Review Article - (2023) Volume 11, Issue 1
Phytochemical Investigation and Antibacterial Assessment of Iraqi Tropaeolum majus L. Extracts (Ethyl Acetate Fraction)
Mazin Saleem Shakir* and Enas Jawad Kadhim
*Correspondence: Mazin Saleem Shakir, Department of Pharmacognosy, Ministry of Health, Babil Health Directorate, Iraq, Email:
Abstract
Tropaeolum majus has a long history of use as a medicinal plant with a wide range of therapeutic applications. The purpose of this study was to evaluate the antibacterial effect of ethyl acetate fraction of Tropaeolum majus plant against four genera of bacteria (Escherichia coli, Salmonella typhimurium, Pseudomonas aeruginosa and Staphylococcus aureus) obtained from nawah scientific center. The antibacterial activity of this plant (0.0003, 0.0007, 0.0015, 0.003, 0.006, 0.012, 0.025, 0.05, 0.1 and 0.2 mg/ml) were evaluated using broth macro dilution method (methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically) and the Minimum Inhibitory Concentration (MIC) values of tested ethyl acetate extract were found from 0.025 to 0.05 mg/ml. Preliminary phytochemical investigation of Tropaeolum majus leaves extract revealed the presence of different chemical constituents like alkaloids, glycosides, tannins, terpenoids, phenols and flavonoids, while for ethyl acetate fraction indicated the presence of glycosides, tannins, phenols and flavonoids. Analysis of ethyl acetate fraction by High Performance Liquid Chromatography (HPLC) showed the presence of four phenolic compounds in the ethyl acetate fraction using (gallic acid, p-coumaric, chlorogenic acid and rutin) as standards. In conclusion, the presence and diversity of secondary metabolites may be responsible for the observed antibacterial activity.
Keywords
Phytochemicals, Antibacterial activity, Tropaeolum majus, Alkaloids, Glycosides
Introduction
For thousands of years, humans have known about the benefits of using plants to treat or alleviate illness. Novel chemical compounds can be found in the plant kingdom which may be significant due to their potential use in medicine (e.g. food and forage conservation) [1].
Plant extracts were regarded as important for the treatment of different ailments by ancient civilizations and natural products account for approximately 30% of global drug sales. It is estimated that there are approximately 2500000 species of higher plants in the world and the majority of them have not been thoroughly investigated for their pharmacological activities. However, there has been a growing interest in plant uses and the identification of plant constituents with antibacterial activity for several decades. The most important reason for the latter was that infections are one of the leading causes of illness and mortality worldwide, particularly infections caused by Enterococcus and Staphylococcus species, which are agents of many intra hospital infections [2]. Furthermore, the emergence of drug resistance and the emergence of undesirable side effects of various antibiotics has encouraged the quest for new antimicrobial agents, especially among plant extracts, to identify new chemical structures to avoid the a fore mentioned disadvantages [3,4].
Despite the fact that pharmaceutical industries have developed several new antibiotics over the last three decades, microorganism resistance to these drugs has increased. Bacteria, in general, have the genetic ability to transmit and acquire resistance to drugs used as therapeutic agents. This is cause for concern, given the number of patients in hospitals who have suppressed immunity, as well as new multi resistant bacterial strains. As a result, new infections can occur in hospitals, resulting in a high mortality rate [5,6].
The problem of microbial resistance is worsening and the future use of antimicrobial drugs remains uncertain. As a result, steps must be taken to address this issue, such as limiting antibiotic use, conducting research to better understand the genetic mechanisms of resistance and continuing studies to develop new drugs, both synthetic and natural. The ultimate goal is to provide patients with appropriate and efficient antimicrobial drugs.
Tropaeolum majus is a member of the tropaeolaceae family and is commonly known as garden nasturtium, Indian cress, or monk's cress. It’s a cultigen that originated in the South American Andes, where it has been grown as a crop plant since ancient times [7]. It had been brought to Europe in 1684 from Peru by a Dutch monk, Pater Beverning [8]. It is cultivated currently in most countries of the world, including Iraq, as an ornamental plant. It's widely cultivated as a decorative as well as a therapeutic plant [9]. It is a bushy (about 30 cm tall) or vining (may stretch out up to 90 cm) plant that grows rapidly. It has tender, rounded, watercress flavored leaves (5 to 15 cm across), held by long fleshy stalks and showy trumpet shaped yellow or orange flowers with reddish patches. The plant as a whole has a spicy peppery flavor. Fresh leaves, flowers and stems are used in salads and green pods can be pickled and used as a caper alternative [10-12].
Tropaeolum majus is prevalently utilized as an antiseptic, diuretic, laxative, hair tonic, antiscorbutic, antiinflammatory, antihypertensive and antidepressant. Likewise, it is applied in the cleaning of skin, eyes and the treatment of skin disorders, furunculosis, acne, pulmonary disorders, amyotrophic lateral sclerosis, psoriasis, eczema and scrofula [13,14].
The few studies that are available indicate the existence of certain chemical components in specific parts of the plant, but no complete characterization has been done. These chemical constituents can be assigned to various classes of polyphenols, flavonoids, alkaloids, saponins, tannins, anthocyanins, carotenoids and terpenoids [15,16].
The purpose of our study was to determine the antibacterial activity of Tropaeolum majus extracts (ethyl acetate fraction). The antibacterial activity from Tropaeolum majus was tested against four genera of bacteria (Escherichia coli, Salmonella typhimurium, Pseudomonas aeruginosa and Staphylococcus aureus).
Materials and Methods
Plant material collection
The leaves of Tropaeolum majus of the family (tropaeolaceae) were collected from Babil in January and February 2021. The plant was identified and authenticated by Prof Dr. Sukaena Abass/Department of Biology/College of Sciences/University of Baghdad. The leaves were thoroughly washed, dried in the shade and ground to a fine powder in a mechanical grinder.
Method of work
The leaves' air dried powder is weighed, then defatted with n-hexane to remove chlorophyll and waxy material, then extracted in soxhlet with 80% ethanol for 18 hours, then mixed and dried using a rotary evaporator, the dry extract is weighted and the yield of extraction is calculated. The dry extract is dissolved in water and treated with various reagents to screen for the type of phytochemicals present and then partitioned 2-3 times with various solvents of different polarities such as petroleum ether, chloroform, ethyl acetate and n-butanol then each fraction is dried and weighted.
Preliminary phytochemical examination of plant extract
Phytochemical analysis for the detection and identification of bioactive chemical components in the medicinal plants studied was carried out using the standard methods described by harborne [17].
The preliminary phytochemical screening tests is also helpful within the detection of the bioactive principles and after might result in the drug discovery and development. In addition, these tests facilitate their quantitative estimation and qualitative separation of pharmacologically active chemical compounds.
In this study we used ethyl acetate fraction to evaluate the antibacterial activity of Tropaeolum majus, since contains several active constituents like flavonoids, phenols, tannins and other bioactive compounds.
HPLC analysis
HPLC was utilized to assess the flavonoids and phenolic acids contained in the ethyl acetate fraction of Tropaeolum maju. The phenol standards used included (gallic acid, p-coumaric acid, chlorogenic acid and rutin). The standards were dissolved in a mobile phase at concentrations of 1 mg/mL and then filtered through a 0.22 μm syringe filter then 10 μl were injected into the HPLC column. The measurements were done using a waters 2690 alliance HPLC system equipped with a waters 996 photodiode array detector. The column type used was column C18 inertsil: 4.6 x 250 mm, 5 μm and the mobile phase consist of buffer (0.1% acetic acid in water) and methanol. The phenols were identified by comparing retention times of standards and calculating absorbance ratios following co-injection of samples and standards.
Test organisms
The test organisms used in this study were obtained from Nawah Scientific Inc. (Mokatam, Cairo, Egypt), which include (Escherichia coli ATCC 25922, Salmonella typhimurium ATCC 14028, Pseudomonas aeruginosa ATCC 25668 and Staphylococcus aureus ATCC 6538).
Preparing inoculum (colony suspension method)
A disc of Escherichia coli ATCC 25922, Salmonella typhimurium ATCC 14028, Pseudomonas aeruginosa ATCC 25668 and Staphylococcus aureus ATCC 6538, were inoculated separately into 100 ml of tryptic Soy broth medium and incubated at 37.0°C ± 1.0°C for 24.0 ± 2.0 hr. For the preparation of fresh (18-24 h) culture agar plate, a loopful from the broth was streaked onto tryptic soy agar medium, incubated at 37.0°C ± 1.0°C for 21.0 ± 3.0 hr. Inoculating 3-4 colonies (from each organism plate) yielded a straight sterile saline solution, which was then adjusted to reach turbidity corresponding to a 0.5 McFarland standard. A 0.5 McFarland standard and the DENSICHEK optical instrument were used to standardize inoculum density. That adjustment results in a suspension containing approximately 1.0 × 108 CFU/mL for each test organism. The suspension was diluted to 1.0 × 107 CFU/mL by taking 1.0 ml and diluting it in 9.0 ml broth. Then, 5.0 ml was added to 5.0 ml sterile broth to yield approximately 5.0 × 106 CFU/ml [18].
Broth macro dilution method
First, 5.0 ml of each sample was added to 5.0 ml tube broth (1:2 dilution), mixed well and then 5.0 ml of the 1:2 dilution was aspirated using a fresh tip and added to the next 5.0 mL broth (1:4). This procedure was repeated for each antibacterial sample to produce at least (9) dilutions. In the first well of the 24 well plates, 1.0 mL of the sample was directly inoculated and 1.0 mL of each dilution was inoculated in the subsequent wells. Each well received 100 μl of prepared inoculum, resulting in a final concentration of 5.0 × 105 CFU/mL (the optimum concentration is 2-8 × 105 CFU/mL). To validate inoculum density, another 100 μl l of each organism solution was diluted and cultivated (externally). Each organism/sample plate received a growth control well containing inoculation broth but no sample. To each organism/sample plate, a negative control well containing only broth and no bacteria was introduced. All plates were incubated for 24.0 ± 2.0 hours at 37.0°C ± 1.0°C. Plates were taken from the incubator and put on a dark surface to monitor growth after incubation [18].
Determination of MIC values
The broth macro dilution technique was used to determine the Minimum Inhibitory Concentrations (MIC) of plant crude extracts [19]. The sample concentration range was prepared by diluting the stock solutions twice in sterile broth. Ten dilutions of the samples were examined, ranging from 0.0003 to 0.2 mg/ml.
Results
Phytochemical screening
Preliminary phytochemical examination of crude leaves extracts of Tropaeolum majus revealed the presence of flavonoids, phenols, tannins, alkaloids and terpenoids, while the preliminary phytochemical examination of ethyl acetate fraction revealed the presence of flavonoids, phenols and tannins. The results of these tests are summarized in Tables 1 and 2.
| Crude extract | Alkaloids | Flavonoids | Glycosides | Phenols | Tannins | Terpenoids |
|---|---|---|---|---|---|---|
| + | + | + | + | + | + |
Table 1: Phytochemical screening results of Tropaeolum majus crude extract.
| Ethyl acetate fraction | Alkaloids | Flavonoids | Glycosides | Phenols | Tannins | Terpenoids |
|---|---|---|---|---|---|---|
| - | + | + | + | + | - |
Table 2: Phytochemical screening results of ethyl acetate fraction.
HPLC analysis
Four phenolic compounds (gallic acid, p-coumaric, chlorogenic acid and rutin) were detected in the ethyl acetate fraction of Tropaeolum majus leaves extract by comparison of their retention time with the retention time of the standards as shown in Figure 1.
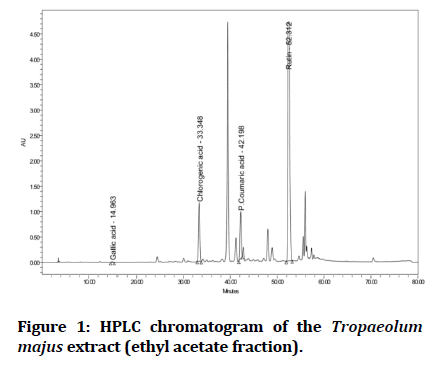
Figure 1: HPLC chromatogram of the Tropaeolum majus extract (ethyl acetate fraction).
Antibacterial activity
The results obtained for Tropaeolum majus leaves extract exhibits a valuable antibacterial activity against the tested microorganisms at a concentration from 0.025 to 0.05 mg/mL as shown in Table 3 and MIC values of the plant extract against these bacterial species are presented in Table 4 and shown in Figures 2 and 3.
| Dilution (mg/mL) |
0.2 | 0.1 | 0.05 | 0.025 | 0.012 | 0.006 | 0.003 | 0.0015 | 0.0007 | 0.0003 |
|---|---|---|---|---|---|---|---|---|---|---|
| E. coli ATCC 25922 |
C | C | C | C | T | T | T | T | T | T |
| S. tyhimurium ATCC 14028 |
C | C | C | C | T | T | T | T | T | T |
| P. aeruginosa ATCC 25668 | C | C | C | C | T | T | T | T | T | T |
| S. aureus ATCC 6538 |
C | C | C | T | T | T | T | T | T | T |
Table 3: Minimal Inhibitory Concentrations (MIC) reading results.
| Test organism | MIC value |
|---|---|
| Escherichia coli ATCC 25922 | 0.025 mg/ml |
| Salmonella typhimurium ATCC 14028 | 0.025 mg/ml |
| Pseudomonas aeruginosa ATCC 25668 | 0.025 mg/ml |
| Staphylococcus aureus ATCC 6538 | 0.05 mg/ml |
Table 4: Minimal Inhibitory Concentrations (MICs) of Tropaeolum majus extract determined by the agar macro dilution method.
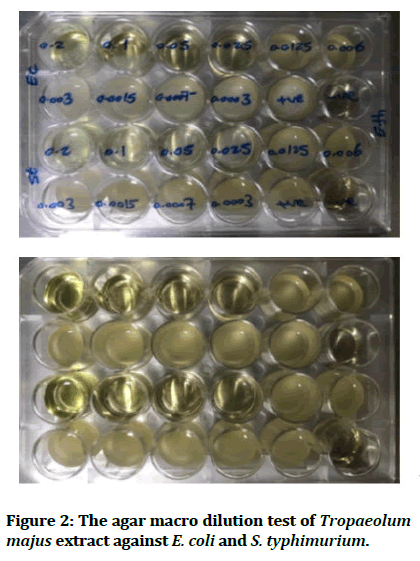
Figure 2: The agar macro dilution test of Tropaeolum majus extract against E. coli and S. typhimurium.
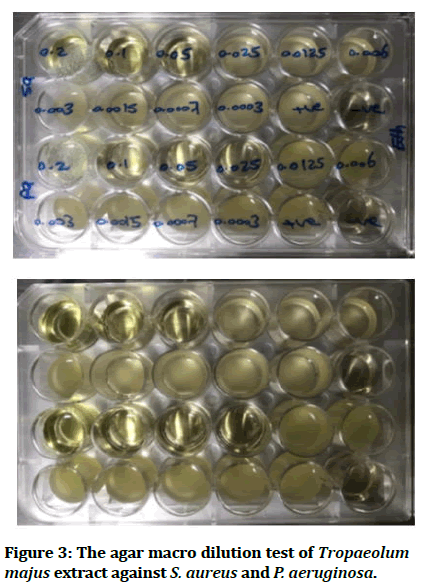
Figure 3: The agar macro dilution test of Tropaeolum majus extract against S. aureus and P. aeruginosa.
Discussion
Natural plant substances may successfully serve as an alternative to synthetic compounds commonly used in medicine. The plant assayed in this study is commonly used as medicinal plants in different parts of the world. The previous studies showed that there is no or weak antibacterial activity of Tropaeolum majus due to the low amount of phenols in the plant extract as a result of the type of methods used for plant extraction (decoctions and sonication), but our study showed that the Iraqi Tropaeolum majus leaves extract (extracted in soxhlet) possesses good antibacterial activity against selected pathogens due to the presence of the phenolic acids and flavonoid mentioned above. Many studies showed that phenolic compounds that have been identified in Tropaeolum majus leave extract (gallic acid, p-coumaric, chlorogenic acid and rutin) possess good antibacterial activity against different types of bacterial strains (gram positive and gram negative bacteria) [20-23]. The current study's findings are promising and may improve the use of Tropaeolum majus leaves extract in the treatment of various bacterial infections especially (Escherichia coli, Salmonella typhimurium, Pseudomonas aeruginosa and Staphylococcus aureus).
Conclusions
In this study, we evaluated the antibacterial activity of the Tropaeolum majus extract (ethyl acetate fraction). And it displayed a good antibacterial activity with MIC<100 mg/mL due to the presence of phenolic compounds in ethyl acetate fraction at a much higher concentration than other fractions, indicating that these plants could be a good source for the antibacterial agents. More research is needed to investigate the additional antimicrobial effectiveness aspects of these powerful plant extracts (e.g. in vivo efficacy, toxicity and anti-mycobacterial, antiviral and anti-parasitic activity).
References
- Soberon JR, Sgariglia MA, Sampietro DA, et al. Antibacterial activity of plant extracts from northwestern Argentina. J Appl Microbiol 2007; 102:1450–1461. [Crossref][Googlescholar][Indexed]
- Maple PAC, Hamilton Miller JMT, Brumfitt W. Worldwide antibiotic resistance in methicillin resistant Staphylococcus aureus. Lancet 1989; 333:537–540. [Crossref][Googlescholar][Indexed]
- Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science 1994; 264:375–382. [Crossref][Googlescholar][Indexed]
- Ordonez AAL, Gomez JD, Cudmani NM, et al. Antimicrobial activity of nine extracts of Sechium edule (Jacq.) Swartz. Microb Ecol Health Dis 2003; 15:33–39. [Crossref][Googlescholar][Indexed]
- Nascimento GGF, Locatelli J, Freitas PC, et al. Antibacterial activity of plant extracts and phytochemicals on antibiotic resistant bacteria. Braz J Microbiol 2000; 31:247–256. [Crossref][Googlescholar][Indexed]
- Cohen ML. Epidemiology of drug resistance: Implications for a post-antimicrobial era. Science 1992; 257:1050–1055. [Googlescholar][Indexed]
- Munir M, Alhajhoj MR, Khakwani AA, et al. Flowering time response of nasturtium (Tropaeolum majus L.) cultivar ‘Empress of India’ to photoperiod, light integral and temperature using photo thermal model. Songklanakarin J Sci Technol 2015; 37:247–254. [Googlescholar][Indexed]
- Christenhusz MJM. 745. Tropaeolum Minus. Curtis’s Bot Mag 2012; 29:324–330. [Crossref][Googlescholar][Indexed]
- Huxley AJ, Griffiths M. Dictionary of gardening. Stockton Press, United States. 1992. [Googlescholar]
- Niizu PY, Rodriguez Amaya DB. Flowers and leaves of Tropaeolum majus L. as rich sources of lutein. J Food Sci 2005; 70:S605-S609. [Crossref][Googlescholar][Indexed]
- Jakubczyk K, Janda K, Watychowicz K, et al. Garden nasturtium (Tropaeolum majus L.) a source of mineral elements and bioactive compounds. Rocz Panstw Zakl Hig 2018; 69:119–126. [Crossref][Googlescholar][Indexed]
- Leila A, Samıra A, Ghozlène A, et al. Anatomical study of garden nasturtium (Tropaeolum majus L.) growing under the climatic conditions of annaba (Eastern Algeria). Int J Innov Approaches Agric Res 2019; 3:257–266. [Crossref][Googlescholar][Indexed]
- Brondani JC, Cuelho CHF, Marangoni LD, et al. Traditional usages, botany, phyto chemistry, biological activity and toxicology of Tropaeolum majus L.-A review. Bol Latinoam Y Del Caribe Plantas Med Y Aromat 2016; 15:264–273. [Googlescholar][Indexed]
- Cock IE, Van Vuuren SF. The traditional use of southern African medicinal plants in the treatment of viral respiratory diseases: A review of the ethnobotany and scientific evaluations. J Ethnopharmacol 2020; 262:113194. [Crossref][Googlescholar][Indexed]
- Jurca T, Baldea I, Filip GA, et al. A phyto complex consisting of Tropaeolum majus L. and Salvia officinalis L. extracts alleviates the inflammatory response of dermal fibroblasts to bacterial lipopolysaccharides. Oxid Med Cell Longev 2020; 2020. [Crossref][Googlescholar][Indexed]
- Bazylko A, Granica S, Filipek A, et al. Comparison of antioxidant, anti-inflammatory, antimicrobial activity and chemical composition of aqueous and hydroethanolic extracts of the herb of Tropaeolum majus L. Ind Crops Prod 2013; 50:88–94. [Crossref][Googlescholar][Indexed]
- Herborne JB. Phytochemical methods. A Guid to Mod Tech plant Anal 1973; 2:5–11. [Crossref]
- Clinical and Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 11th edition, Pennsylvania 19087, USA. 2018.
- Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the Minimal Inhibitory Concentration (MIC) of antimicrobial substances. Nat Protoc 2008; 3:163–175. [Crossref][Googlescholar][Indexed]
- Borges A, Ferreira C, Saavedra MJ, et al. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb Drug Resist 2013; 19:256–265. [Crossref][Googlescholar][Indexed]
- Lou Z, Wang H, Rao S, et al. P-coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012; 25:550–554. [Crossref][Googlescholar][Indexed]
- Lou Z, Wang H, Zhu S, et al. Antibacterial activity and mechanism of action of chlorogenic acid. J Food Sci 2011; 76:M398-M403. [Crossref][Googlescholar][Indexed]
- Adamczak A, Ozarowski M, Karpinski TM. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J Clin Med 2019; 9:109. [Crossref][Googlescholar][Indexed]
Author Info
Mazin Saleem Shakir* and Enas Jawad Kadhim
Department of Pharmacognosy, Ministry of Health, Babil Health Directorate, IraqCitation: Mazin Saleem Shakir, Enas Jawad Kadhim, Phytochemical Investigation and Antibacterial Assessment of Iraqi Tropaeolum majus L. Extracts (Ethyl Acetate Fraction), J Res Med Dent Sci, 2023, 11 (01): 138-143.
Received: 23-Oct-2022, Manuscript No. JRMDS-23-71573; , Pre QC No. JRMDS-23-71573 (PQ); Editor assigned: 26-Oct-2022, Pre QC No. JRMDS-23-71573 (PQ); Reviewed: 06-Nov-2022, QC No. JRMDS-23-71573; Revised: 28-Dec-2022, Manuscript No. JRMDS-23-71573 (R); Published: 10-Jan-2023
