Research - (2021) Volume 9, Issue 4
Pain Modulation through Opioid and Monoaminergic Pathways in Aloe Vera Induced Analgesia in Rats
Snigdha Misra1 and Rajesh Kumar Suman2*
*Correspondence: Rajesh Kumar Suman, Department of pharmacology, Hind Institute of medical sciences, India, Email:
Abstract
Objective: To study analgesic activity and to evaluate the involvement of opioid and monoaminergic pain mechanisms in the antinociceptive activity of aqueous extract of aloe vera (AEAV)
Materials and Methods: Analgesic activity of AEAV alone (100, 200 and 400 mg/kg orally) and in combination with pentazocine or venlafaxine ( sub analgesic doses) were studied using tail immersion test and acetic acid induced writhing in rats. The effect of pre-treatment with opioid antagonist naltrexone 1 mg/kg was also studied.
Results: AEAV produced a dose dependent significant antinociceptive activity in tail immersion test and acetic acid induced writhing in rats (P< 0.01) which is suggestive of possible central as well as peripheral action. Administration of sub analgesic dose of AEAV with pentazocine or venlafaxine resulted in significant (P< 0.01) antinociceptive activity in both the pain models. This demonstrated the involvement of opioid receptors and serotonergic and noradrenergic systems (descending pathway) leading to synergistic action between the drugs. The central pathway was further confirmed by pre-treatment with naltrexone which inhibited antinociceptive activity induced by AEAV alone and combination with pentazocine or venlafaxine in both experimental models.
Conclusion: Aqueous extract of Aloe vera induced antinociception is mediated through both opioid and monoaminergic pain mechanisms suggesting its potential use in neuropathic and chronic pain conditions.
Keywords
Aloe vera, Analgesia, Opioid, Monoamines
Introduction
Chronic pain is one of the most prevalent, costly and disabling conditions in both clinical practice and the workplace, yet it often remains inadequately treated [1]. It is commonly associated with anxiety, depression, mood and sleep disorders [1]. The pharmacologic treatments for chronic pain include opioids, Tricyclic antidepressants, Anticonvulsants, NSAIDS & others [1]. Opioid & Non–opioid analgesics are associated with side effects such as drug abuse, somnolence or cognitive impairment, gastrointestinal bleeding and renal impairment [2].
The uses of herbal medicines continue to expand rapidly across the world. Aloe barbadensis commonly known as aloe vera is being used in traditional practice to treat pain, inflammation, ulcer and diabetes [3]. A recent study has reported antidepressant effect of aloe vera [4]. Low doses of antidepressants are often prescribed to relieve chronic pain in conditions such as diabetic neuropathy, migraine and tension headaches, osteoarthritis, and fibromyalgia [5]. Several studies have shown that aqueous extract of aloe vera (AEAV) has analgesic and anti-inflammatory effect [6]. The anti- inflammatory action of AEAV corrects the underlying inflammatory condition and reduces the pain. However, the mechanisms underlying chronic pain are complex. Synaptic transmission in the spinal cord is regulated by the actions of local inhibitory interneurons and projections that descend from the brainstem to the dorsal horn. These systems can limit the transfer of incoming sensory information to the brain. The major inhibitory neurotransmitters in the spinal cord are opioid peptides, NE & 5HT. There is ample evidence which suggests that many analgesics cause pain modulation by affecting descending pain inhibition which involves opioid receptors and noradrenergic and serotonergic receptors [7]. A better understanding of central pain modulation may allow new insights into antinociceptive mechanisms of aloe vera that may ultimately result in improved pain therapy. Therefore, the present study was undertaken to confirm its antinociceptive activity and explore the involvement of opioid system and descending pain inhibitory pathway involving monoamines in the analgesic effect of aloe vera in experimental models.
Materials and Methods
Preparation of extract
Fresh leaves of Aloe vera were collected and brought to the laboratory and thoroughly washed in running water. The leaves were extracted with different solvents in the order of increasing polarity. The solvents were aqueous, ethanol, methanol, and petroleum ether.
For the preparation of aqueous extract ten grams of fresh plant leaves were soaked in 100 ml in distilled water and homogenized, then filtered through Whattman filter paper no 1 & used for further experiment.
For the preparation of ethanol, methanol, & petroleum ether extracts, fresh leaf gel was dried in the oven at 80 degree Celsius for 48 hrs and then powdered. 10 grams of this powder was soaked in 100ml of each of the solvents ethanol, methanol and petroleum ether for 24 hrs. The contents were then filtered through Whattman filter paper no 1 & the filtrate was evaporated to dry. This dried extract was further powdered and then dissolved in distilled water.
Drugs
Pentazocine (Taj Pharmaceuticals, India), Venlafaxine (Sun pharmaceutical, Baroda, India), Naltrexone (Sigma Aldrich, Canada) and normal saline. From the results of the pilot study it was indicated that the aqueous extract of Aloe vera( AEAV) was most effective among all extracts and was used in the experiment for further study.
Selection of animals
Wistar albino rats (150-200gms) of either sex were acclimatized to standard laboratory conditions for 15 days. They were maintained under standard laboratory conditions at 24 ± 2°C and relative humidity 50 ± 55% on 12 h-day/ night cycle with free access to food and water. The rats were arranged into test and control groups. The doses of Pentazocine, Venlafaxine, Aspirin and Naltrexone were selected as per available literature. The study was approved by Institutional Animal Ethics Committee.
Part 1: Evaluation of central analgesic activity of aloe vera extract by tail immersion method [8]
Experiment 1: The procedure is based on the observation that morphine-like drugs are selectively capable of prolonging the reaction time of the typical tail- withdrawal reflex in rats induced by immersing the end of the tail in warm water of 55°C.
The lower 5 cm portion of the tail will be marked. This part of the tail will be immersed into the water bath of exactly 55°C. The reaction time will be recorded. The reaction time will be determined before and periodically at 0, 30, 60, 90 and 120 min after oral administration of the test and standard substance.
Thirty rats were divided into five different experimental groups of 6 animals each group.
Group 1–Negative control with gum acacia
Group 2–Standard group treated with pentazocinei.p(10mg/kg)
Group 3–Test group treated with aloe vera extract p.o(100mg/kg)
Group 4–Test group treated with aloe vera extract p.o(200mg/kg)
Group 5–Test group treated with aloe vera extract p.o(400mg/kg)
Experiment 2
To study the effect of combination treatment on antinociception induced by aqueous extract of aloe vera (AEAV)
Group 1–Negative control: Gum acacia10ml/kg p.o.
Group 2–Standard control treated with pentazocinei.p( 10mg/kg).
Group 3–Standard control treated with venlafaxine (25mg/kg) p.o.
Group 4–Sub effective dose of aqueous extract of aloe vera, 100mg/kg p.o.
Group 5–Sub effective dose of pentazocine, 2.5 mg/kg p.o.
Group 6–Sub effective dose of venlafaxine, (6mg/ kg)p.o.
Group 7–Sub effective dose of Aq. Ext. of Aloe vera+subeffective dose of pentazocine.
Group 8-Subeffective dose of Aq. Ext. of Aloe vera+subeffective dose of venlafaxine.
Experiment 3
To study the effect of naltrexone pretreatment on antinociception induced by aqueous extract of aloe vera(AEAV)
Group 1–Negative control: Gum acacia 10ml/kg p.o.
Group 2–Naltrexone treated group: Naltrexone 1 mg/kgp.o.
Group 3–Pretreatment of naltrexone and aqueous extract of aloe vera (AEAV) 400 mg/kg.
Group 4–Pretreatment of naltrexone and venlafaxine 25 mg/kg.
Group 5–Pretreatment of naltrexone and pentazocine 10 mg/kg i.p.
Group 6–Pretreatment of naltrexone and subeffective dose of venlafaxine and AEAV.
Group 7-Pretreatment of naltrexone and subeffective dose of pentazocine and AEAV.
Part II : Evaluation of peripheral analgesic activity of aqueous extract of aloe vera using writhing assay [9]
Writhing assay
The acetic acid-induced abdominal writhing test will be performed by intraperitoneal injection of acetic acid (10ml/kg,1% v/v in normal saline) according to the procedure described [9].
The mice will receive treatments 30min before intraperitoneal injection of acetic acid and observed for constriction of abdominal muscles together with stretching of the hind limbs. The writhes will be cumulatively counted for 30 min following acetic acid injection A significant reduction in the number of acetic acid-induced abdominal constrictions of the treated mice, compared to that of control group of mice, will be taken as an indication of analgesic effect.
The writhing assay will be performed in three sets of experiments
Experiment 1: To study the effect of different doses of aqueous extract of aloe vera (AEAV) on the number of writhes in rats over a period of 30 min. The treatment groups will be similar to part I except group 2 will receive aspirin 25mg/kg ip and serve as positive control.
Experiment 2: Effect of combination treatment on the number of writhes in rats. The treatment groups are similar to Part I and experiment 2.
Experiment 3: Effect of Naltrexone pre-treatment on antinociception induced by aqueous extract of aloe vera (AEAV) when administered alone or in combinations. The treatment groups are similar to Part I and experiment 3.
Statistical analysis
Data is represented as Mean ± SEM and analyzed using one - way ANOVA followed by Turkey multiple comparison tests. P < 0.05 was considered statistically significant.
Results
Effects of aqueous extract of aloe vera (AEAV) on mean reaction time and number of writhes at different doses in rats
AEAV produced dose dependent significant increase in mean reaction time as compared to normal control at 200mg/kg and 400mg/kg at 60 min and 90 min (Table 1). The antinociceptive effect produced by AEAV at 400mg/kg is more significant as compared to AEAV 200mg/kg and is considered as the effective dose of AEAV. Similarly, the reduction in the number of writhes with aqueous extract of aloe vera was highly significant at 400mg/kg. The effective dose of aqueous extract of aloe vera was considered as 400mg/kg (Figure 1 and Table 1).
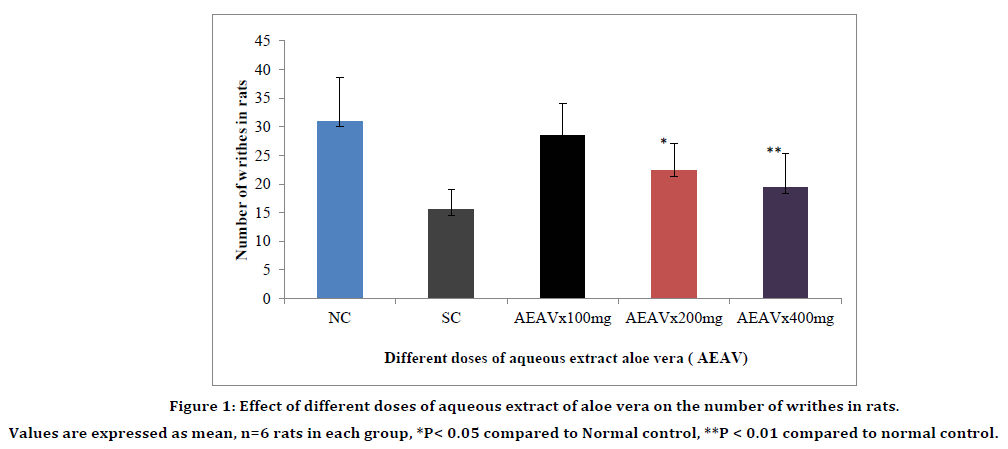
Figure 1. Effect of different doses of aqueous extract of aloe vera on the number of writhes in rats.
Values are expressed as mean, n=6 rats in each group, *P< 0.05 compared to Normal control, **P < 0.01 compared to normal control.
| Groups | 0 min | 30 min | 60 min | 90min | 120 min |
|---|---|---|---|---|---|
| NC | 2 ± 0.4 | 2.16 ± 0.52 | 2.5 ± 0.46 | 2.33 ± 0.46 | 2.33 ± 0.46 |
| Standard control (PTZ) | 2.66± 0.46 | 3.83± 0.65 | 8.16 ± 0.65** | 5.66 ± 0.61* | 3.0 ± 0.63 |
| AEAV 100mg/kg | 2.5 ± 0.46 | 2.83 ± 0.65 | 3.0 ± 0.4 | 2.66 ± 0.54 | 2.5 ± 0.46 |
| AEAV 200mg/kg | 2.66 ± 0.61 | 3.16 ± 0.52 | 6.5 ± 0.83* | 5.33 ± 0.81* | 4.66 ± 0.61 |
| AEAV 400mg/kg | 2.5 ± 0.46 | 3.33 ± 0.73 | 7.16 ± 0.77** | 6.0 ± 0.8* | 4.66 ± 0.54 |
| **P < 0.001 compared to Normal Control, *P < 0.01 compared to Normal Control | |||||
| PTZ: Pentazocine, AEAV100mg /kg= Aloe vera extract at a dose of 100mg/kg, AEAV200mg/kg=Aloe vera extract at a dose of 200mg/kg | |||||
| AEAV400mg/kg=Aloe vera extract at a dose of 400mg/kg | |||||
Table 1: Effect of aqueous extract of Aloe vera (AEAV) leaves on mean reaction time in rats.
Effects of combination treatment on mean reaction time and writhing
Sub effective doses of AEAV, PTZ and VLF did not show any significant pain inhibition when compared with normal control. Combined treatment with sub effective doses of AEAV and PTZ significantly (P<0.01) increased mean reaction time when compared to normal control, sub effective doses of AEAV or sub effective doses of pentazocine. Indicating a synergistic action between the two drugs resulting from combination therapy. The antinociceptive effect was comparable to effective doses of PTZ. Similarly combined treatment with sub effective doses of AEAV and sub effective doses of VLF produced significant (P<0.01) inhibition in pain perception when compared with normal control or sub effective doses of venlafaxine or sub effective doses of AEAV. The analgesic effect observed by combination therapy is comparable to effective doses of VLF (Table 2). Similiar results were observed on writhing with the combination treatments (Figure 2).
| Groups | 0 min | 30 min | 60 min | 90 min | 120 min |
|---|---|---|---|---|---|
| Negative control | 2 ± 0.4 | 2.16 ± 0.52 | 2.5 ± 0.46 | 2.33 ± 0.46 | 2.33 ± 0.46 |
| PTZ 2.5 mg/kg | 2.33 ± 0.46 | 2.5 ± 0.46 | 3.66 ± 0.61 | 3.33 ± 0.54 | 2.5 ± 0.46 |
| VLF 6 mg/kg | 2.66 ± 0.36 | 2.83 ± 0.52 | 2.83 ± 0.65 | 2.5 ± 0.46 | 2.83 ± 0.95 |
| AEAV100mg/kg | 2.5 ± 0.46 | 2.5 ± 0.73 | 3.0 ± 0.4 | 3.0 ± 0.63 | 2.5 ± 0.61 |
| PTZ 10 mg/kg | 3.06 ± 0.81 | 4 ± 0.63 | 8.16 ± 0.65*ab | 5.66 ± 0.61* | 3.0 ± 0.63 |
| VLF 25 mg/kg | 2.66 ± 0.46 | 3.83 ± 0.65 | 5.16 ± 0.95*ac | 5.0 ± 1.01* | 3.16 ± 0.52 |
| PTZ 2.5 mg/kg+AEAV100mg/kg | 3.5 ± 0.46 | 4.5 ± 0.46 | 6.33 ± 0.73*ab | 4.83 ± 0.65 | 3.16 ± 0.65 |
| VLF 6mg/kg+AEAE 100mg/kg | 3.0 ± 0.4 | 4.16 ± 0.52 | 5.83 ± 0.86*ac | 4.16 ± 0.52 | 3.0 ± 0.63 |
| All values are Mean ± SEM, *P<0.01 compared to Normal control, aP<0.01 compared to AEAV 100 mg/kg | |||||
| bP<0.01 compared to PTZ2.5 mg/kg, cP<0.01 compared to VLF6mg/kg | |||||
| AEAV–Aqueous extract of aloe vera, PTZ-Pentazocine, VLF-Venlafaxine | |||||
Table 2: Effect of combination treatment on mean reaction time in rats.
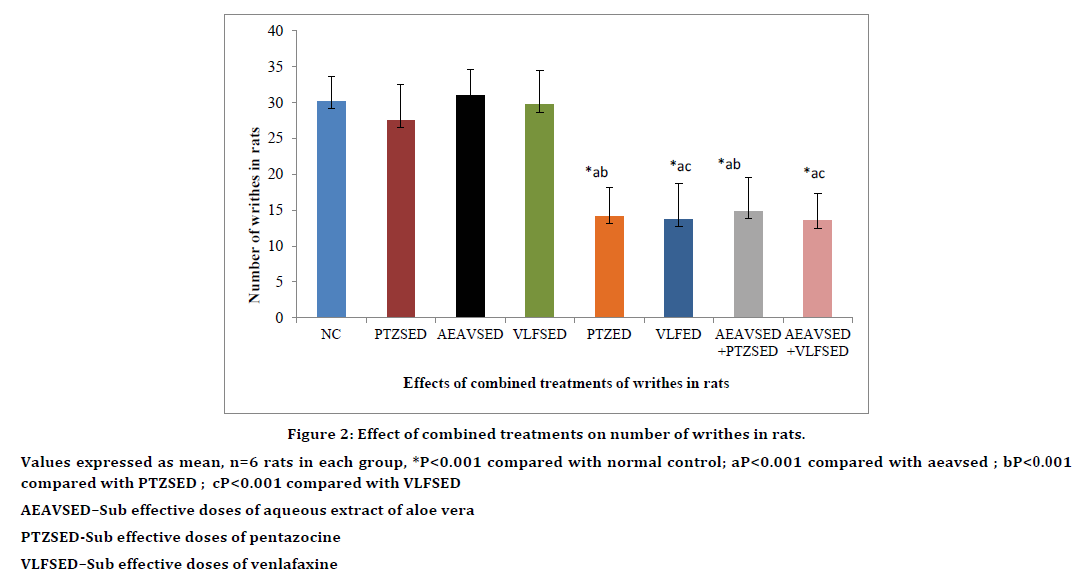
Figure 2. Effect of combined treatments on number of writhes in rats.
Values expressed as mean, n=6 rats in each group, *P<0.001 compared with normal control; aP<0.001 compared with aeavsed ; bP<0.001
compared with PTZSED ; cP<0.001 compared with VLFSED
AEAVSED–Sub effective doses of aqueous extract of aloe vera
PTZSED-Sub effective doses of pentazocine
VLFSED–Sub effective doses of venlafaxine
Effect of Naltrexone pre-treatment
Administration of naltrexone alone does not produce any significant antinociceptive effect when compared with normal control. Naltrexone administered prior to treatment with effective doses of pentazocine, venlafaxine, aqueous extract of aloe vera did not produce any significant pain inhibition. NTX administered prior to combination therapy also did not produce any significant antinociceptive effect (Table 3).
| Groups | 0 min | 30 min | 60 min | 90 min | 120 min |
|---|---|---|---|---|---|
| Negative control | 3 ± 0.4 | 2.83 ± 0.52 | 3 ± 0.63 | 2.6 ± 0.36 | 2.83 ± 0.52 |
| NTX1 mg/kg | 2.83 ± 0.33 | 2.16 ± 0.52 | 2.33 ± 0.54 | 2.5 ± 0.46 | 2.16 ± 0.43 |
| NTX1 mg/kg+AEAV 400mg/kg | 2.5 ± 0.46 | 2.33 ±0.46 | 2.33 ± 0.54 | 2.5 ± 0.54 | 2.66 ± 0.36 |
| NTX + VLF 25mg/kg | 2.5 ± 0.46 | 3.33 ± 0.46 | 2.83 ± 0.52 | 3.0 ± 0.63 | 2.83 ± 0.52 |
| NTX + VLF6mg/kg+AEAV100mg/kg | 2.66 ± 0.54 | 2.5 ± 0.67 | 2.5 ± 0.46 | 3.0 ± 0.63 | 2.16 ± 0.33 |
| NTX + PTZ2.5mg/kg +AEAV100mg/kg | 3.1 ± 0.59 | 2.21 ± 0.32 | 2.72 ± 0.52 | 2.5 ± 0.43 | 2.45 ± 0.63 |
| Values are expressed as Mean ± SEM, GA=Gum acacia 10ml/kg | |||||
| NTX=Naltrexone, VLF=Venlafaxine, PTZ=Pentazocine, AEAV=Aqueous extract of aloe vera | |||||
Table 3: Effect of Naltrexone pre-treatment on aqueous extract of aloe vera (AEAV) in rats used alone or in combination with either venlafaxine or pentazocine.
Discussion
The present study has been undertaken to investigate the central pain mechanism which involves the opioid path way and noradrenergic and serotonergic pathway. The thermal stimulation induced pain model selectively screens out centrally acting analgesic activity [10]. AEAV showed dose dependentantinociceptive action using the tail immersion method. It was observed that AEAV at a dose of 400mg/kg produced significant antinociceptive effect and was considered as the effective dose. Previous studies have demonstrated the antinociceptive effect of AEAV and have indicated possible involvement of central pain pathways [6]. To further explore the role of opioid pathway and descending pain inhibitory pathway in the analgesic action of AEAV, antinociceptive effect of sub analgesic doses of AEAV alone and combination with sub analgesic doses of pentazocine or venlafaxine was studied. It was observed that sub analgesic doses of AEAV alone did not show any antinociceptive effect but the combination therapy with pentazocine or venlafaxine showed significant antinociceptive effect. This could be explained by the synergistic action between AEAV and pentazocine or venlafaxine. It is well documented that PTZ produces analgesia by acting as an agonist at the κ receptors (opioid receptor). The synergism between AEAV and pentazocine is suggestive of possible participation of opioid receptor in the mechanism of action of AEAV. Similarly the synergism between AEAV and venlafaxine is suggestive of possible role of descending pain inhibiting pathway which involves monoamines such as noradrenaline (NA) and 5 hydroxytryptamine (5-HT). Among the antidepressants of newer generation, venlafaxine, a presynaptic drug which blocks the synaptosomal uptake of noradrenaline and serotonin and to a lesser degree of dopamine, was selected for our study Currently available preclinical and clinical data indicate that serotonin- norepinephrine reuptake inhibitors (SNRI) may be the most promising agents for the modulation of pain symptoms [5]. The increased concentration of norepinephrine and serotonin in the synaptic cleft acts both presynaptic ally and postsynaptic ally to inhibit neurotransmission of pain signals.
The contribution of opioid, noradrenergic and serotonergic systems in the analgesic activity of AEAV was further confirmed by naltrexone pre-treatment. Naltrexone pre-treatment inhibited the antinociceptive effect produced by effective doses of AEAV alone or combination therapy with pentazocine or venlafaxine. This demonstrates that naltrexone acting as an opioid antagonist blocks the opioid receptors which result in inhibition of antinociceptive effect and underlies the role of opioid receptors in the mechanism of action of AEAV. Naltrexone pre-treatment also inhibits the antinociceptive effect produced by combination of AEAV and venlafaxine. This is in agreement with earlier reports which have shown that venlafaxine exhibits a dose dependent antinociceptive effect which was reversed with naloxone [11]. The results strongly point towards the participation of opioid receptors in the mechanism of action of venlafaxine. It appears that venlafaxine produces antinociceptive effect or augments the antinociceptive effect of opioids effect by acting through opioid-mediated descending pain inhibition in the PAG [11]. It has been reported that opioids not only activate cortical and subcortical receptors, but also descending inhibitory circuits. Thus, the overall effect of venlafaxine is stimulation of the descending inhibitory pathway by acting through the opioid receptors and increasing the concentration of inhibitory neurotransmitters like NE and 5HT by acting as a SNRI (serotonin norepinephrine reuptake inhibitor).
Analgesics can also act on the peripheral nervous system to block pain perception due to acetic acid -induced writhing in experimental animals [12]. In the present study, aqueous extract of aloe vera inhibited acetic acid induced writhing in rats when used at a dose of 200mg/kg and 400mg/kg alone as well as in combination of sub analgesic doses with that of pentazocine. This result is in accordance with the earlier reports that acetic acid induced writhing is readily reversed by non-steroidal anti-inflammatory drugs as well as by opioid agonist such as pentazocine.[13] Further our study showed that combination of sub analgesic dose of AEAV with that venlafaxine also inhibited acetic acid-induced writhing response which is in agreement with previous reports that the selective serotonergic and noradrenergic reuptake inhibitor produced a dose-dependent antinociceptive effect in the acetic acid-induced writhing test in mice [11]. These results are consistent with the hypothesis that venlafaxine produces analgesia by blocking norepinephrine and serotonin uptake and therefore enhancing the action of the inhibitory neurotransmitters at the spinal terminals of an opioid-mediated intrinsic analgesia system. Also naltrexone pre-treatment displays antagonism of opioid receptors blocking pain induced by acetic acid induced writhing. Naloxone hydrochloride and Naltrexone hydrochloride in lower dose have been reported to be effective antagonists of opioid analgesia in Acetic acid induced writhing [14]. Thus, the study demonstrates antinociceptive effect of aqueous extract of aloe vera in central and peripheral experimental pain models.
Conclusion
The aqueous extract of aloe vera acts through peripheral pain mechanisms and also alters central pain related systems. This could explain the use of plant extract of aloe vera individually or in combination with synthetic drugs due to its synergistic action and this may result in dose reduction of opioid or non-opioid analgesics leading to lesser side effects and improving the outcome for pharmacologic management of chronic pain. Isolated principles from aloe vera needs to be evaluated in scientific manner to understand its mechanism of action.
Funding Source
Intramural funding has been acquired to conduct the study.
References
- Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: A synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry 2009; 31:206–219.
- Labianca R, Sarzi-Puttini P, Zuccaro SM. et al. Adverse effects associated with non-opioid and opioid treatment in patients with chronic pain. Clin Drug Investig 2012; 32:53.
- Vogler BK, Ernst E. Aloe vera: A systemic reiview of its clinical effectiveness. Br J Gen Practice 1999; 49:823-828.
- Salehi B, Biazar E, Jahromi MH, et al. Antidepressant effects of aloe vera hydroalcoholic extract. Archives Adv Biosci 2011; 2.
- McQuay HJ, Tramèr M, Nye BA, et al. A systematic review of antidepressants in neuropathic pain. Pain 1996; 68:217-227.
- Egesie UG, Chima KE, Galam NZ. Anti-inflammatory and analgesic effects of aqueous extract of Aloe Vera (Aloe barbadensis) in Rats Afr. J Biomed Res 2011; 14:209-212.
- Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest 2010; 120:3779–3787.
- Aydin S, Demir T, Öztürk Y, et al. Analgesic activity of Nepeta italica L. Phytother Res 1999; 13:20-23 .
- Koster R, Anderson M, De-Beer EJ. Acetic acid analgesic screen federation proceedings. 1959; 18:418-420.
- Wong CH, Day P, Yarmush JG, et al. Nifedipine induced analgesia after epidural injections in rats. Anesth Analg 1994; 79:303–306.
- Schreiber S, Backer MM, Pick CG. The antinociceptive effect of venlafaxine in mice is mediated through opioid and adrenergic mechanisms. Neurosci Lett 1999; 273:85–88.
- Turner RA. Screening methods in pharmacology. New York: Academic Press 2009; 152–63.
- Laura MB, Fei X, Raul RG, et al. Potentiated opioid analgesia in norepinephrine transporter knock-out mice. J Neurosci 2000; 20:9040–9045.
- Bhargava HN, Larsen AK, Rahmani NH, et al. Naltrexone-induced alterations of the distribution of morphine in brain regions and spinal cord of the rat. Brain Res 1993; 607:1–8.
Author Info
Snigdha Misra1 and Rajesh Kumar Suman2*
1Department of Pharmacology, MGM Medical College, Navi Mumbai, India2Department of pharmacology, Hind Institute of medical sciences, Atria, Sitapur, India
Citation: Snigdha Misra, Rajesh Kumar Suman, Pain Modulation through Opioid and Monoaminergic Pathways In Aloe Vera Induced Analgesia in Rats, J Res Med Dent Sci, 2021, 9 (4):60-66.
Received: 03-Feb-2021 Accepted: 29-Mar-2021
