Review Article - (2022) Volume 10, Issue 8
Periodontal Diseases Affecting Cardiovascular Health
Shivani Singh Rawat1*, Khushboo Durge1, Pavan Bajaj1, Bhairavi Kale1 and Anjali Borle2
*Correspondence: Shivani Singh Rawat, Department of Periodontics, Sharad Pawar Dental College & Hospital, Datta Meghe Institute of Medical Sciences, Sawangi (Meghe) Wardha, India, Email:
Abstract
Oral health is the most underappreciated part of one's health, and it plays a role in bad health. Medical emergencies can happen to anyone, although they are more common in elderly and medically vulnerable patients. Physical or mental difficulties may be linked to dental pathology in a rapidly rising proportion of the population. In patients with Periodontitis, the microbiota supplies a reservoir of gram-negative bacteria. Bacteria are the increases the cause of periodontal inflammation. The penetration of these germs destroys the tooth-supporting system, ending into disease. Any infection triggers inflammation in inflammatory cells. Inflammatory variables that predispose to vascular damage include C reactive proteins, fibrinogen, interleukins, and tumour necrosis factor. As a result of such reactions, several cardiovascular illnesses such as atherosclerosis, coronary heart disease, and myocardial infarction might develop. As a result, to avoid such outcomes, interventional periodontal therapy is performed. Adequate brushing, mechanical therapies like as interdental assistance, host modulation therapies, and antibacterial therapy are examples of interventional therapies. It is always preferable to treat illness and cure it promptly.Keywords
Tooth-supporting system, Atherosclerosis, Coronary heart disease, Myocardial infarction
Introduction
Benjamin Franklin’s remark “An ounce of prevention is worth a pound of cure” [1]. Underlines the importance of prevention in terms of disease prevalence and its effect of causation into the physiologic functioning. Oral health is the most overlooked aspect of one’s health contributing ill health. It is an essential and vital component of overall health and is associated with much more than just healthy teeth [2].
Periodontal disease is a complex, multifaceted, chronic inflammatory condition that affects 10% to 15% of the population. Gingiva and teeth’s supporting structures, such as bone and periodontium, are degraded by bacteria (microbial film) and inflammation.
Periodontitis is the most common disease of the oral cavity which not only deranges the oral health but also overall altogether.
Periodontitis is initiated by various Gram-negative bacteria characterized by the destruction of the periodontal tissue. Intracellular proteases, caspases that are the key mediators of apoptosis, are shown to involve in disease progression [3].
The inflammation of periodontium is caused by bacteria, primarily by gram negative anaerobic bacteria (It may consist of Actinomycetes species, P micros, P intermedia, P gingivalis and trepanoma species, etc.) The infiltration of these bacteria causes the destruction of tooth supporting apparatus and cause the dis-ease. When left untreated it can sabotage the underlying bone. Diabetes, smoking and chewing tobacco are the two most major risk factors/ventures.
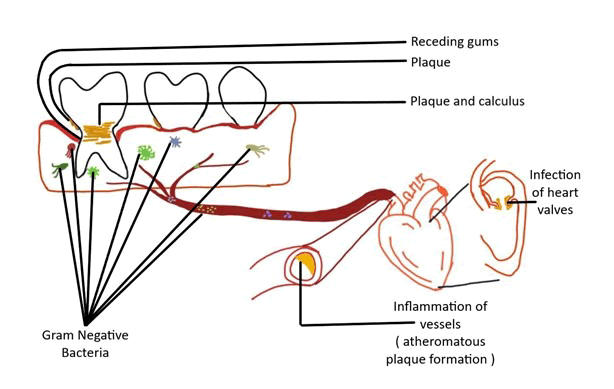
The link between periodontal disease and cardiovascular health is strong, and it's clear that the two are intertwined. Researchers discovered that the bacteria causing disease can spread throughout the blood via blood stream producing inflammation in heart vessels and infection in heart valves. Therefore the upcoming part of this review article will emphasizing on strong correlation between periodontal disease and cardiovascular disorders.
Literature Review
As a corollary, the next section of this review will focus on the substantial link between periodontal disease and cardiovascular disease.
The sub gingival environment as a reservoir of Bacteria
Oral flora complexity should be taken into account as a further complicating factor. Only 5% of the bacteria found in the oral cavity are thought to be closely linked to periodontal disease. The sub gingival microbiota in patients suffering from Periodontitis provides a pool of gram negative bacteria. These bacteria and their products have easy access to periodontal tissues and the circulatory system through the sulcular epithelium, which is often ulcerated and discontinuous [4].
Figure 1: Representation of relationship between periodontitis and cardiovascular disease.
At the turn of the twenty-first century, medicine and dentistry were investigating why people become affected with a wide range of systemic illnesses. Thanks to two men, WD Miller and William Hunter, the assumption that oral germs and infection were the likely origins of most of a person's systemic illness gained a lot of traction. Infections, particularly those originating in the mouth, have been the source of the man's pain and illness for the past 40 years. Willoughby D Miller, a microbiologist in Philadelphia, and William Hunter, a London physician, are credited with pioneering this era, which became known as the "age of focused infection" [5].
"Focal infection" meant a nidus of infection anywhere in the body, such as Periodontitis, that might impact distant sites and organs via the circulation.
Dentists and physicians felt that germs on the teeth and the infectious disorders that followed, including as dental caries, gingivitis, and Periodontitis, were a "focal of infection" that led to a wide range of systemic problems in the 1920 s and 1930 s. During this time, tooth extraction became popular as a technique of removing oral bacteria from the body and preventing disorders of the joints, as well as diseases of the heart, liver, kidneys, and pancreas.
By 1940, however, medicine and dentistry had realized that a patient's general systemic condition was explained by much more than bacteria in his or her mouth. Dentists and doctors recognized that: Dentists and doctors recognized that:
- Teeth extraction did not always make a person feel better or cure their ailment.
- Systemic disorders arose in people who had a healthy mouth and no evident oral infection.
- People who didn't have teeth and so didn't have an obvious oral infection had systemic ailments [6].
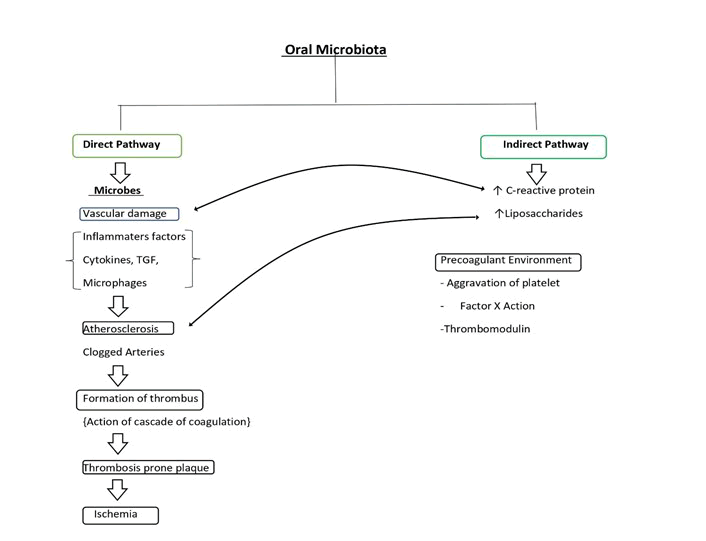
Pathophysiology of Periodontal disease: Periodontal disease is caused by bacteria that build up on the tooth surface and release cell wall components including LPS and endotoxins. The immune cells in the body are activated as a result of this (tumor necrosis factor-a TNFa, monocytes, IL-6, and interleukin-1b). Early gingival inflammation is assumed to be a bacterial invasion defense mechanism. The acute phase of inflammation becomes a chronic pathogenic condition when it does not resolve in an individual [7].
Figure 2: Possible pathways of disease causation.
Impact of periodontal pathogens on cardiovascular well being
Atherosclerosis: Atherosclerosis is a narrowing of the arterial intima. Damage to the vascular endothelium causes an inflammatory response from circulating monocytes sticking to the endothelium, which leads to the formation of atherosclerotic plaques. The presence of intravascular bacteria and their products can induce chemical damage to the vascular endothelium [8]. The growth of atheromatous plaques and thickening of vessel walls narrows the lumen and reduces blood flow through arteries substantially. After an atheromatous plaque ruptures, arterial thrombosis is common. Platelet and coagulation pathways are activated when a plaque is repaired. Platelet fibrin builds up cause’s thrombus, which can obstruct the vessel and cause an ischemia event like angina or a heart attack. The thrombus may break from the vessel wall and create an embolus, which can obstruct the vessels and cause serious complications including stroke [9].
Periodontal patients associated with stroke
A systemic bacterial or viral infection is frequently the cause of strokes. Thromboembolic events are the most common cause of stoke. Periodontitis was found to be a larger risk of stroke than smoking, regardless of any recognized risk factors. Poor dental health was found to be a significant risk factor for cerebrovascular ischemia in case control studies. In one study, male stroke patients had considerably more bleeding on probing, suppuration, sub gingival calculus, and the number of periodontal and periapical lesions than controls [10-12].
Thrombogenesis: Thromboembolism is the most common cause of acute myocardial infarction, and platelet aggregation plays an important role. Streptococcus sanguins, the most prevalent component of supragingival plaque, and Porphyromonas gingivalis, with which platelets adhere, are intimately associated with Periodontitis [13-14]. Approximately 8% of all occurrences of infective endocarditis are thought to be linked to periodontal or dental disorders, with no prior dental procedure.
Myocardial Infraction: Gram negative bacteria and their accompanying lipopolysaccharides have been discovered to produce infiltration of inflammatory cells into the artery wall, proliferation of arterial smooth muscle cells, and intravascular coagulation in animal models. Chronic system exposure to these organisms' products occurs as a result of periodontal illnesses. Endothelial and arterial wall integrity, as well as platelet function, may be induced by low-level bacteraemia, resulting in atherogenic alterations and possibly thrombogenic events [15-16].
Coronary heart disease: Ischemic Heart disease is one of the leading causes of mortality in the world [17]. Periodontal disease has been linked to coronary heart disease, suggesting that it is an independent, albeit minor, and risk factor. According to studies, there are two possible causes for coronary heart disease. For starters, research has revealed that periodontal disease is a persistent infection that leads to a chronic inflammatory response. Second, a biological consideration is the intermittent bacteraemia associated with periodontal disease and its possible role in the chronic inflammatory state or more directly on endothelial tissue surfaces [18-19].
Influence of periodontal inflammatory stimulants
A biomarker is an indicator of a natural state and therefore could assist in distinguishing between normal and pathologic processes [20].
Reactive proteins: C-Reactive Protein (CRP) is an acute phase protein produced by the liver and used as a response biomarker diagnostic. It's a type 1 acute phase protein that's involved in inflammation and infection. A few studies have found a substantial increase in CRP levels following nonsurgical periodontal therapy, whereas others have seen a decrease [21].
Fibrinogen: Blood viscosity is influenced by fibrinogen, a soluble plasma glycoprotein generated by the liver and converted to fibrin by thrombin during blood coagulation. By damaging artery endothelial cells, increasing vascular muscle cell proliferation, and activating inflammatory cells, it can increase early plaque formation. It also affects platelet adhesion and aggregation, which is important in thrombosis. As a result, a high fibrinogen level can increase the risk of coronary heart disease by causing platelet aggregation and thrombosis [22].
Tumour necrosis factor: Interleukin-1, Tumour necrosis factor, interleukin-6, and interleukin-8 have different causes. Cellular activation involving cellular adhesion molecules, toll-like receptors, matrix metalloproteinase, and nuclear factor-B activation is connected to systemic inflammation. In individuals suffering Periodontitis caused by atheromatous plaque rupture, the following interaction between endothelium, monocytes, and platelets may be proatherogenic, resulting in atherogenesis or poor cardiovascular outcomes [23].
Influence of interventional periodontal therapy
Disease is merely a healthy response to an unhealthy oral cavity environment. It is preferable to prevent a sickness than to treat it later in life. The following are the goals of interventional periodontal therapy:
- Remove soft deposits such as tooth plaque, material Alba, and food debris.
- Gingival massage promotes keratinization and antibacterial protection while also improving circulation.
- Preventing the formation of calculus.
Brushing teeth has a specific goal
Brushing and flossing are the first-line treatments, as they dramatically lower the amount of bacteria in the mouth. Brushing for two minutes twice a day and flossing once a day is recommended by the American Dental Association. It's fantastic for gingivitis and periodontitis treatment. However, a considerable section of the population does not follow the above-mentioned regimen.
- The plaque can be removed.
- Removal food particles and stains from the teeth.
- Stimulation of gingival tissue
Mechanical therapies for the treatment of periodontal diseases
Interdental aids such as dental floss, toothpicks, and an interdental brush can help enhance oral health. Plaque control has been proven to be significantly reduced when breath malodor is controlled. It is the most reliable technique to eliminate oral malodor, especially when combined with daily tongue scarping to lower oral cavity bacterial burden.
Due to the drawbacks of mechanical plaque management aids, chemical aids like as antiseptics and antibiotics have been developed, with chlorhexidine being the most often used antiseptic.
Scaling removes enough plaque and calculus from the enamel to leave a smooth, clean surface, whereas root planning removes plaque and calculus from the root surfaces. Cemental irregularities may contain calculus. Subgingival debridement should leave enough plaque free space for junctional epithelium and epithelial attachment to regenerate. The amount of subgingival root surface washing required to do this is likely to differ from patient to patient and site to site. Although it is well known that non-surgical instrumentation frequently fails to achieve complete removal of plaque and calculus, it may still be associated with periodontal health, as failure to remove all plaque and calculus can result in recolonization of the root surface and inflammation. Of course, good supra gingival plaque control is still necessary to allow pocket healing. Occlusal realignment has also been advocated as a method of preventing periodontal tissue damage caused by occlusal trauma. Damaged or necrotic bone and periodontal tissue are repaired using guided tissue regeneration and periodontal surgery. All of these treatments aid in reducing the number of bacteria in the biofilm as well as controlling the accelerating variables. To limit periodontal inflammation and disease recurrence, patients with periodontitis should accept such maintenance and preventative procedures as a lifelong commitment [24-25].
Chemotherapeutic agents used for the treatment of periodontal diseases
Host Modulation Therapy (HMT): Host modulatory therapy (HMT) aims to alter the host's response in order to lessen damage. Sub-antimicrobial doxycycline, bisphosphonates, anti-inflammatory medicines, enamel matrix compounds, and growth factors are among the host modifying drugs accessible. All of these medications have the ability to alter the host response and prevent the toxic elements produced by the immune system from entering the body. Prostaglandins and cytokinins are inhibited by anti-inflammatory medicines, whereas collagenase is inhibited by tetracyclines, and osteoclast activity is reduced by biphosphonates [26].
Antimicrobial therapy: Mechanical and surgical debridement are the most popular treatments for slowing disease development. However, they are unable to eliminate all germs from the periodontal pocket and tissues. As a result, after 8 weeks of treatment, remaining microorganisms in the periodontal environment recolonize. Chemotherapeutic medicines used in combination with mechanical and surgical debridement may be more effective as a result.
Discussion
Periodontal medicine is now a recognized specialty that employs periodontal therapy to treat systemic diseases associated to periodontal disease [26].
Evolution is continuous process of changes in structural and physiological mechanism in living being. Microbes/pathogens can evolve naturally or artificially and become resistant to various medicines [27]. As a result, rather than treating the condition, prevention must have been prioritised.
Conclusion
The contemporary information gap about the link between periodontal disease and systemic disease could have an impact on the dental care that patients get. The presence of periodontal disease may enhance the risk of a variety of systemic illnesses. Due to a plethora of variables, clinical evidence of causality will be extremely difficult to obtain in this case. Because systemic disease is typically asymptomatic in its early stages, the cause of the disease may be overlooked. The infection and inflammation that are thought to play a role in the development of systemic disease could originate elsewhere. These reactions connected to periodontal disease may play a role in systemic disease, according to the best available evidence. Individuals are not healthy until they have good dental health, so periodontal treatment must be administered based on the value of its advantages to patients' oral health. The recognition of periodontal disorders as a risk factor for cardiovascular disease, on the other hand, is leading to a convergence in dental and medical care that can only benefit patients and the general public. It's imperative to remember that even the most important priority is prevention; nonetheless, there are there are ways to improve oral health. Various ways include dental floss, toothpicks, and chlorhexidine mouthwash. Root planning eliminates plaque and calculus from the root surfaces, whereas scaling removes enough plaque and calculus from the enamel to leave a smooth, clean surface.
References
- McConnell S. An ounce of prevention. IEEE software. 2001; 18:5.
- Ikhar A, Chandak M, Motwani N, et al. Baseline Assessment of Oral Health Status of Ashram Schools in Wardha District. Int J Cur Res Rev 2020; 12:32.
[Crossref]
- Baliga V, Dhadse P, Ragit G, et al. Role of caspases in periodontal diseases. J Datta Meghe Inst Med Sci Univ 2019; 14:268.
- Colombo AP, Magalhães CB, Hartenbach FA, et al. Periodontal-disease-associated biofilm: A reservoir for pathogens of medical importance. Microb Pathog 2016; 94:27-34.
- Rocca JP, Fornaini C, Wang Z, et al. Focal Infection and Periodontitis: A Narrative Report and New Possible Approaches. Int J Microbiol 2020; 2020.
- REILLY PG, Claffey NM. A history of oral sepsis as a cause of disease. Periodontol 2000; 23:13-18.
- Mealey BL. Influence of periodontal infections on systemic health. Periodontology 1999; 21:197-209.
- Leishman SJ, Ford PJ, Do HL, et al. Periodontal pathogen load and increased antibody response to heat shock protein 60 in patients with cardiovascular disease. J Clin Periodontol 2012; 39:923-930.
- Beck J, Garcia R, Heiss G, et al. Periodontal disease and cardiovascular disease. J Periodontol 1996; 67:1123-1137.
- Sfyroeras GS, Roussas N, Saleptsis VG, et al. Association between periodontal disease and stroke. J Vasc Surg 2012; 55:1178-84.
- Grau AJ, Becher H, Ziegler CM, et al. Periodontal disease as a risk factor for ischemic stroke. Stroke 2004; 35:496-501.
- Dorfer CE, Becher H, Ziegler CM, et al. The association of gingivitis and periodontitis with ischemic stroke. J Periodontol 2004; 31:396-401.
- Scannapieco FA, Genco RJ. Association of periodontal infections with atherosclerotic and pulmonary diseases. J Periodontal Res 1999; 34:340-345.
- Hamilton JA, Hasturk H, Kantarci A, et al. Atherosclerosis, periodontal disease, and treatment with resolvins. Curr Atheroscler Rep 2017; 19:1-0.
- Emingil G, Buduneli E, Aliyev A, et al. Association between periodontal disease and acute myocardial infarction. J Periodontal 2000; 71:1882-1886.
- Mattila KJ, Nieminen MS, Valtonen VV, et al. Association between dental health and acute myocardial infarction. BMJ 1989; 298:779-781.
- Thakare P, Ankar R, Wavare S, et al. Ischemic heart disease: Case report. Indian J Forensic Med Toxicol 2020; 14:6618â??6622.
[Crossref][Google Scholar][Indexed]
- Hujoel PP, Drangsholt M, Spiekerman C, et al. Periodontal disease and coronary heart disease risk. Jama 2000; 284:1406-1410.
- Beck JD, Offenbacher S, Williams R, et al. Periodontitis: a risk factor for coronary heart disease? Ann Periodontol 1998; 3:127-1241.
- Jain P, Jain M, Gaikwad R, et al. Role of inflammation and inflammatory biomarkers in dental implant procedures: A comprehensive review. J Datta Meghe Inst Med Sci Univ 2020; 15:715â??718.
- Kanaparthy R, Kanaparthy A, Mahendra M. C-reactive protein as a marker of periodontal disease. Gen Dent 2012; 60:e1-5.
- Mattila K, Vesanen M, Valtonen V, et al. Effect of treating periodontitis on C-reactive protein levels: a pilot study. BMC infectious diseases. 2002; 2:1-3.
- Craandijk J, Van Krugten MV, Verweij CL et al. Tumor necrosis factorâ?α gene polymorphisms in relation to periodontitis. J Clin Periodontol 2002; 29:28-34.
- Forner L, Larsen T, Kilian M, et al. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol 2006; 33:401-417.
- Attin T, Hornecker E. Tooth brushing and oral health: how frequently and when should tooth brushing be performed? Oral Health Prev Dent 2005; 3.
- Tariq M, Iqbal Z, Ali J, et al. Treatment modalities and evaluation models for periodontitis. Int J Pharm Investig 2012; 2:106.
- Rajput DS. Evolution, ayurveda, immunity, and preventive aspects for emerging infectious diseases such as COVID-19. Int J Pharm. Sci Res 2020; 11:86â??93.
Author Info
Shivani Singh Rawat1*, Khushboo Durge1, Pavan Bajaj1, Bhairavi Kale1 and Anjali Borle2
1Department of Periodontics, Sharad Pawar Dental College & Hospital, Datta Meghe Institute of Medical Sciences, Sawangi (Meghe) Wardha, India2Department of Prosthodontics and Implantology, Sharad Pawar Dental College & Hospital, Datta Meghe Institute of Medical Sciences, Sawangi (Meghe) Wardha, India
Citation: Shivani Singh Rawat, Khushboo Durge, Pavan Bajaj, Bhairavi Kale, Anjali Borle, Periodontal Diseases Affecting Cardiovascular Health,” J Res Med Dent Sci, 2022; 10 (5):000-000.
Received: 27-May-2022, Manuscript No. JRMDS-22-47343; , Pre QC No. JRMDS-22-47343; Editor assigned: 30-May-2022, Pre QC No. JRMDS-22-47343; Reviewed: 13-Jun-2022, QC No. JRMDS-22-47343; Revised: 26-Jul-2022, Manuscript No. JRMDS-22-47343; Published: 05-Aug-2022