using the following equation:
Antiviral activity=(Mean optical density of cell controls−Mean optical density of virus controls)/(Optical density of the test-Mean optical density of virus controls) × 100%, using the Dynex Immuno Assay System (DIAS) reader. Based on the results, the 50% CPE Inhibitory Dose (ID50) was calculated.
Cytotoxicity test: Before this assay, we assessed the cytotoxicity; Vero cells were seeded at a density of 2 × 104 cells/well in a 96 well culture plate. The next day, the culture medium containing serially diluted samples was added to the cells and incubated for 48 hours before being removed and the cells washed with PBS. The following steps were carried out in the same manner as described above for the antiviral activity assay [50].
The 50% Cytotoxic Concentrations (CC50) and the 50% Inhibitory Concentration (IC50) were determined using GraphPad PRISM Software (Graph Pad Software, San Diego, USA).
GraphPad PRISM Software (Graph Pad Software, San Diego, USA) was used to calculate the 50% Cytotoxic Concentrations (CC50) and 50% Inhibitory Concentrations (IC50).
Results
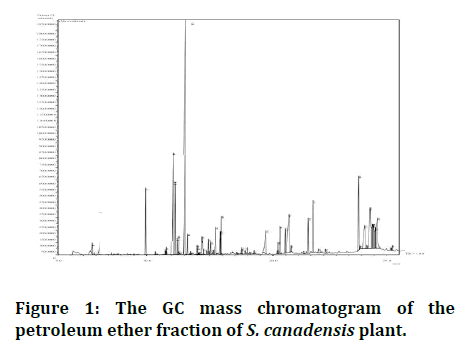
The weight of the dried extract was 3.5 gm and the result of the preliminary test for terpenoids was positive, the GC-MS analysis of petroleum ether fraction from S. canadensis is demonstrated (Figure 1) and the compounds are listed (Table 2).
Figure 1: The GC mass chromatogram of the petroleum ether fraction of S. canadensis plant.
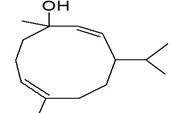
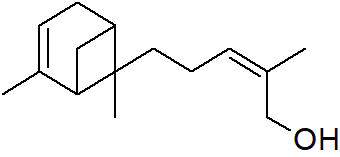
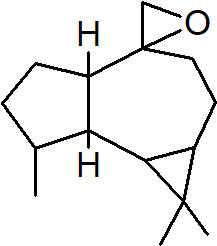
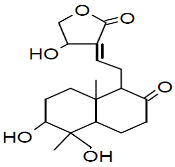
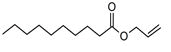
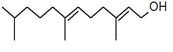
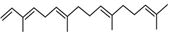
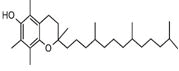
| Peak number | Retention time | Area | Area % | Mol. weight | Mol. formula | Compounds name | Structure |
|---|---|---|---|---|---|---|---|
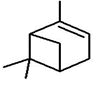
| 1 | 5.649 | 2184956 | 0.48 | 136 | C10H16 | alpha-Pinene |
|
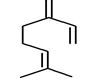
| 2 | 5.870 | 280275 | 0.06 | 136 | C10H16 | beta-Myrcene |
|
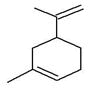
| 3 | 6.254 | 8277130 | 1.81 | 136 | C10H16 | (+)-Sylvestrene |
|
| 4 | 7.230 | 339490 | 0.07 | 152 | C10H16O | cis-Verbenol |
|
| 5 | 9.936 | 13218526 | 2.89 | 196 | C12H20O2 | (-)-Bornyl acetate |
|
| 6 | 10.694 | 486752 | 0.11 | 136 | C10H16 | Terpinolene |
|
| 7 | 11.455 | 1184103 | 0.26 | 204 | C15H24 | beta.-Bourbonene |
|
| 8 | 11.517 | 828204 | 0.18 | 190 | C14H22 | Benzene, 1-(1-ethylpropyl)-2-propyl- |
|
| 9 | 11.566 | 1327065 | 0.29 | 204 | C15H24 | beta.-Elemene |
|
| 10 | 12.040 | 27558413 | 6.03 | 204 | C15H24 | (+)-Ylangene |
|
| 11 | 12.196 | 14691074 | 3.22 | 204 | C15H24 | Copaene |
|
| 12 | 12.376 | 2809466 | 0.62 | 204 | C15H24 | 4,11,11-Trimethyl-8-methylenebicyclo [7.2.0] undec-4-ene |
|
| 13 | 12.496 | 3757497 | 0.82 | 204 | C15H24 | Bicyclo [5.2.0] nonane, 4-methylene-2,8,8-trimethyl-2-vinyl- |
|
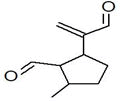
| 14 | 12.550 | 447213 | 0.10 | 204 | C15H24 | alpha.-Caryophyllene, alpha.-Humulene |
|
| 15 | 13.064 | 117884411 | 25.81 | 204 | C15H24 | Germacrene D |
|
| 16 | 13.224 | 3886668 | 0.85 | 204 | C15H24 | 1R,3Z,9s-4,11,11-Trimethyl-8-methylenebicyclo [7.2.0] undec-3-ene |
|
| 17 | 13.463 | 565549 | 0.12 | 204 | C15H24 | Bicyclo [4.1.0]-3-heptene, 2-isopropenyl-5-isopropyl-7,7-dimethyl- |
|
| 18 | 13.967 | 2128997 | 0.47 | 222 | C15H26O | Elemol |
|
| 19 | 14.051 | 1376543 | 0.30 | 346 | C22H34O3 | Kauran-18-al, 17-(acetyloxy)-, (4.beta.)- |
|
| 20 | 14.158 | 448803 | 0.10 | 238 | C15H26O2 | trans-beta-Terpinyl pentanoate |
|
| 21 | 14.372 | 6800969 | 1.49 | 220 | C15H24O | Caryophyllene oxide |
|
| 22 | 14.511 | 1048911 | 0.23 | 152 | C10H16O | trans-Chrysanthemal |
|
| 23 | 14.732 | 719321 | 0.16 | 164 | C12H20 | Bicyclo [6.1.0] nonane, 9-(1-methylethylidene)- |
|
| 24 | 14.874 | 3196901 | 0.70 | 360 | C24H40O2 | 10,12-Tricosadiynoic acid, methyl ester |
|
| 25 | 14.932 | 594225 | 0.13 | 222 | C15H26O | Germacrene D-4-ol |
|
| 26 | 15.059 | 2471474 | 0.54 | 220 | C15H24O | Bergamotol |
|
| 27 | 15.268 | 1058847 | 0.23 | 220 | C15H24O | Aromadendrene oxide-(2) |
|
| 28 | 15.452 | 7595443 | 1.66 | 350 | C20H30O5 | Andrographolide |
|
| 29 | 15.809 | 6656828 | 1.46 | 302 | C20H30O2 | Icosapent |
|
| 30 | 15.873 | 7349568 | 1.61 | 220 | C15H24O | Cis-Lanceol |
|
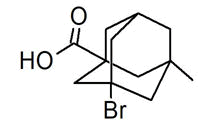
| 31 | 17.137 | 500030 | 0.11 | 272 | C12H17BrO2 | 3-Bromo-7-methyl-1-adamantanecarboxylic acid |
|
| 32 | 17.476 | 1370888 | 0.30 | 296 | C20H40O | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol |
|
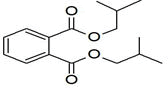
| 33 | 17.561 | 1233530 | 0.27 | 166 | C10H14O2 | Cyclopentane acetaldehyde, 2-formyl-3-methyl-. alpha. -methylene- |
|
| 34 | 17.808 | 732776 | 0.16 | 278 | C16H22O4 | Palatinol IC |
|
| 35 | 17.972 | 1445865 | 0.32 | 536 | C37H76O | 1-Heptatriacotanol |
|
| 36 | 18.146 | 681054 | 0.15 | 190 | C15H22 | 11,11-Dimethyl-spiro [2,9] dodeca-3,7-dien |
|
| 37 | 18.507 | 631046 | 0.14 | 270 | C17H34O2 | Palmitic acid, methyl ester |
|
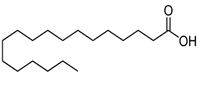
| 38 | 19.422 | 11378057 | 2.49 | 256 | C16H32O2 | Palmitic acid |
|
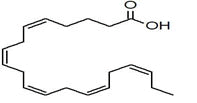
| 39 | 20.301 | 753950 | 0.17 | 294 | C19H34O2 | 9,12-Octadecadienoic acid, methyl ester, Linoleic acid methyl ester |
|
| 40 | 20.386 | 2276448 | 0.50 | 268 | C17H32O2 | 7-Hexadecenoic acid, methyl ester, (Z)- |
|
| 41 | 20.528 | 8292856 | 1.82 | 182 | C12H22O | Ethyl linalool |
|
| 42 | 20.954 | 5635379 | 1.23 | 152 | C10H16O | cis-Carveol |
|
| 43 | 21.237 | 30424178 | 6.66 | 224 | C15H28O | (Z)6,(Z)9-Pentadecadien-1-ol |
|
| 44 | 21.414 | 1362383 | 0.30 | 284 | C18H36O2 | Octadecanoic acid, stearic acid |
|
| 45 | 22.421 | 773866 | 0.17 | 222 | C15H26O | Viridiflorol |
|
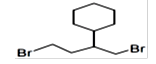
| 46 | 22.775 | 7579156 | 1.66 | 310 | C11H20Br | 1,6-Dibromo-2-cyclohexylpentane |
|
| 47 | 23.168 | 11921464 | 2.61 | 210 | C13H22O2 | 3,7-Dimethyl-2,6-non-adien-1-ol acetate |
|
| 48 | 23.596 | 1371898 | 0.30 | 204 | C15H24 | Cycloheptane, 4-methylene-1-methyl-2-(2-methyl-1-propen-1-yl)-1-vinyl- |
|
| 49 | 24.140 | 1043273 | 0.23 | 288 | C18H37Cl | Octadecane, 1-chloro- |
|
| 50 | 26.755 | 22549397 | 4.94 | 410 | C30H50 | 2,6,10,14,18,22-Tetracosahexaene, 2,6,10,15,19,23-hexamethyl-, (all-E)-, Spinacene, Squalen |
|
| 51 | 26.851 | 651682 | 0.14 | 222 | C15H26O | (-)-Isolongifolol |
|
| 52 | 27.236 | 17175198 | 3.76 | 178 | C10H18O2 | Angelicoidenol, 1,7,7-trimethylbicyclo [2.2.1] heptane-2,5-diol |
|
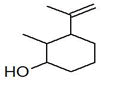
| 53 | 27.421 | 845858 | 0.19 | 154 | C10H18O | Isopropenyl-2-methyl cyclohexanol. |
|
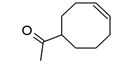
| 54 | 27.669 | 26729205 | 5.85 | 152 | C10H16O | 1-(4-Cycloocten-1-yl) ethanone |
|
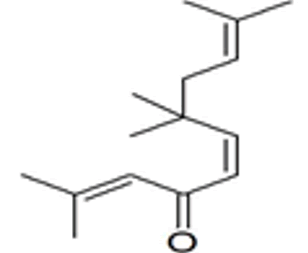
| 55 | 27.864 | 14315021 | 3.13 | 220 | C15H24O | (5Z)-2,7,7,10-Tetramethyl-2,5,9-undecatrien-4-one. |
|
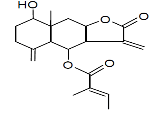
| 56 | 27.917 | 11657608 | 2.55 | 346 | C20H26O5 | 8-Hydroxy-8a-methyl-3,5-dimethylene-2-oxododecahydronaphtho [2,3-b] furan-4-yl (2E)-2-methyl-2-butenoate |
|
| 57 | 28.108 | 10347338 | 2.27 | 212 | C13H24O2 | Decanoic acid, 2-propenyl ester, Allyl caprate |
|
| 58 | 28.246 | 19332679 | 4.23 | 210 | C15H28O | (2E,6E)-3,7,11-Trimethyl-2,6-dodecadien-1-ol, farnesol |
|
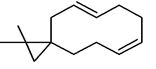
| 59 | 29.315 | 832202 | 0.18 | 272 | C20H32 | (E,E,E)-3,7,11,15-Tetramethylhexadeca-1,3,6,10,14-pentaene, alpha- springene |
|
| 60 | 29.418 | 1786871 | 0.39 | 430 | C29H50O2 | Vitamin E |
|
| Total | 456804778 | 100.00 |
Table 2: The GC mass profile of S. canadensis L. aerial part.
The GC mass analysis shows the presence of many terpenes and terpenoids that fall within the monoterpenes and sesquiterpenes. The results also showed the presence of fatty acids as well as vitamin E.
The most important compounds detected were α-pinene, bornyl acetate, myrcene, verbenol, terpinolene, germacrene-D andrographolide, caryophyllene oxide, palmitic acid and linoleic acid methyl ester.
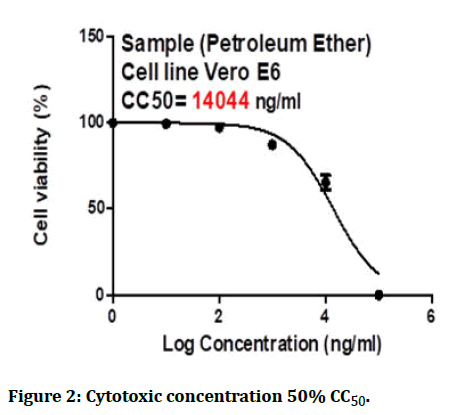
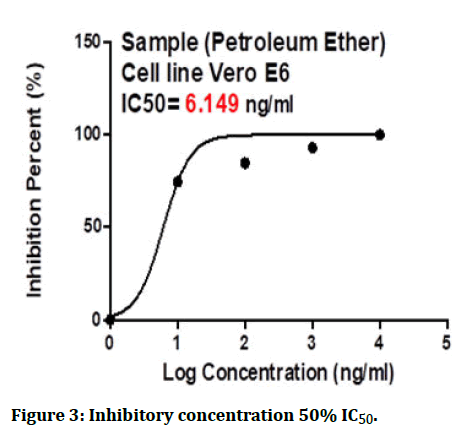
The cytotoxic effects of the petroleum ether fraction had to be tested on Vero cells to rule out non-specific activities and the maximal non-toxic concentration for the Vero cells was also calculated (Figures 2 and 3).
Figure 2: Cytotoxic concentration 50% CC50.
Figure 3: Inhibitory concentration 50% IC50.
The results of the 50% Cytotoxic Concentrations (CC50) and the 50% Inhibitory Concentration (IC50) were determined using Graph Pad PRISM Software (Graph Pad Software, San Diego, USA).
Graphs of Cytotoxicity Concentration 50 (CC50) and the 50% Inhibitory Concentration (IC50) on Vero E6 cells and 229E.
Discussion
Terpenes are secondary metabolites that are generated by a broad group of plants and animals. Terpenes have shown effectiveness against viruses, bacteria, fungus and protozoa, making them a prospective source of novel antimicrobial agents [51].
By dividing the supplied Antiviral Activity (AVA) value by the cytotoxicity (TOX) value (AVA/TOX), the Selectivity Index (SI) quantifies the window between cytotoxicity and antiviral activity. The greater the SI ratio, the more successful and safer a medicine would be in vivo during viral infection therapy. The ideal medicine would be cytotoxic only at very high doses but antiviral at extremely low concentrations, resulting in a high SI value (high AVA/low TOX) and the ability to destroy the target virus at concentrations much below its cytotoxic concentration. A compound's selectivity index is a commonly accepted metric for expressing a compound's in vitro performance in inhibiting viral reproduction [52].
The tested samples (petroleum ether) showed high antiviral activities against COVID-19 (229E virus). The determination of Selective Index (SI) for the tested sample is very important, for biologically active compounds it is recommended to obtain SI ≥ 10 [53].
The selective index=Estimated CC50/Estimated IC50=2,283.9. Consequently, the tested samples are good candidates for further experiments as anti-COVID-19.
The experiment shows that the CC50 of the petroleum ether fraction from S. canadensis was 14044 ng/ml while the IC50 was 6.149 ng/ml only, which indicates a potent activity against the Human Coronavirus 229E.
It has been found that the compounds in petroleum ether fraction have antiviral activity at a concentration below the cytotoxic concentration.
The GC mass analysis revealed the presence of about 60 compounds like terpenes, terpenoids, fatty acid, fatty acid esters and vitamin E.
Most of them possess pharmacological and biological activities, concerning the compounds with a relatively good concentration in the fraction, a-pinene, terpineol have high SI against Herpes Simplex Virus type 1 (HSV-1) in vitro, it has been suggested that when herpes virus was incubated with essential oils and isolated monoterpenes before host cell infection and may interfere with virion envelope structures or mask the viral structure required for adsorption or entrance into host cells [54]. It has been demonstrated using molecular docking techniques that several major monoterpenes found in essential oils for medicinal use (-pinene and -terpineol) can exert relatively strong binding and inhibit the active site of the Mpro protein, a key homodimeric cysteine protease enzyme that cleaves polyproteins into individual proteins required for SARS-CoV-2 replication and transcription [55].
Andrographolide is a bicyclic diterpenoid lactone, it has been found to have antiviral properties in several earlier researches against human immunodeficiency virus HIV, hepatitis-B virus, HPV16 pseudovirus and hepatitis-C virus, herpes simplex virus HSV-1, flaviviruses and pestiviruses Dengue virus (DENV1) and Chikungunya Virus (CHIKV) [56-63].
Several types of research report the mechanism of action of andrographolide against influenza virus. The Retinoic acid Inducible Gene-1 (RIG-1) like receptors signaling pathway have been identified as potential targets for andrographolide's antiviral effect against influenza A [64]. NF-kB was identified as a particular andrographolide binding target in a recent proteomic investigation [65]. It significantly suppresses the activation of NF-kB in vivo in a dose dependent manner, preventing NF-kB from binding to DNA [66].
Bornyl acetate a bicyclic monoterpene is a lung inflammatory disease preventative agent. It reduced the levels of proinflammatory cytokines in vivo and in vitro by inhibiting the activation of MAPKs and NF-kB signaling pathway, through repressing the activation of extracellular regulated protein kinases, c-Jun N-terminal kinase and p38 Mitogen Activated Protein Kinase (MAPK), also the number of total cells, neutrophils and macrophages, histologic changes in the lungs were decreased [67].
Ylangene a sesquiterpenoid, has anti-inflammatory properties it inhibits (inducible nitric oxide synthase) and COX-2 (cyclooxygenase-2) activity and promotes the pro-inflammatory proteins iNOS and COX-2 to be downregulated [68]. It has been found that oxygenated ylangene sesquiterpenoids be cytotoxic against HepG2, MDA-MB231 and A549 cancer cell lines With IC50 values of 16.0, 16.3 and 15.8 g/mL, respectively [69].
α-Copaene a sesquiterpene possesses antileishmanial activity, crude extracts and fractions rich in α-copaene (38.8%) from Copaifera paupera, had the most activity against L. amazonensis (IC50=62.5 g/mL) and L. infantum (IC50=65.9 g/mL) [70]. α- Copaene has been proved as non-genotoxic with a significant antioxidant activity [71].
Germacrene-D a sesquiterpene hydrocarbon is one of the major constituents of Cedrus libani (29.40%), Loizzo, Monica Rosa, et al. reported that leaves ethanolic extract exhibited an interesting antiviral activity against Herpes Simplex Virus type 1 (HSV-1) with no cytotoxic effect [72]. Essential oils with a high concentration of geranial and germacrene-D from 13 plants demonstrated antimicrobial activity towards Candida albicans [73]. Another study on the essential oils of Lippia javanica containing a high concentration of germacrene-D demonstrates potent antimicrobial activity against all the tested gram positive, gram negative bacteria and the pathogenic fungi strains [74]. In our study Germacrene-D was the major compound in the tested fraction about (25.81%).
Icosapent an Eicosapentaenoic Acid (EPA) fatty acid, Icosapent has proven successful in the treatment of individuals with moderate rises in blood triglyceride levels who have already been treated with statins, as well as in the treatment of patients with severe hypertriglyceridemia [75].
The use of icosapent ethyl as a part of supportive therapy in patients with mild to severe COVID-19 pneumonia has been documented in various clinical trials, in individuals with SARS-CoV-2 infection, icosapent ethyl provides antiinflammatory benefits by lowering inflammatory markers such as CRP, ILs, granulocyte colony stimulating factor, interferons and TNFs, which promote inflammation and lung damaging [76,77].
Lanceol cis a sesquiterpene alcohol is the most prevalent component (20.22%) in Rubus fruticosus L. essential oil. Lanceol has been shown to have substantial antioxidant and antibacterial action against resistant strains obtained from patients suffering from respiratory infections [78]. Lanceol was also reported to possess antibacterial activity against Helicobacter pylori [79].
Palmitic acid a saturated fatty acid demonstrated antioxidant, potent inhibition of gastrointestinal motility and considerable anti-proliferative effects against different cancers cell lines [80]. Methyl Palmitate (MP) and MP like compounds possess anti-inflammatory and anti-fibrotic activity due to NF-kB suppression [81].
Ethyl linalool show bacteriostatic activity against gram negative Escherichia coli bacteria [82].
(-)-Carveol, a natural product, has significant gastroprotective activity involving multiple mechanisms of action, including cyto protection, anti-secretory, antioxidant and immune regulation [83].
(Z)6, (Z)9-pentadecadien-1-ol one of the major components of essential oil of Nigella sativa L. seeds (13.52%), possess antibacterial activity against Bacillus subtilis, Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa, as well as antifungal activity against Candida albicans [84]. The presence of (Z)6, (Z)9-pentadecadien-1-ol as one of the major compounds in the methanol extract of Psydrax dicoccos leaves showed significant antifungal activity against Candida albicans and other fungal tested species, this activity has been attributed mostly to its antioxidant properties as a result of free radical scavenging [85].
Squalene a triterpene compound has been shown to have many biological and pharmacological activities such as antioxidant, immune stimulant, hepato protective, anticancer, antigenotoxic, atherosclerosis, antiproliferative, anti-inflammatory, preventing skin oxidative damage caused by free radicals and an intermediate in cholesterol biosynthesis [86,87].
Angelicoidenol derivative like 2-O-b-D-glucopyranosyl- (+)-angelicoidenol is a mono-terpenoid compound known to inhibit Viral Protein R (Vpr) activity which is important for HIV viral replication [88]. But only weak anticancer activity against hepatoma cancer, epidermoid carcinoma, prostate cancer and breast cancer and melanoma cancer cell lines [89].
A silico study showed that farnesol was the best docking ligand from the 171 tested essential oils compounds for the SARS-CoV-2 target proteins; Main Protease (Mpro), endoribonuclease (Nsp15/NendoU), ADP-Ribose-1- Phosphatase (ADRP), RNA Dependent RNA Polymerase (RdRp), Spike protein (rS) and Human Angiotensin Converting Enzyme (hACE2), which are essential for a viral infection to be effective indicating that it can limit viral replication when administered alone or in combination [90-92].
Natural products especially essential oils from several plants have shown antiviral activity to Coronaviruses, although the mechanism of action of these herbal products is mainly through the inhibition of viral replication [93]. Another study revealed that the antiviral activity was strongest in the pretreatment system, suggesting that the compounds' antiviral action is dependent on viral attachment and/or entrance prevention [94].
In general Essential oil components, on the other hand, may function synergistically with each other; they may enhance other antiviral medicines, or give some alleviation from COVID-19 symptoms.
Conclusion
Coronavirus COVID-19 is a dangerous viral disease caused by a new Coronavirus (SARS-CoV-2), medicinal plants have been used in the treatment of a large number of diseases since ancient times, Solidago canadensis L. belongs to the asteraceae family, which includes approximately one hundred species, widely distributed in North American wildflowers and greater than a dozen species inhabiting South America, Europe and Asia. It has been cultivated in Iraq in the provinces of Babylon and Diyala recently. 750 gm has been extracted with ethanol 85% then fractionated after drying with petroleum ether, then GC/MS analysis by Shimadzu GCMS-QP2020 show the detection of 60 compounds. The results showed the presence of a variety of compounds; fatty acids like palmitic acid and linoleic acid methyl ester. Terpenes and terpenoids like α-pinene, bornyl acetate, myrcene, verbenol, terpinolene, germacrene-D andrographolide, caryophyllene oxide, as well as vitamin E.
References
- Belouzard S, Millet JK, Licitra BN, et al. Mechanisms of Coronavirus cell entry mediated by the viral spike protein. Viruses 2012; 4:1011-1033. [Crossref][Googlescholar][Indexed]
- Weiss SR, Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome Coronavirus. Microbiol Mol Biol Rev 2005; 69:635-664. [Crossref][Googlescholar][Indexed]
- Signer J, Jonsdottir HR, Albrich WC, et al. In vitro virucidal activity of Echinaforce, an Echinacea purpurea preparation, against Coronaviruses, including common cold Coronavirus 229E and SARS-CoV-2. Virol J 2020; 17:136. [Crossref][Googlescholar][Indexed]
- World Health Organisation (WHO). WHO Coronavirus (COVID-19) Dashboard. 2021.
- Chaachouay N, Douira A, Zidane L. COVID-19, prevention and treatment with herbal medicine in the herbal markets of sale prefecture, North-Western Morocco. Eur J Integr Med 2021; 42:101285. [Crossref][Googlescholar][Indexed]
- Bagetta G, Cosentino M, Corasaniti MT, et al. Herbal medicines: Development and validation of plant derived medicines for human health. Clin Pharmacogn 2012; 519. [Crossref][Googlescholar][Indexed]
- Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect 2001; 109:69–75. [Crossref][Googlescholar][Indexed]
- Lillehoj H, Liu Y, Calsamiglia S, et al. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet Res 2018; 49:76. [Crossref][Googlescholar][Indexed]
- de Souza DMF, Sa RD, Araujo EL, et al. Anatomical, phytochemical and histochemical study of Solidago chilensis Meyen. An Acad Bras Cienc 2018; 90:2107–2120. [Crossref][Googlescholar][Indexed]
- Amtmann M. The chemical relationship between the scent features of goldenrod (Solidago canadensis L.) flower and its unifloral honey. J Food Compos Anal 2010; 23:122–129. [Crossref][Googlescholar][Indexed]
- Evans WC. Trease and evans pharmacognosy. Saunders: Elsevier, 16th edition, Edinburgh, New York, 2009. [Googlescholar][Indexed]
- Reznicek G, Jurenitsch J, Plasun M, et al. Four major saponins from Solidago canadensis. Phytochemistry 1991; 30:1629–1633. [Crossref][Googlescholar][Indexed]
- Reznicek G, Jurenitsch J, Freiler M, et al. Isolation and structure elucidation of further new saponins from Solidago canadensis. Planta Med 1992; 58:94–98. [Crossref][Googlescholar][Indexed]
- Papp I, Apati P, Andrasek V, et al. LC-MS analysis of antioxidant plant phenoloids. Chromatographia 2004; 60:93–100. [Crossref][Googlescholar][Indexed]
- Zielinka M, Kostrzewa A, Ignatowicz E, et al. The flavonoids, quercetin and isorhamnetin 3-O-acylglucosides diminish neutrophil oxidative metabolism and lipid peroxidation. Acta Biochim Pol 2001; 48:183–189. [Googlescholar][Indexed]
- Dobjanschi L, Paltinean R, Vlase L, et al. Comparative phytochemical research of genus: S. graminifolia note I. flavonoids. Acta Biol Marisiensis 2018; 1:18-26. [Crossref][Googlescholar][Indexed]
- Abdel Baki PM, El-Sherei MM, Khaleel AE, et al. Aquaretic activity of Solidago canadensis L. cultivated in Egypt and determination of the most bioactive fraction. Iran J Pharm Res 2019; 18:922–937. [Crossref][Googlescholar][Indexed]
- Li YK, Zhao QJ, Hu J, et al. Two new quinoline alkaloid mannopyranosides from Solidago canadensis. Helv Chim Acta 2009; 92:928–931. [Crossref][Googlescholar][Indexed]
- Beserra FP, Rozza AL, Vieira AJ, et al. Antiulcerogenic compounds isolated from medicinal plants. Stud Nat Prod Chem 2016; 47:215–234. [Crossref][Googlescholar][Indexed]
- Yang W, Chen X, Li Y, et al. Advances in pharmacological activities of terpenoids. Nat Prod Commun 2020; 15:1–13. [Crossref][Googlescholar][Indexed]
- Fursenco C, Calalb T, Uncu L, et al. Solidago virgaurea L.: A review of its ethno medicinal uses, phyto chemistry and pharmacological activities. Biomolecules 2020; 10:1619. [Crossref][Googlescholar][Indexed]
- Elshafie HS, Grulova D, Baranova B, et al. Antimicrobial activity and chemical composition of essential oil extracted from Solidago canadensis L. growing wild in Slovakia. Molecules 2019; 24:1206. [Crossref][Googlescholar][Indexed]
- Kolodziej B, Kowalski R, Kedzia B. Antibacterial and antimutagenic activity of extracts aboveground parts of three solid ago species: Solidago virgaurea L., Solidago canadensis L. and Solidago gigantea Ait. J Med Plant Res 2011; 5:6770–6779. [Crossref][Googlescholar][Indexed]
- Kalemba D, Marschall H, Bradesi P. Constituents of the essential oil of Solidago gigantea Ait. (giant goldenrod). Flavour Fragr J 2001; 16:19-26. [Crossref][Googlescholar][Indexed]
- Thiem BA, Wesolowska MA, Skrzypczak LU, et al. Phenolic compounds in two Solidago L. species from in vitro culture. Acta Pol Pharm 2001; 58:277-281. [Googlescholar][Indexed]
- Lawson SK, Sharp LG, Powers CN, et al. Volatile compositions and antifungal activities of Native American medicinal plants: Focus on the asteraceae. Plants 2020; 9:126. [Crossref][Googlescholar][Indexed]
- Weyerstahl P, Marschall H, Christiansen C. Constituents of the essential oil of Solidago canadensis (goldenrod) from Poland a correction. Planta Med 1993; 59:281–282. [Crossref][Googlescholar][Indexed]
- Schmidt CO, Bouwmeester HJ, Bulow N, et al. Isolation, characterization and mechanistic studies of (-)-a-gurjunene synthase from Solidago canadensis. Arch Biochem Biophys 1999; 364:167–177. [Crossref][Googlescholar][Indexed]
- Kasali AA, Ekundayo O, Paul C, et al. Epi-cubebanes from Solidago canadensis. Phytochemistry 2002; 59:805–810. [Crossref][Googlescholar][Ineddex]
- Li D, Pan S, Zhu X, et al. Anticancer activity and chemical composition of leaf essential oil from Solidago canadensis L. in China. Adv Mater Res 2012; 347–353:1584–1589. [Crossref]
- Starks CM, Williams RB, Goering MG, et al. Antibacterial clerodane diterpenes from Goldenrod (Solidago virgaurea). Phytochemistry 2010; 71:104–109. [Crossref][Googlescholar][Indexed]
- Toiu A, Vlase L, Vodnar DC, et al. Solidago graminifolia L. Salisb. (asteraceae) as a valuable source of bioactive polyphenols: HPLC profile, in vitro antioxidant and antimicrobial potential. Molecules 2019; 24:2666. [Crossref][Googlescholar][Indexed]
- Abdel Motaal A, Ezzat SM, Tadros MG, et al. In vivo anti-inflammatory activity of caffeoylquinic acid derivatives from Solidago virgaurea in rats. Pharm Biol 2016; 54:2864–2870. [Crossref][Googlescholar][Indexed]
- Demir H, Acik L, Bali EB, et al. Antioxidant and antimicrobial activities of Solidago virgaurea extracts. African J Biotechnol 2009; 8:274–279. [Googlescholar][Indexed]
- Bucciarelli A, Minetti A, Milczakowskyg C, et al. Evaluation of gastro protective activity and acute toxicity of Solidago chilensis Meyen (asteraceae). Pharm Biol 2010; 48:1025–1030. [Crossref][Googlescholar][Indexed]
- Huang Y, Hao YL, Mai XY. Chemical constituents from Solidago canadensis with hypolipidemic effects in HFD fed hamsters. J Asian Nat Prod Res 2013; 15:319–324. [Crossref][Googlescholar][Indexed]
- Sanad FA, Ahmed SF, El-Tantawy WH. Antidiabetic and hypolipidemic potentials of Solidago virgaurea extract in alloxan induced diabetes type 1. Arch Physiol Biochem 2022; 128:716-723. [Crossref][Googlescholar][Indexed]
- Guimaraes AG, Quintans JS, Quintans-Junior LJ. Monoterpenes with analgesic activity: A systematic review. Phyther Res 2012; 27:1–15. [Crossref][Googlescholar][Indexed]
- Mahizan NA, Yang SK, Moo CL, et al. Terpene derivatives as a potential agent against antimicrobial resistance pathogenes. Molecules 2019; 24:2631. [Crossref][Googlescholar][Indexed]
- Astani A, Schnitzler P. Antiviral activity of monoterpenes beta-pinene and limonene against herpes simplex virus in vitro. Iran J Microbiol 2014; 6:149–155. [Googlescholar][Indexed]
- Yang Z, Wu N, Zu Y, et al. Comparative Anti-Infectious Bronchitis Virus (IBV) activity of (-)-pinene: Effect on Nucleocapsid (N) protein. Molecules 2011; 16:1044-1054. [Crossref][Googlescholar][Indexed]
- Zamora AP, Edmonds JH, Reynolds MJ, et al. The in vitro and in vivo antiviral properties of combined monoterpene alcohols against West Nile virus infection. Virology 2016; 495:18–32. [Crossref][Googlescholar][Indexed]
- Bicchi C, Rubiolo P, Ballero M, et al. HIV-1 inhibiting activity of the essential oil of Ridolfia segetum and Oenanthe crocata. Planta Med 2009; 75:1331–1335. [Crossref][Googlescholar][Indexed]
- Cetkovic GS, Dilas SM, Canadanovic-Brunet JM, et al. Thin layer chromatography analysis and scavenging activity of marigold (Calendula officinalis L.) extracts. Acta Period Technol 2003; 34:93–102. [Crossref][Googlescholar][Indexed]
- Hamad MN. Detection and isolation of flavonoids from Calendula officinalis (F. asteraceae) cultivated in Iraq. Iraqi J Pharm Sci 2016; 25:1–6. [Crossref][Googlescholar]
- Harborne JB. Phytochemical methods; a guide to modern techniques of plant analysis. J Chem Inf Model 1998; 3:317. [Crossref][Googlescholar]
- Alfekaik DF, AL-Hilfi SA. Fatty acids composition by (GC-MS) and most important physical chemicals parameters of seed oil pomegranate and grape seeds. J Biol Agric Healthc 2016; 6:25-32. [Googlescholar]
- Schmidtke M, Schnittler U, Jahn B, et al. A rapid assay for evaluation of antiviral activity against cox sackie virus B3, influenza virus A and herpes simplex virus type 1. J Virol Methods 2001; 95:133–143. [Crossref][Googlescholar][Indexed]
- Khiralla A, Spina R, Varbanov M, et al. Evaluation of antiviral, antibacterial and anti-proliferative activities of the endophytic fungus Curvularia papendorfii and isolation of a new polyhydroxyacid. Microorganisms 2020; 8:1353. [Crossref][Googlescholar][Indexed]
- Pauwels R, Balzarini J, Baba M, et al. Rapid and automated tetrazolium based colorimetric assay for the detection of anti-HIV compounds. J Virol Methods 1988; 20:309–321. [Crossref][Googlescholar][Indexed]
- Paduch R, Kandefer-Szerszen M, Trytek M, et al. Terpenes: Substances useful in human healthcare. Arch Immunol Ther Exp 2007; 55:315–327. [Crossref][Googlescholar][Indexed]
- Joshua C. Treating HHV-6 infections: The laboratory efficacy and clinical use of ati-HHV-6 agents. Hum Herpes viruses HHV-6A, HHV-6B HHV-7. 3rd Edition, Elsevier 2014; 311–331. [Crossref] [Googlescholar]
- Indrayanto G, Putra GS, Suhud F. Validation of in vitro bioassay methods: Application in herbal drug research. Profiles Drug Subst Excip Relat Methodol 2021; 273–307. [Crossref][Googlescholar][Indexed]
- Astani A, Reichling J, Schnitzler P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phyther Res 2009; 24:673–679. [Crossref][Googlescholar][Indexed]
- Mieres-Castro D, Ahmar S, Shabbir R, et al. Antiviral activities of eucalyptus essential oils: Their effectiveness as therapeutic targets against human viruses. Pharm Rev 2021; 14:1210. [Crossref][Googlescholar][Indexed]
- Calabrese C, Berman SH, Babish JG, et al. A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phyther Res 2000; 14:333–338. [Crossref][Googlescholar][Indexed]
- Niranjan Reddy VL, Malla Reddy S, Ravikanth V, et al. A new bis-andrographolide ether from Andrographis paniculata nees and evaluation of anti-HIV activity. Nat Prod Res 2005; 19:223–230. [Crossref][Googlescholar][Indexed]
- Chen H, Ma YB, Huang XY, et al. Synthesis, structure activity relationships and biological evaluation of dehydroandrographolide and andrographolide derivatives as novel anti-hepatitis B virus agents. Bioorg Med Chem Lett 2014; 24:2353–2359. [Crossref][Googlescholar][Indexed]
- Ekalaksananan T, Sookmai W, Fangkham S, et al. Activity of andrographolide and its derivatives on HP6 pseudovirus infection and viral oncogene expression in cervical carcinoma cells. Nutr Cancer 2015; 67:687–696. [Crossref][Googlescholar][Indexed]
- Lee JC, Tseng CK, Young KC, et al. Andrographolide exerts anti-hepatitis C virus activity by up regulating haeme oxygenase-1 via the p38 MAPKNrf2 pathway in human hepatoma cells. Br J Pharmacol 2014; 171:237-252. [Crossref][Googlescholar][Indexed]
- Seubsasana S, Pientong C, Ekalaksananan T, et al. A Potential andrographolide analogue against the replication of herpes simplex virus type 1 in vero cells. Med Chem 2011; 7:237–244. [Crossref][Goooglescholar][Indexed]
- Jayakumar T, Hsieh CY, Lee JJ, et al. Experimental and clinical pharmacology of Andrographis paniculata and its major bioactive phyto constituent andrographolide. Evid Based Complement Alternat Med 2013; 2013. [Crossref][Goooglescholar][Indexed]
- Wintachai P, Kaur P, Lee RC, et al. Activity of andrographolide against chikungunya virus infection. Sci Rep 2015; 5:14179. [Crossref][Goooglescholar][Indexed]
- Yu B, Dai CQ, Jiang ZY, et al. Andrographolide as an Anti-H1N1 drug and the mechanism related to retinoic acid inducible gene I like receptors signaling pathway. Chin J Integr Med 2014; 20:540–545. [Crossref][Goooglescholar][Indexed]
- Wang J, Tan XF, Yang P, et al. A quantitative chemical proteomics approach to profile the specific cellular targets of andrographolide, a promising anticancer agent that suppresses tumor metastasis. Mol Cell Proteomics 2014; 13:876-886. [Crossref][Goooglescholar][Indexed]
- Chen JX, Xue HJ, Ye WC, et al. Activity of andrographolide and its derivatives against influenza virus in vivo and in vitro. Biol Pharm Bull 2009; 32:1385–1391. [Crossref][Goooglescholar][Indexed]
- Chen N, Sun G, Yuan X, et al. Inhibition of lung inflammatory responses by bornyl acetate is correlated with regulation of myeloperoxidase activity. J Surg Res 2014; 186:436–445. [Crossref][Goooglescholar][Indexed]
- Cheng SY, Lin EH, Huang JS, et al. Ylangene type and nardosinane type sesquiterpenoids from the soft corals Lemnalia flava and Paralemnalia thyrsoides. Chem Pharm Bull 2010; 58:381–385. [Crossref][Goooglescholar][Indexed]
- Wu Q, Sun J, Chen J, et al. Terpenoids from marine soft coral of the genus Lemnalia: Chemistry and biological activities. Mar Drugs 2018; 16:320. [Crossref][Goooglescholar][Indexed]
- Rodrigues IA, Ramos AD, Falcao DQ, et al. Development of nanoemulsions to enhance the antileishmanial activity of Copaifera paupera oleoresins. Biomed Res Int 2018; 2018. [Crossref][Goooglescholar][Indexed]
- Turkez H, Celik K, Togar B. Effects of copaene, a tricyclic sesquiterpene, on human lymphocytes cells in vitro. Cytotechnology 2014; 66:597–603. [Crossref][Goooglescholar][Indexed]
- Loizzo MR, Saab A, Tundis R, et al. Phytochemical analysis and in vitro evaluation of the biological activity against Herpes Simplex Virus type 1 (HSV-1) of Cedrus libani A. Rich. Phytomedicine 2008; 15:79–83. [Crossref][Goooglescholar][Indexed]
- Duarte MC, Figueira MG, Sartoratto A, et al. Anti-Candida activity of Brazilian medicinal plants. J Ethnopharmacol 2005; 97:305–311. [Crossref][Goooglescholar][Indexed]
- Ngassapa O, Runyoro DK, Harvala E, et al. Composition and antimicrobial activity of essential oils of two populations of Tanzanian lippia javanica (Burm. F.) spreng (verbenaceae). Flavour Fragr J 2003; 18:221–224. [Crossref][Goooglescholar][Indexed]
- Scherer DJ, Nicholls SJ. Lowering triglycerides to modify cardiovascular risk: Will icosapent deliver? Vas Health Risk Manag 2015; 11:203–209. [Crossref][Goooglescholar][Indexed]
- Berger AA, Sherburne R, Urits I, et al. Icosapent ethyl: A successful treatment for symptomatic COVID-19 infection. Cureus 2020; 12:e10211. [Crossref][Goooglescholar][Indexed]
- Suh W, Urits I, Viswanath O, et al. Three cases of COVID-19 pneumonia that responded to icosapent ethyl supportive treatment. Am J Case Rep 2020; 21:e928422. [Crossref][Goooglescholar][Indexed]
- Thanina AC, Bendahou M, Arab K. Antibacterial activity of two extracts from Rubus fruticosus L. against resistant pathogens and their antioxidant potential. Afr J Microbiol Res 2015; 9:1255–1262. [Crossref][Goooglescholar]
- Phukan H, Bora CR, Mitra PK. Phytochemical screening and GC-MS analysis of methanolic leaf extract of an endemic plant Kayea assamica. IOSR J Pharm Biol Sci 2017; 12:7-16. [Crossref][Goooglescholar]
- Elagbar ZA, Naik RR, Shakya AK, et al. Fatty acids analysis, antioxidant and biological activity of fixed oil of Annona muricata L. Seeds. J Chem 2016; 2016. [Crossref][Goooglescholar][Indexed]
- El-Demerdash E. Anti-inflammatory and anti-fibrotic effects of methyl palmitate. Toxicol Appl Pharmacol 2011; 254:238-244. [Crossref][Goooglescholar][Indexed]
- Oladimeji OH, Onu NO, Attih EE. Ethyl linalool and diethyl phthalate from Pycnanthus angolensis (Welw.) Warb. Afr J Pharmacol Ther 2017; 6:76–78. [Crossref][Goooglescholar][Indexed]
- Serafim CA, Araruna ME, Alves Junior EB, et al. (-)-Carveol prevents gastric ulcers via cytoprotective, antioxidant, antisecretory and immuno regulatory mechanisms in animal models. Front Pharmacol 2021; 12. [Crossref][Goooglescholar][Indexed]
- Tahir F, Sonibare M, Yagi SM. Comparative chemical profiling and antimicrobial activity of Nigella sativa seeds oils obtained from different sources. Nat Resour Hum Health 2022; 2:194–199. [Crossref]
- Umaiyambigai D, Saravanakumar K, Raj AG. Phytochemical profile and antifungal activity of leaves methanol extract from the Psydrax dicoccos (Gaertn) Teys. and Binn. rubiaceae family. Int J Pharmacol Phytochem Ethnomed 2017; 7:53-61. [Crossref]
- Lou-Bonafonte JM, Martinez-Beamonte R, Sanclemente T, et al. Current insights into the biological action of squalene. Mol Nutr Food Res 2018; 62:e1800136. [Crossref][Goooglescholar][Indexed]
- Huang ZR, Lin YK, Fang JY. Biological and pharmacological activities of squalene and related compounds potential uses in cosmetic dermatology. Molecules 2009; 14:540–554. [Crossref][Goooglescholar][Indexed]
- Nam Hoang N, Kodama T, Nwet Win N, et al. A new monoterpene from the rhizomes of Alpinia galanga and Its anti-VPR activity. Chem Biodivers 2021; 18:e2100401. [Crossref][Goooglescholar][Indexed]
- Hanh TT, Giang VH, Trung NQ, et al. Chemical constituents of Blumea balsamifera. Phytochem Lett 2021; 43:35–39. [Crossref][Goooglescholar][Indexed]
- Chen S, Luo H, Chen L, et al. An overall picture of SARS Coronavirus (SARS-CoV-2) genome encoded major proteins structures, functions and drug development. Curr Pharm Des 2006; 12:4539-4553. [Crossref][Goooglescholar][Indexed]
- Asif M, Saleem M, Saadullah M, et al. COVID-19 and therapy with essential oils having antiviral, anti-inflammatory and immunomodulatory properties. Inflammopharmacology 2020; 28:1153-1161. [Crossref][Goooglescholar][Indexed]
- da Silva JK, Figueiredo PL, Byler KG, et al. Essential oils as antiviral agents potential of essential oils to treat SARS-CoV-2 infection an in-silico investigation. Int J Mol Sci 2020; 21:3426. [Crossref][Goooglescholar][Indexed]
- Loizzo MR, Saab AM, Tundis R, et al. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem Biodivers 2008; 5:461–470. [Crossref][Goooglescholar][Indexed]
- Chatow L, Nudel A, Nesher I, et al. In vitro evaluation of the activity of terpenes and cannabidiol against human Coronavirus E229. Life 2021; 11:290. [Crossref][Goooglescholar][Indexed]
Author Info
Hayder T Hasan* and Enas J Kadhim
Department of Pharmacognosy and Medicinal Plants, College of Pharmacy, University of Baghdad, Baghdad, IraqCitation: Hayder T Hasan, Enas J Kadhim, Phytochemical Investigation and Pharmacological Activity of Petroleum Ether Fraction of Solidago canadensis L. against COVID-19 Activity, J Res Med Dent Sci, 2023, 11 (01):215-231
Received: 25-Oct-2022, Manuscript No. JRMDS-22-71571; , Pre QC No. JRMDS-22-71571 (PQ); Editor assigned: 28-Oct-2022, Pre QC No. JRMDS-22-71571 (PQ); Reviewed: 11-Nov-2022, QC No. JRMDS-22-71571; Revised: 26-Nov-2022, Manuscript No. JRMDS-22-71571 (R); Published: 23-Nov-2022