Commentary Article - (2024) Volume 12, Issue 4
Optimization of Fibrinolytic Enzyme from Bacillus KDm 99 Using Response Surface Method
Zahra Mirzaeia, Arastoo Badoei Dalfard* and Zahra Karami
*Correspondence: Arastoo Badoei Dalfard, Department of Biology, Shahid Bahonar University, Iran, Email:
Abstract
The purpose of this study is to employ the surface response approach to maximize the production of bacterial fibrinolytic enzyme. The Bacillus KDm99 bacterium stock was cultivated in nutrient broth, solid medium (nutrient agar-casein), and a special medium containing: peptone (0.5g), casein (0.1g), MgSO4 (0.02g), NaCl (0.02g), and KH2PO4 (0.05g). It was shown that the solid media had protease activity. The greatest protease activity was seen at a concentration of 50 microliters of enzyme with concentration (66.02 micro mole/min), 50 microliters of casein substrate with concentration (0.1%), 200 microliters of PBS-40 mm buffer with pH=8, and 300 microliters (Trichloroacetic acid)(5%) In order to optimize the bacterial growth environment for optimum enzyme synthesis, variables including supplies (carbon, nitrogen sources, metal ions, temperature, pH, agricultural residues, and animal fertilizers) were investigated. Following inoculation and 48 hours of incubation in a shaker incubator, the greatest efficiency of bacterial growth and enzyme production was assessed. The response method of variables at various levels has been modeled using the software (Design-Expert v13.0.0.0) in the presence of 0.5 mm NaCl concentration, Tween-20 organic solvent; body shampoo detergent (Zi- Musk) and ion’s KH2PO4, FeSO4 enzyme Maximum activity and stability are displayed. Also, it was determined that Km is equivalent to 0.3 micromole and that Vm is equal to 0.8 micromole/min. The first hour saw an 80% maintenance of the temperature stability of the enzyme activity at 60°C, and the greatest enzyme activity was observed at pH=6 of the PBS buffer
Keywords
Fibrinolytic, Protease, RSM, Enzyme, Surface response method
Introduction
In the industrial sector, Bacillus sp. is the most active and dynamic extracellular alkaline protease producer. of the three largest groups of industrial enzymes, proteases are one of them, and their global market is drastically increasing annually. Of the 60% of enzymes marketed worldwide, proteases account for 20% [1]. Proteases from microbial sources are preferred to the enzymes from plant and animal sources since they posse’s almost all characteristics desired for their biotechnological applications, Among the various proteases, bacterial protease was the most significant compared with animal, fungi and plant protease. Bacillus species were specific producers of extracellular protease [2]. The most common class of proteases produced by bacterial, fungi, yeast, and actinomycetes are alkaline proteases. Microbial generated enzymes are superior to their plant- and animal-derived counterparts in terms of benefits. The possibility of mass production in industrial fermenters, cheaper production costs, a wide range of chemical and physical properties, and genetic engineering are a few of these benefits. Microbial enzymes are suitable biocatalysts for a variety of industrial applications because of their qualities. Another benefit of microbial enzymes is that they are typically extracellular in nature, which makes it simpler to isolate them in their purest form [3]. Despite the fact that there are several microbiological sources for the creation of proteases only a small number of these have been identified as commercial manufacturers [4-6]. Extracellular proteases have also been applied commercially to assist in the protein cleavage process in a variety of industrial operations [7].
In addition to acting as blood pressure regulators and molecular expectorants, fibrinolytic enzymes have anti-inflammatory, analgesic, and antibacterial properties [8]. Based on the method of proteolysis and functional group in the active site, proteases are categorized into four groups: serine, aspartic, cysteine, and metalloprotease [9]. They are divided into groups of neutral proteases, alkaline proteases, and acidic protease groups according to the pH range [10]. The hydrolysis of all proteins is catalyzed by these degrading enzymes, whose molecular weight is typically between (18-90) Kilogram. Both fermentation on a solid bed and fermentation in a sub merged culture are ways used by bacteria to manufacture proteases [11]. Fibrinolytic enzymes are a subclass of hydrolases that have an impact on peptidases. The blood's endothelial cells often produce thrombolytic enzymes. The body's ability to produce these enzymes begins to decline as it ages. Weak thrombolytic enzyme production can result in thrombolytic circumstances since endothelial cells are found in all body cells, including blood arteries and the lymphatic system [12]. The diversity of bacteria's biochemical and physiological makeup may make it easier to target stronger fibrinolytic proteases for conceivable medicinal uses. Proteases are used in several industries such as pharmaceutical, food, deter-gent, silk, and leather and also for recovery of silver from used X-ray.
The quest for more fibrinolytic enzymes from other sources has been underway for the past ten years. Earthworms, vampire bat saliva and venom which have all been found to be potential thrombolytic agents from animals, have all had their effects verified in clinical studies Moreover, the characteristics of fibrinolytic enzymes extracted from fermented food products including Chinese dochi and Japanese natto have been determined, Plasminogen can be transformed into plasmin by these enzymes. These resources include agricultural and industrial wastes like (corn powder, cow manure, fish waste from canning enterprises along the docks and beaches, soybean meal, wheat bran, banana peel, tapioca peel, and rice bran). It is now vital to employ techniques to cut down on the number of tests required to hasten the logical conclusion due to the continuously expanding research and development activities in industrial units. Using these techniques will boost output and streamline processes in manufacturing. Achieving accurate results is made possible by proper experiment design, which also lowers expenses.
This is where it's important to highlight that the designed experiments are just as valuable as the way they're carried out because the data can be analysed more easily and precisely. As a result, using software that makes it easier to design and analyze experiments might be useful. of course, using experimental design software requires having a solid grasp of the physics of the issue being studied. Also, a professional designer and analyst should be aware of the significance and, more crucially, the use of each software result acquired from the analysis of experimental data in order to provide a logical and trustworthy interpretation of the results. For optimal output and cost savings, the environment must be optimized. Based on this, the goal of this study is to use the response surface method to maximize the production of bacterial fibrinolytic enzyme.
What is Experimental Design?
Designed activities that are carried out with the aim of finding out the influencing factors and how they affect the process are called designed experiments. In the design of experiments, the term matrix is used, the rows of which show how to conduct the experiment and the columns of which show the values of different factors. The effort of all researchers in the field of experiment design has been to reduce the size of the matrix (reducing the number of experiments) by selecting a part of all experiments necessary to reach the goal. The main problem is the selection of this number of tests from all the tests, which has caused various types of test design. The proper name for this branch of science is "statistically optimal testing usually denoted by (Design of Experiments), and it can be said that a designed experiment is a test in which targeted changes are applied to the input variables of the process.
Statistical Optimization through Design-Expert software
In this research, the Response Surface Method (RSM) software (Design-Expert V.13.0.0.0) was used to optimize the bacterial fibrinolytic enzyme from the Bacillus KDm99. The use of different statistical tools to optimize the enzyme is a common method. It is considered in recent years. The mutual effect of different pairs of variables on the rate of enzyme production, determining the relative contribution of each factor in the optimization process by means of variance analysis, determining the optimal conditions and finally measuring the rate of enzyme production under different conditions are evaluated. To optimize the concentration of environmental components used, to reduce consumption costs and to obtain fibrinolytic enzymes in order to increase their production performance, it is appropriate to use software for statistical optimization. In 2020: Chavi Sharma and colleagues were able to optimize the fibrinolytic enzyme with the activity of 30.75 u/ml from Bacillus cereus RSA1 using RSM, CCD method [12].
In 2019: Ponnuswamy Vijayaraghavan and colleagues were able to optimize the fibrinolytic enzyme with the activity of 2296U/ml from Xanthomonas oryzae-IND3 using RSM, CCD method. In 2019: Shihan Pan and colleagues were able to optimize a fibrinolytic enzyme with a molecular weight 38kDa of Bacillus subtilis D21-8 using RSM method [13]. In 2019: Moharam Maysa and colleagues were able to optimize the fibrinolytic enzyme with the activity 43KDa of from Bacillus subtilis Egy using RSM, CCD method. In 2017: K.V. Smitha and colleagues were able to optimize the fibrinolytic enzyme with the activity of 40KDa from Bacillus altitudinis-S-CSR 0020, using RSM, BBD method [14].
Why is Enzyme Optimization done?
The use of chemicals worldwide in various industries has increased tremendously and affects people's health. The modern world plans to replace these harmful chemicals with environmentally friendly products to improve life on the planet. Creating enzymatic processes despite chemical processes has been one of the main goals of scientists, in order to increase enzymatic processes, to optimize an ideal and alternative method to reduce chemical harms and a suitable method to produce as much enzyme as possible and to check enzyme activity through n- Invoice and invoice design with statistical and software methods with accuracy [99.9%] [15].
Materials and Methods
Bacterial growth conditions for the synthesis of fibrinolytic enzyme
Initially, solid culture medium and medium (nutrient broth) were used to culture Bacillus KDm99 (nutrient agar-casein). Casein with a pH of 7.5 was added to the plate before chilling, and then 50 microliters of the bacterial stock were added to the plate after it had finished cooling and the solid medium had been completely sterilized [16]. The culture spent 24 hours at 30°C in a shaker incubator. 100 microliters of bacterial stock were added to the medium (Nutrient Broth), which had been autoclaved and thoroughly sanitized, and it was then put in a shaker incubator for 24 hours at 35°C to promote bacterial growth. he Target media was placed in a shaker incubator at a temperature of 35°C for 48 hours before around 50 microliters of the medium (nutrient broth) to the special medium contained peptone (0.5g), NaCl (0.02g), MgSO4 (0.02g), KH2PO4 (0.05g), and casein (0.1g) was added measuring enzyme activity [17].
The Dedicated culture media was added to the Falcon after 48 hours of incubation, and after 10 minutes of centrifugation at 4°C and 6000 rpm, the supernatant was used to quantify the enzyme activity. The enzyme was quantified in microliters in amounts of (10, 50, 100). The enzyme control and substrate were placed separately for each sample, then the enzyme was added to the micro tube along with casein substrate in amounts of 50 microliters and PBS buffer in amounts of (240, 200, 250) microliter. The micro tubes was then placed in a Bain-Marie for 1 hour at a temperature of 37°C.After this step, 300 microliters of (Trichloroacetic acid) enzyme activity inhibitor with a concentration of 5% was added. The mixture was then stored in the refrigerator at 4°C for 30 minutes to precipitate the undissolved casein, and After 10 minutes of centrifugation at 10,000 rpm, the enzyme absorption was measured with a spectrophotometer at 28nm [18].
To maximize the first pH factor, 5 Arlen-containing materials with varying pH values (5, 4, 6, 7, 8, and 9) were taken into consideration. These materials included peptone (0.5g), NaCl (0.02g), KH2PO4 (0.05g), and MgSO4 (0.02g), and were brought to a volume of 100 ml. The medium containing the bacteria grown in the nutrient broth medium was transferred to the production medium in the same amount of Arlen’s. These Arlen’s were incubated at a temperature of 35°C for 48 hours in a shaker incubator, and then the supernatant was centrifuged at a speed of 6000 rpm for 10 minutes at the temperature 4°C was centrifuged and separated. and enzyme assay of the production environments at different pH levels using 50 microliters of enzyme produced from the supernatant of each of the production 280 nm [19].
Optimum pH determination
Environments mixed with 50 microliters of casein substrate (1%) and 200 microliters of PBS phosphate buffer and after adding 300 microliters (Trichloroacetic acid) with a concentration of (5%)was placed at 4°C temperature for 30 minutes. The supernatant's absorbance was then measured at 280 nm after 10 minutes of centrifugation at 10,000 rpm. As a control, a mixture containing 50 microliters of casein substrate, 50 microliters of distilled water, and 200 microliters of PBS buffer was used, as well as a mixture containing 50 microliters of produced enzyme with a different pH, 50 microliters of distilled water, and 200 microliters of PBS buffer [21,22].
Determining the Optimal Temperature
A 100 mL production medium containing 5 Arlene containing peptone (0.05g), KH2PO4 (0.05g), MgSO4 (0.02g), and NaCl (0.02g) at pH=7 and several temperatures (25, 30, 35, 40 and 45) °C were considered in order to optimize the temperature. 50 microliters of casein substrate, 50 mic roliters of distilled water and 200 microliters of PBS buffer, as well as 50 microliters of enzyme produced at different temperatures, 50 microliters of distilled water and 200 microliters of PBS buffer to the title of the witness was used [23].
Choosing the best carbon source
In five Arlen containing peptone, KH2PO4 (0.05g), MgSO4 (0.02g), and NaCl (0.02g) and sucrose, glucose, galactose, maltose and starch in the amount of 10% along with casein (an enzyme inducer) as a medium. Production was used. The production medium was incubated at 35°C for 48 h and then centrifuged at 6000 rpm for 10 min in 4°C. The supernatant was separated and a volume of 50 Microliter of enzyme from each production condition with carbon sources was used for enzyme testing. The absorbance of the supernatant was measured at a wavelength of 280 nm [24].
Optimizing the culture medium for enzyme production with RSM surface response method - Design-Expert softwareV13.0.0.0
The best sources of carbon and nitrogen, pH, temperature and waste were defined for the software at three levels, the software predicted 40 experiments and 40 falcons from the prepared environment and one falcon as a control in a volume of 25 ml with casein after inoculation of the medium (broth) Nutrient) was prepared with production medium for 48 hours. Centrifugation at 10,000 rpm was performed for 10 minutes and the absorbance of the supernatant was read at 280 nm. The effect of each parameter was examined in three-dimensional models and the test of glucose (1.25g), yeast extract (0.75g), Chicken manure (0.85g), and KH2PO4 (0.65g) was selected and mass produced [25,26].
Mass Production
After selecting the most optimal factors (PH, temperature, carbon sources, nitrogen sources, metal ions, agricultural and animal residues), the mass production of the enzyme was investigated. In this way, a 100cc Erlenmeyer flask containing optimized medium of glucose (0.24g), yeast extract (0.12g), chicken manure (0.15g), KH2PO4 (0.12g) was prepared at a temperature of 25°C, PH=6, and the volume was 50cc delivered and from the stock of bacteria that had grown in the medium (nutrient broth) for 24 hours in the incubator shaker, it was inoculated into the optimal medium and the optimal medium was placed in the incubator shaker for 48 hours and after the desired time, the supernatant soup was made from Sedimentation was done in a refrigerated centrifuge at 6000 rpm for 10 minutes [27,28].
Purification of protease (fibrinolytic) enzyme
Sedimentation and concentration
Ammonium sulfate was used for protein precipitation; this method of precipitation based on increasing ionic strength is known as salting-out.
Dialysis
To separate the salt from the protein, dialysis is performed. Dialysis is a separation technique that separates small and unwanted molecules from macromolecules through a selected semi-permeable membrane, and the dialysis buffer is placed on one side of the membrane and the sample selected for dialysis. is placed on the other side and the passage of small molecules and salts through the membrane pores is possible and this causes the concentration of these molecules to decrease in the protein and as a result the protein returns to its original state, changing the dialysis buffer causes More molecules pass through the membrane and remove other molecules that have passed through the membrane [29].
Chromatography
In this method, the composition of a solution between the stationary and mobile phase is measured, and the mobile phase carries the sample. Based on the conditions of the dissolved substances, cation (positive charge), anion (negative charge), cation and anions are connected on the stationary phase (fine surface of the chromatograph resin), one of the most famous ion exchange resins can be called (Q-Sepharose). In this method, the unloaded proteins are removed from the chromatographic column and the proteins with a negative charge are added to the column from the PBS loading buffer with pH = 8. 1 M NaCl is used to remove the proteins and replace Column load To separate the proteins separately in the chromatography process, the output of the column is separated into volumes of 2 ml and the absorbance of all of them is read at a wavelength of 280 nm [30].
Using Design-ExpertV.13.0.0.0 software, RSM surface response method and optimized design parameters, experiments were carried out
The basis of the work is to present a standard table that examines the variables with or without mutual effects, based on this method, (40-50) cc containers containing optimized culture medium including (glucose, yeast extract, casein (as an enzyme inducer), chicken manure, KH2PO4 in Three levels (1g), (1.25g), (0.75g) and temperature (25, 30, 35)°C, pH= (6, 7, 8) were proposed based on the compositions provided by this software. from the autoclave, and bacterial inoculation from the pre-cultivation medium to the production medium was placed in the shaker incubator and after 48 hours of inoculation, the amount of fibrinolytic enzyme production was checked among the 40 tests proposed by the software to optimize the culture medium which includes Glucose (1.25g), yeast extract (0.75g), chicken manure (0.75g), KH2PO4 (0.75g)) which showed the best optimization in the production environment were selected. In the first stage of optimization The material in this environment with temperature 25°C and pH = 6 the amount of enzyme production was (136.6%) and we defined this test in three other levels inside the software and 30 other tests were suggested, which were glucose test (1.25g) extract Yeast (0.65g), chicken manure (0.75g), KH2PO4 (0.65g) was selected from among 30 experiments and the amount of enzyme production (141.56%) was calculated and through this test mass production was carried out. Biochemistry of the enzyme was investigated, (Table 1) [31].
| Factor (1) | Factor (2) | Factor (3) | Factor (4) | Factor (5) | Factor (6) | Response | |
|---|---|---|---|---|---|---|---|
| Temperature (A) | PH (B) | Glucose (C) | Yeast extract (D) | Chicken manure (E) | Potassium phosphate (F) | Enzyme production | |
| °C | % | % | % | % | % | ||
| 1 | 25 | 7 | 0/75 | 0/75 | 0/75 | Jan-25 | 67/08 |
| 2 | 30 | 7 | 0/75 | 1 | 1 | 1 | 65/75 |
| 3 | 35 | 7 | 1 | Jan-25 | Jan-25 | 1 | 62/02 |
| 4 | 25 | 6 | 0/75 | 0/75 | Jan-25 | Jan-25 | 65/75 |
| 5 | 30 | 6 | 1 | 1 | 0/75 | Jan-25 | 82/23 |
| 6 | 35 | 6 | 0/75 | 0/75 | 1 | Jan-25 | 72/73 |
| 7 | 25 | 6 | 1 | 1 | 1 | 0/75 | 46/03 |
| 8 | 25 | 8 | 1 | Jan-25 | 0/75 | 1 | 96/93 |
| 9 | 35 | 6 | 1 | Jan-25 | 0/75 | 0/75 | 82/41 |
| 10 | 35 | 7 | 1 | Jan-25 | Jan-25 | 1 | 68/77 |
| 11 | 35 | 7 | 1 | 1 | 1 | 0.75 | 72/30 |
| 12 | 25 | 7 | 1 | Jan-25 | 1 | Jan-25 | 88/28 |
| 13 | 25 | 6 | Jan-25 | 0/75 | 0/75 | Jan-25 | 62/52 |
| 14 | 30 | 7 | 1 | 0/75 | Jan-25 | Jan-25 | 92/66 |
| 15 | 30 | 6 | Jan-25 | Jan-25 | Jan-25 | 0/75 | 40/93 |
| 16 | 35 | 6 | 0/75 | 0/75 | Jan-25 | 0/75 | 39/12 |
| 17 | 30 | 7 | 0/75 | 1 | 1 | 1 | 57/03 |
| 16 | 35 | 8 | 0/75 | Jan-25 | 0/75 | Jan-25 | 99/60 |
| 19 | 35 | 8 | 0/75 | Jan-25 | 1 | 0/75 | 68/24 |
| 20 | 25 | 8 | 1 | 0/75 | 1 | 0/75 | 59/42 |
| 21 | 25 | 6 | 1 | 1 | 1 | 0/75 | 64/37 |
| 22 | 25 | 8 | Jan-25 | 1 | Jan-25 | 1 | 108/21 |
| 23 | 30 | 7 | 1 | 0/75 | Jan-25 | Jan-25 | 76/10 |
| 24 | 30 | 6 | Jan-25 | 0/75 | 1 | 1 | 65/48 |
| 25 | 25 | 7 | Jan-25 | Jan-25 | 1 | 0/75 | 102/88 |
| 26 | 30 | 8 | Jan-25 | Jan-25 | 1 | Jan-25 | 80/30 |
| 27 | 30 | 7 | Jan-25 | 1 | 0/75 | 0/75 | 91/88 |
| 28 | 35 | 6 | Jan-25 | 1 | Jan-25 | Jan-25 | 93/34 |
| 29 | 25 | 6 | Jan-25 | 0/75 | 0/75 | 0/75 | 124/01 |
| 30 | 30 | 7 | 0/75 | 1 | 1 | 1 | 77/05 |
| 31 | 25 | 7 | 0/75 | Jan-25 | Jan-25 | 0/75 | 85/96 |
| 32 | 25 | 8 | Jan-25 | 0/75 | 0/75 | Jan-25 | 119/32 |
| 33 | 30 | 6 | Jan-25 | 0/75 | 1 | 1 | 187/04 |
| 34 | 35 | 8 | Jan-25 | 0/75 | Jan-25 | 0/75 | 49/48 |
| 35 | 30 | 6 | 0/75 | 0/75 | 0/75 | 0/75 | 80/81 |
| 36 | 25 | 6 | Jan-25 | Jan-25 | 0/75 | 1 | 98/07 |
| 37 | 30 | 6 | 0/75 | Jan-25 | Jan-25 | Jan-25 | 93/34 |
| 38 | 35 | 8 | 1 | 0/75 | 0/75 | 1 | 52/38 |
| 39 | 25 | 7 | 0/75 | Jan-25 | 0/75 | 0/75 | 91/77 |
| 40 | 30 | 8 | 0/75 | 1 | Jan-25 | Jan-25 | 72/87 |
Table 1: Designing 40 experiments suggested by Design-ExpertV.13.0.0.0 software and obtaining the amount of enzyme production according to the optimized conditions.
Identification of the Significant Variables Using the 26 Factorial Designs
The 26 factorial designs is one of the efficient methods to screen out the important nutrient and physiochemical parameters from a large number of process variables affecting fibrinolytic enzyme production. Six important factors such as Glucose (carbon source), Yeast extract (nitrogen source), NaH2PO4 (mineral salt), Chicken manure, pH and Temperature was selected for analysis, the (26) factorial design allows the evaluation of N (6) variables in N (40) experiments, (Table 2).
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 0.5276 | 30 | 0.0176 | 7.19 | 0.002 | significant |
| A-Temperature | 0.0341 | 1 | 0.0341 | 13.93 | 0.0047 | |
| B-pH | 0.0125 | 1 | 0.0125 | 5.09 | 0.0505 | |
| C-Glucose | 0.0217 | 1 | 0.0217 | 8.88 | 0.0154 | |
| D-Yeast extract | 0.0953 | 1 | 0.0953 | 38.96 | 0.0002 | |
| E-Poultry manure | 0.0539 | 1 | 0.0539 | 22.04 | 0.0011 | |
| F-KH2PO4 | 0.0749 | 1 | 0.0749 | 30.62 | 0.0004 | |
| AB | 0.0406 | 1 | 0.0406 | 16.58 | 0.0028 | |
| AC | 0.0411 | 1 | 0.0411 | 16.78 | 0.0027 | |
| AD | 0.0472 | 1 | 0.0472 | 19.29 | 0.0017 | |
| AE | 0.0816 | 1 | 0.0816 | 33.35 | 0.0003 | |
| AF | 0.0572 | 1 | 0.0572 | 23.39 | 0.0009 | |
| BC | 0.0753 | 1 | 0.0753 | 30.8 | 0.0004 | |
| BD | 0.0156 | 1 | 0.0156 | 6.39 | 0.0323 | |
| BE | 0.1123 | 1 | 0.1123 | 45.9 | 0.0001 | |
| BF | 0.0189 | 1 | 0.0189 | 7.73 | 0.0214 | |
| CE | 0.0314 | 1 | 0.0314 | 12.82 | 0.0059 | |
| CF | 0.0967 | 1 | 0.0967 | 39.52 | 0.0001 | |
| DE | 0.0084 | 1 | 0.0084 | 3.43 | 0.097 | |
| DF | 0.0647 | 1 | 0.0647 | 26.46 | 0.0006 | |
| A2 | 0.0713 | 1 | 0.0713 | 29.14 | 0.0004 | |
| B2 | 0.0454 | 1 | 0.0454 | 18.57 | 0.002 | |
| C2 | 0.0585 | 1 | 0.0585 | 23.91 | 0.0009 | |
| D2 | 0.0776 | 1 | 0.0776 | 31.71 | 0.0003 | |
| E2 | 0.0514 | 1 | 0.0514 | 21.02 | 0.0013 | |
| F2 | 0.0187 | 1 | 0.0187 | 7.66 | 0.0218 | |
| ABC | 0.0953 | 1 | 0.0953 | 38.96 | 0.0002 | |
| ABE | 0.0654 | 1 | 0.0654 | 26.72 | 0.0006 | |
| ACD | 0.0814 | 1 | 0.0814 | 33.27 | 0.0003 | |
| ACE | 0.0706 | 1 | 0.0706 | 28.85 | 0.0004 | |
| ADE | 0.0106 | 1 | 0.0106 | 4.33 | 0.0672 | |
| Residual | 0.022 | 9 | 0.0024 | |||
| Lack of Fit | 0.0026 | 3 | 0.0009 | 0.2678 | 0.8465 | not significant |
| Pure Error | 0.0194 | 6 | 0.0032 | |||
| Cor Total | 0.5496 | 39 |
Table 2: The proposed variance analysis model presented for experiments with 95% confidence.
Comparison of Selected Models
The software for this test is linear vs Mear. It compares 2FI vs Linear with 95% probability for enzyme production and optimization (Table 3).
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Mean vs Total | 30.05 | 1 | 30.05 | |||
| Linear vs Mean | 0.2339 | 6 | 0.039 | 4.07 | 0.0036 | Suggested |
| 2FI vs Linear | 0.2066 | 15 | 0.0138 | 2.27 | 0.0497 | Suggested |
| Quadratic vs 2FI | 0.0124 | 6 | 0.0021 | 0.2561 | 0.9472 | |
| Cubic vs Quadratic | 0.0773 | 6 | 0.0129 | 3.98 | 0.0585 | Aliased |
| Residual | 0.0194 | 6 | 0.0032 | |||
| Total | 30.6 | 40 | 0.765 |
Table 3: Comparison of selected models with 95% probability.
ANOVA results for the production of fibrinolytic enzymes by CCD and RSM
According to the ANOVA table and the values of P-Value, R2 and Transform type of analysis results The following was obtained, it is necessary to mention some explanations about the mentioned items before stating the analysis will be expressed: 1. P-Value: Initially, it was the amount of error in the data and by creating a Transform Appropriately, we reduced the amount of this error significantly, then by removing the data an optimal model was obtained [32]. In other words, we continued this work until most of the parameters reached 0.05 P-Value & lt; reach So that the model has sufficient accuracy. In the beginning, R2 value was about 0.804, but after doing step 1, its value was changed to 0.9599, which means if the impact of the changes is taken into account. It should be noted that the closer the value of R2 is to 1, the more optimal the model is. Data obtained from RSM on fibrinolytic enzyme production were subjected to anova (Table 4).
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Linear | 0.2963 | 27 | 0.011 | 3.39 | 0.066 | Suggested |
| 2FI | 0.0897 | 12 | 0.0075 | 2.31 | 0.1568 | Suggested |
| Quadratic | 0.0773 | 6 | 0.0129 | 3.98 | 0.0585 | |
| Cubic | 0 | 0 | Aliased | |||
| Pure Error | 0.0194 | 6 | 0.0032 |
Table 4: Lack of Fit Tests values obtained for the model.
The fitting quality of the equation of the second order polynomial model was expressed through the coefficient of determination, R2, and the adjusted R2 value (Table 5).
| Std. Dev. | 0.0495 | R2 | 0.9599 |
|---|---|---|---|
| Mean | 0.8667 | Adjusted R2 | 0.8264 |
| C.V. % | 5.71 | Predicted R2 | -0.855 |
| Adeq Precision | 12.8755 |
Table 5: Correlation coefficient values for the proposed square mode.
Then, the fitted polynomial equation was expressed as three-dimensional (3D) surface diagrams to show the relationship between the response and the experimental levels of each of the variables. Statistical software (Design-Expert V13.0.0.0) was used to draw 3D diagrams. A combination of different optimized parameters, which provides the maximum response, i.e. the maximum fibrinolytic activity, was experimentally tested to confirm the validity of the model (Table 6).
| Factor | Coefficient Estimate | df | Standard Error | 95% CI Low | 95% CI High | VIF |
|---|---|---|---|---|---|---|
| Intercept | 1.04 | 1 | 0.0531 | 0.917 | 1.16 | |
| A-Temperature | -0.0504 | 1 | 0.0135 | -0.0809 | -0.0198 | 1.91 |
| B-pH | 0.036 | 1 | 0.016 | -0.0001 | 0.0722 | 2.62 |
| C-Glucose | -0.0617 | 1 | 0.0207 | -0.1085 | -0.0149 | 4.73 |
| D-Yeast extract | 0.1381 | 1 | 0.0221 | 0.088 | 0.1881 | 5.8 |
| E-Poultry manure | -0.0693 | 1 | 0.0148 | -0.1027 | -0.0359 | 2.32 |
| F-KH2PO4 | -0.168 | 1 | 0.0304 | -0.2367 | -0.0993 | 10.92 |
| AB | -0.0936 | 1 | 0.023 | -0.1455 | -0.0416 | 3.86 |
| AC | 0.2143 | 1 | 0.0523 | 0.096 | 0.3326 | 17.45 |
| AD | -0.0946 | 1 | 0.0215 | -0.1433 | -0.0459 | 3.98 |
| AE | 0.35 | 1 | 0.0606 | 0.2129 | 0.4871 | 25.67 |
| AF | -0.1489 | 1 | 0.0308 | -0.2185 | -0.0793 | 7.75 |
| BC | 0.2891 | 1 | 0.0521 | 0.1712 | 0.4069 | 21.05 |
| BD | 0.0431 | 1 | 0.0171 | 0.0045 | 0.0817 | 2.33 |
| BE | 0.2224 | 1 | 0.0328 | 0.1481 | 0.2967 | 7.93 |
| BF | 0.0438 | 1 | 0.0158 | 0.0082 | 0.0795 | 2.02 |
| CE | -0.0809 | 1 | 0.0226 | -0.1319 | -0.0298 | 3.73 |
| CF | -0.3595 | 1 | 0.0572 | -0.4888 | -0.2301 | 26.59 |
| DE | -0.0345 | 1 | 0.0186 | -0.0766 | 0.0076 | 2.97 |
| DF | -0.2278 | 1 | 0.0443 | -0.3279 | -0.1276 | 17.31 |
| A² | -0.3466 | 1 | 0.0642 | -0.4918 | -0.2013 | 15.33 |
| B² | 0.2271 | 1 | 0.0527 | 0.1078 | 0.3463 | 10.33 |
| C² | -0.3082 | 1 | 0.063 | -0.4508 | -0.1657 | 14.25 |
| D² | 0.3018 | 1 | 0.0536 | 0.1806 | 0.4231 | 9.36 |
| E² | -0.2895 | 1 | 0.0632 | -0.4324 | -0.1467 | 14.83 |
| F² | -0.0943 | 1 | 0.0341 | -0.1714 | -0.0172 | 3.78 |
| ABC | -0.5305 | 1 | 0.085 | -0.7227 | -0.3382 | 35.42 |
| ABE | -0.5774 | 1 | 0.1117 | -0.8301 | -0.3247 | 65.15 |
| ACD | 0.2028 | 1 | 0.0352 | 0.1233 | 0.2823 | 7.07 |
| ACE | 0.3011 | 1 | 0.0561 | 0.1743 | 0.428 | 15.9 |
| ADE | -0.0623 | 1 | 0.0299 | -0.13 | 0.0054 | 5.82 |
Table 6: The value of the obtained coefficients for the proposed square mod.
According to the remaining parameters in the system; a model was presented to predict the efficiency of enzyme production. The following relationship shows the proposed model: (enzyme production) =%:
04/1-0503653/0 *A+0/0360274 *B-0617/0 051*C+13809/0*D0693/0 234 * E-1680/0 09 *F-093/0 5568 *AB+214/ 0 293 *AC-0946/0 162 *AD+3500/0 03 *AE -148/0 891 *AF +289091/0*BC+043/0 1446 *BD+222/0 399 *BE+0438/0 273 *BF-080/0 8568 *CE-359/0 453 *CF-034/04933 *DE-227/0 764 *DF-346/0 57 *A2+227/0 05 *B23082/0 45 *C2+ 3018/0 34 *D22895/0 3*E2-0943/0 091 *F2530/0 455 *ABC577/0 381 *ABC +20/0 2782 *ACD +3011/0 4 *ACE-062/02598 *ADE, (Table 7).
| (% Enzyme activity) | = |
|---|---|
| 91.82323 | |
| -2.31681 | Temperature |
| -21.63923 | pH |
| -9.1595 | Glucose |
| 4.80655 | Yeast extract |
| -54.87378 | Poultry manure |
| 6.59317 | KH2PO4 |
| 0.560214 | Temperature * pH |
| 0.511317 | Temperature * Glucose |
| -0.206583 | Temperature * Yeast extract |
| 1.93776 | Temperature * Poultry manure |
| -0.11331 | Temperature * KH2PO4 |
| 6.58614 | pH * Glucose |
| 0.26362 | pH * Yeast extract |
| 13.207 | pH * Poultry manure |
| 0.448322 | pH * KH2PO4 |
| -29.54973 | Glucose * Poultry manure |
| -4.18134 | Glucose * KH2PO4 |
| -6.74028 | Yeast extract * Poultry manure |
| -3.69772 | Yeast extract * KH2PO4 |
| -0.010997 | Temperature² |
| 0.173081 | pH² |
| -3.68265 | Glucose² |
| 2.70171 | Yeast extract² |
| -3.58966 | Poultry manure² |
| 0.50951 | KH2PO4² |
| -0.181742 | Temperature * pH * Glucose |
| -0.420636 | Temperature * pH * Poultry manure |
| -0.068217 | Temperature * Glucose * Yeast extract |
| 0.960681 | Temperature * Glucose * Poultry manure |
| 0.235244 | Temperature * Yeast extract * Poultry manure |
Table 7: A model was presented to predict the efficiency of enzyme
Importance and ranking of factors optimized during testing by the software According to the influence of the optimized factors on the amount of enzyme production and optimization, the software shows the most important optimal factor with rank and the least qualified factor with rank [33], (Table 8).
| Name | Goal | Lower Limit | Upper Limit | Lower Weight | Upper Weight | Importance |
|---|---|---|---|---|---|---|
| A:Temperature | minimize | 25 | 35 | 1 | 1 | 5 |
| B:pH | minimize | 6 | 7 | 1 | 1 | 5 |
| C:Glucose | maximize | 0.75 | 1.25 | 1 | 1 | 4 |
| D:Yeast extract | minimize | 0.75 | 1.25 | 1 | 1 | 3 |
| E:Poultry manure | minimize | 0.75 | 1.25 | 1 | 1 | 3 |
| F:KH2PO4 | minimize | 0.75 | 1.25 | 1 | 1 | 3 |
| (Enzyme activity)% | maximize | 0.3912 | 1.43815 | 1 | 1 | 5 |
Table 8: Values obtained from optimization of enzyme production efficiency
Suggested software tests to ensure the researcher's performance in the laboratory In order to ensure the performance and accuracy, the researcher re-defined the optimized factors with the highest enzyme production from Table 1 in three levels and suggested the software for 10 more tests, test number 10 with the enzyme production rate of 88.93% as the final stage.
Table 9 was used to compare the optimization of enzyme production in two normal and optimal environments at 24, 48, and 72 hours.
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Factor 6 | Response | |
|---|---|---|---|---|---|---|---|
| Row | temperature | pH | Glucose | Yeast extract | chicken manure | KH 2 PO 4 | Enzyme production |
| °C | (%) | (%) | (%) | (%) | (%) | ||
| 1 | 27 | 6 | 45658 | 0/75 | 0/65 | 0/65 | 93/41 |
| 2 | 27 | 6 | 12785 | 0/85 | 0/75 | 0/85 | 93/24 |
| 3 | 27 | 6 | 12785 | 0/85 | 0/85 | 0/65 | 93/55 |
| 4 | 23 | 45328 | 42005 | 0/65 | 0/85 | 0/75 | 94/10 |
| 5 | 23 | 6/2/ | 12785 | 0/85 | 0/85 | 0/65 | 93/70 |
| 6 | 25 | 45328 | 12785 | 0/75 | 0/75 | 0/75 | 93/60 |
| 7 | 23 | 45328 | 45658 | 0/85 | 0/65 | 0/85 | 93/39 |
| 8 | 23 | 45509 | 42005 | 0/85 | 0/85 | 0/75 | 93/80 |
| 9 | 27 | 45328 | 42005 | 0/75 | 0/65 | 0/85 | 93/52 |
| 10 | 23 | 45509 | 45658 | 0/75 | 0/75 | 0/85 | 93/88 |
Table 9: The tests suggested by the software and the amount of enzyme production
3D graphs of RSM response surface
Temperature and pH affect enzyme production
A little bit of this analysis is that if we increase the amount of X1= Temperature and X2=PH at the same time, X1 is equal to 30 and X2 is equal to 8, we see the highest amount of enzyme production, and then if we continue to increase the amount of X1, X2 is at its highest level and we see a decrease. We have a high amount of enzyme production, so that the lowest production rate occurs at X1=35 and X2=8, and this decrease is very significant after the temperature of 25, in other words, if we want to express the effect of these two parameters, we can say The best results are obtained at 30°C and pH=8, (Figure 1).
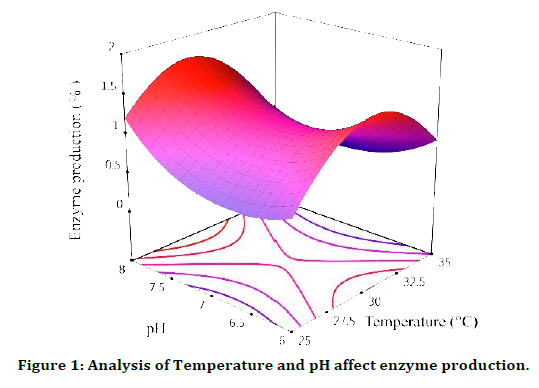
Figure 1: Analysis of Temperature and pH affect enzyme production
Temperature and Yeast Extract Affect Enzyme Production
Quantitatively, in all X1=Temperature, when the amount of X2= yeast extract is at its lowest, we see enzyme production. But with the increase of X2 to about 1, the process of enzyme production decreases, which is more significant in lower X1. Also, when X1 is equal to 25 and X2=1, we will have the highest amount of enzyme production, (Figure 2).
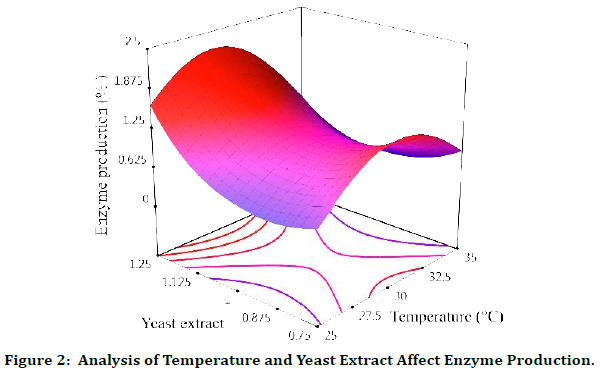
Figure 2: Analysis of Temperature and Yeast Extract Affect Enzyme Production.
PH and Glucose parameters affect enzyme production
From this analysis, it can be concluded that in order to have a suitable efficiency, the environment must be acidic or acidic, with the difference that the efficiency is higher in an acidic environment. Regarding X2=glucose, it can be said that when X1=PH is at its lowest level, with the increase of X2, the amount of production increases, and this increase reaches its maximum until X2 is about 9.0, and after that, it decreases sharply. The amount of production decreases, but this trend in X1=8, it shows a completely different behavior, in this way, by increasing the amount of X2 to 1.1, we have the highest amount of production, and if we continue to increase the amount of X2, we see a decrease in the amount of enzyme production, with this difference that this decrease is insignificant and it can be said that the more X1 is, the more effective X2 will be in the enzyme (Figure 3).
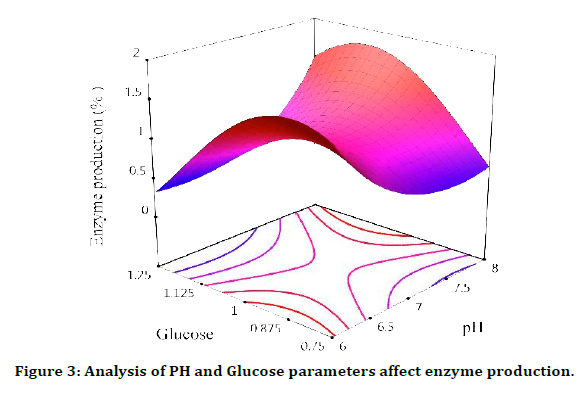
Figure 3: Analysis of PH and Glucose parameters affect enzyme production.
Chicken manure and Glucose affect enzyme production
X1=Glucose, X2=Chicken manure, we have enzyme production in the lowest and highest amount of both parameters X1, X2, but it is very small for both parameters at the beginning, when their amount starts to increase, we see production and this trend is in the middle Both parameters reach the maximum amount of enzyme production and then with a further increase, a decreasing trend is observed again. They are in the middle of their average, X2 = Chicken manure, in the lowest and highest amount of both parameters X1, X2, we have enzyme production, but it is very small for both parameters, at the beginning, when their amount starts to increase, we see production and this The trend towards the middle of both parameters reaches the maximum amount of enzyme production and then with a further increase, a decreasing trend is observed again. Two parameters are in the middle of their mean, (Figure 4).
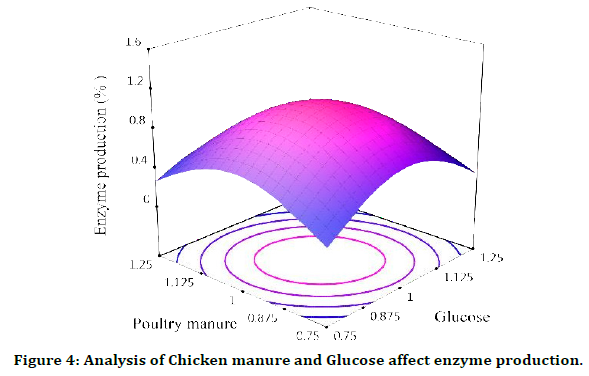
Figure 4: Analysis of Chicken manure and Glucose affect enzyme production
Results and Discussion
Screening average components with a one-variable-at-a-time approach
Single agent experiments at a time showed an increase in enzyme production up to 48 h of the fermentation period (1084 U/mL) and a decrease after 72h at 37°C (942 U/mL). In an enzymatic biological process, the humidity of the substrate and the pH of the fermentation environment are very important for the production of the enzyme. The production of fibrinolytic enzyme was high in the presence of glucose and 1654 U/mL of fibrinolytic activity was observed, while the activity was relatively lower. (1359, 1201, 1128 and 1001 U/mL, respectively) were observed with other carbon sources (starch, sucrose, maltose, galactose). Among nitrogen sources, higher fibrinolytic enzyme activity was obtained with yeast extract (1424 U/mL) compared to peptone, tryptone and urea (1365, 1255, 1098 U/mL, respectively). Among mineral salts, the level of enzyme production is higher with potassium dihydrogen phosphate (1434 units/ml) compared to iron sulfate, disodium phosphate, dipotassium phosphate, magnesium sulfate Sodium chloride, Calcium chloride was obtained (887, 727, 614, 427, 314 and 122 U/mL, respectively). Among food sources, glucose supports maximum enzyme production (1659 units/ml) among all factors, the most important factors. (Temperature, pH, glucose, yeast extract, potassium dihydrogen phosphate and chicken manure) were selected for statistical optimization.
Response Surface Methodology
In this research, the effects of various factors on the production of fibrinolytic enzyme and its optimization by RSM method were investigated in the strain. RSM statistical method is one of the most widely used test design methods. In this method, a number of possible combinations between the factors are selected and then with the statistical evaluation of the results, the best conditions, which may exist among the initially proposed conditions, are introduced as optimal conditions. Another advantage of the RSM method is the investigation of the interaction between factors and also the reduction of the number of tests in the industrial and laboratory scale.
The effect of 6 factors of temperature, pH, glucose, yeast extract, KH2PO4 concentration and chicken manure on three levels of fibrinolytic enzyme was investigated. According to the results obtained from the analysis of variance of 6 factors, four factors of chicken manure, glucose, temperature and pH have the greatest main effect on the production of fibrinolytic enzyme compared to other factors, as well as between the factors "chicken manure and glucose" and "temperature and PH, There was a lot of interference. According to the results of variance analysis, the mean effect showed the greatest effect on enzyme efficiency, which can be further, investigated in future studies.
Therefore, in this report, by performing more than 70 tests, optimal conditions for enzyme production were obtained through the proposed tests of RSM statistical method. As a result, by using this method, it is possible to avoid heavy costs of optimization of industrial products and to perform fewer tests and at the same time obtain better results. In this research, the effects of various factors on the production of fibrinolytic enzyme and its optimization by RSM method were investigated in the strain. RSM statistical method is one of the most widely used test design methods. In this method, a number of possible combinations between factors are selected and then with statistical evaluation of the results, the best possible conditions. If it exists between the initially proposed conditions, it is known as optimal conditions. Another advantage of the RSM method is the investigation of the interaction between factors and also the reduction of the number of tests in the industrial and laboratory scale.
Biochemical Properties of Fibrinolytic Enzyme
Physiochemical properties such as optimal pH/temperature, molecular mass, effect of metal ions/inhibitors and substrate while some fibrinolytic enzymes possess optimal specific activity at extreme acidic or basic conditions, This fibrinolytic enzyme was active at neutral and alkaline pH values, and optimal reaction for fibrinolytic enzyme was obtained at pH=6 while some fibrinolytic enzymes possess optimal specific activity at extreme acidic or basic conditions This optimum pH was similar to that of Bacillus amyloliquefaciens CH51 [34] and Fibrinolytic enzymes from Flammulina velutipes and Pseudomonas baetica SUHU25 were found to exhibit optimal activity at pH=6 The enzyme was stable at pH=6-9 and decreased considerably at higher pH’s. At varying temperatures, this enzyme exhibited maximal activity at 40°C, and was stable up to 40°C after 1 h incubation. The effect of metal ions on the fibrinolytic activity was also examined. K+, Ca2+ and Mg2+ activated the fibrinolytic activity and the relative enzyme activity was 108%, and 128%, respectively. Other than these two ions, none of the ions activated the fibrinolytic activity [35].
Most aspartic proteases have molecular weights in the range 30-45 kDa and their isoelectric points are usually in the range pH 3.4-4.6 (34). Enzyme activity was found to be higher in plasminogen- rich plate than the plasminogen-free plate. Dunia A. Al Farraj and colleagues were able to optimize and report fibrinolytic enzyme with a molecular weight of 32KDa from Bacillus flexus bacteria and maltose, peptone, MgCl2, pH=8, temperature 30-70°C and RSM method [36,37].
Some of the people were able to optimize and report fibrinolytic enzyme with a molecular weight of 42KDa from Bacillus cereus RSA1 bacteria and maltose, peptone, Mn2+ pH=8, temperature 50°C and RSM method [38]. Some of the people were able to optimize and report fibrinolytic enzyme with a molecular weight of 59KDa from Bacillus subtilis WR350 bacteria and sucrose, CSP, MgSO4 pH=7, temperature 60°C and RSM method [20]. In 2019, Li-Jung Yin et al and colleagues were able to optimize and report fibrinolytic enzyme with a molecular weight of 27/5KDa from Bacillus subtilis YJ1 bacteria and Glucose, rice husk, NaCl pH=8/5, temperature 50°C and RSM method [39].
Some of the people were able to optimize and report fibrinolytic enzyme with a molecular weight of 60KDa from Bacillus subtilis D21-8 bacteria and Glucose, Trypton, KH2PO4, pH=7, temperature 37°C and RSM method. To optimize and report fibrinolytic enzyme with a molecular weight of 97/4KDa from Bacillus sp. IND12 bacteria and socruse, pepton, MgSO4, pH=7, temperature 90°C and RSM method. To optimize and report fibrinolytic enzyme with a molecular weight of 250/41U/ml from Serratia sp.KG -2-1 bacteria and Maltose, Yeast extract and pepton, KH2PO4, pH=8, 40°C and RSM meth od [23]. Optimize and report fibrinolytic enzyme with a molecular weight of 47KDa from Bacillus cereus IND5 bacteria and Sucrose, Casein, MgSO4, pH=8, 50°Cand RSM method [40].
To optimize and report fibrinolytic enzyme with a molecular weight of 29/5KDa from Bacillus cereus IND1 bacteria and beef extract NaH2PO4, pH =8, 60°C and RSM method. Optimize and report fibrinolytic enzyme with a molecular weight of 4683 U/ml from Paenibacillus sp. IND8 bacteria and Sucrose, Yeast extract, NaH2PO4, pH= 7/4, 37°C and RSM method. To optimize and report fibrinolytic enzyme with molecular weight of 47KDa from Pseudoalteromona ssp IND11 bacteria and Maltose, Casein, NaH2PO4, pH=7, 80°C and RSM method [41]. In 2018, Priyanka Verma et al and colleagues were able to optimize and report fibri nolytic enzy me with a molecular weight of 45KDa from Streptomyces rubiginosus VITPSS1 bacteria and glycerol, Soyabean meal, pH=7/2, 37°C and RSM method.
To optimize and report fibrinolytic enzyme with a molecular weight of 27-70 KDa from Bacillus amyloliquefaciens CH51 bacteria and CaCl2, pH= 6, 45°C and RSM method. To optimize and report fibrinolytic enzyme with a molecular weight of KDa from Bacillus sp. JER02 bacteria and maltose, pH=8, 45°C and RSM method. To optimize and report fibrinolytic enzyme with a molecular weight of 25-45KDa from Bacillus subtilis (E. coli) bacteria and glucose, soybean hydrolysate, (NH4)2SO4, glutamate, K2HPO4, CaCl2, pH= 8/5, 37°C and RSM method. To optimize and report fibrinolytic enzyme with a molecular weight of 42-60KDa fromBacillus sub tilis I-2 bacteria and Soybean meal, maltose, pH=8, 50°C and RSM method [42]. To optimize and report fibrinolytic enzyme with a molecular weight of 1573U/ml from by Pseudoalteromonas sp. IND11 bacteria and casein, maltose, NaH2PO4, pH=7, 37°C and RSM method. To optimize and report fibrinolytic enzyme with a molecular weight of 50KDa from Pseudomonas aeruginosa KU1 bacteria and Soybean meal, maltose, pH=8, 50°C and RSM method. In 2020, Bijender Kumar Bajaj et al and colleagues were able to optimize and report fibrinolytic enzyme with a molecular weight of 72KDa from Artrhospira (Spirulina) platensis bacteria and Fe2+, pH=6, 40°C and RSM method. In 2021, The current project Optimize and report fibrinolytic enzyme with a molecular weight of (141/56%) from Bacillus KDm99 bacteria and Glucose, Yeast extract, Chicken manure, KH2PO4, pH=6, 40°C and RSM method [43].
Conclusion
Bacillus KDm99 purified and isolated from slaughterhouse wastewater in Kerman province (Iran) was used to produce fibrinolytic enzyme. This optimized enzyme may be widely used in the enzyme industry in the future. This study is very important to investigate the new source of fibrinolytic enzyme from Bacillus KDm 99 for industrial and commercial use.
References
- Razzaq A, Shamsi S, Ali A, et al. Microbial proteases applications. Front bioeng biotechnol. 2019; 7:110.
- Padmapriya B, Rajeswari T, Nandita R, et al. Production and purification of alkaline serine protease from marine Bacillus species and its application in detergent industry. Eur J Appl Sci 2012; 4:21-6.
- Ali AM, Bavisetty SC, Gullo M, et al. Production of fibrinolytic enzymes during food production. InCurrent Develop Biotech Bioeng 2022; 157-187.
- Sun MZ, Liu S, Greenaway FT. Characterization of a fibrinolytic enzyme (ussurenase) from Agkistrodon blomhoffii ussurensis snake venom: insights into the effects of Ca2+ on function and structure. Biochim Biophys Acta Proteins Proteomics 2006; 1764:1340-8.
- Zhang S, Wang Y, Zhang N, et al. Purification and Characterisation of a Fibrinolytic Enzyme from Rhizopus micro sporus var. tuberosus. Food Technol Biotech 2015; 53:243-8.
- Han SM, Weaver FA, Comerota AJ, et al. Efficacy and safety of alfimeprase in patients with acute peripheral arterial occlusion (PAO). J Vasc Surg 2010; 51:600-9.
- Kim W, Choi K, Kim Y, et al. Purification and characterization of a fibrinolytic enzyme produced from Bacillus sp. strain CK 11-4 screened from Chungkook-Jang. Appl Environ Microbiol 1996; 62:2482-8.
- Mirzaei Z, Karami Z. Production and Optimization of Bacterial Fibrinolytic Enzyme Bacillus KDm 99. NeuroQuantol 2022;20:9196.
- Vijayaraghavan P, Vincent SP. A low cost fermentation medium for potential fibrinolytic enzyme production by a newly isolated marine bacterium, Shewanella sp. IND20. BiotechnolRep 2015; 7:135-42.
- Sumi H, Hamada H, Tsushima H, et al. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia 1987;43:1110-1.
- Wang F, Wang C, Li M, et al. Purification, characterization and crystallization of a group of earthworm fibrinolytic enzymes from Eisenia fetida. Biotechnol Lett 2003; 25:1105-9.
- Wiman B. The fibrinolytic enzyme system: basic principles and links to venous and arterial thrombosis. Hematol Oncol Clin North Am 2000; 14:325-38.
- Jhample SB, Bhagwat PK, Dandge PB. Statistical media optimization for enhanced production of fibrinolytic enzyme from newly isolated Proteus penneri SP-20. Biocatal Agric Biotechnol 2015; 4:370-9.
- de Souza FA, Sales AE, Costa e Silva PE, et al. Optimization of production, biochemical characterization and in vitro evaluation of the therapeutic potential of fibrinolytic enzymes from a new Bacillus amyloliquefaciens. Macromol Res 2016; 24:587-95.
- Moharam ME, El-Bendary MA, El-Beih F, et al. Optimization of fibrinolytic enzyme production by newly isolated Bacillus subtilis Egy using central composite design. Biocatal Agric Biotechnol 2019;17:43-50.
- Vijayaraghavan P, Arasu MV, Rajan RA, et al. Enhanced production of fibrinolytic enzyme by a new Xanthomonas oryzae IND3 using low-cost culture medium by response surface methodology. Saudi J Biol Sci 2019; 26:217-24.
- Taneja K, Kumar Bajaj B, Kumar S, et al. Process optimization for production and purification of novel fibrinolytic enzyme from Stenotrophomonas sp. KG-16-3. Biocatal Biotransfor 2019; 37:124-38.
- Vijayaraghavan P, Vincent SG, Arasu MV, et al. Bioconversion of agro-industrial wastes for the production of fibrinolytic enzyme from Bacillus halodurans IND18: Purification and biochemical characterization. Electron J Biotechnol 2016;20:1-8.
- Sharma C, Salem GE, Sharma N, et al. Thrombolytic potential of novel thiol-dependent fibrinolytic protease from Bacillus cereus RSA1. Biomol 2019; 10:3.
- Wu R, Chen G, Pan S, et al. Cost-effective fibrinolytic enzyme production by Bacillus subtilis WR350 using medium supplemented with corn steep powder and sucrose. Scientific Rep 2019; 9:6824.
- Pan S, Chen G, Wu R, et al. Non-sterile submerged fermentation of fibrinolytic enzyme by marine Bacillus subtilis harboring antibacterial activity with starvation strategy. Front Microbiol 2019; 10:1025.
- Yin LJ, Lin HH, Jiang ST. Bioproperties of potent nattokinase from Bacillus subtilis YJ1. J Agric Food Chem 2010; 58:5737-42.
- Taneja K, Bajaj BK, Kumar S, et al. Production, purification and characterization of fibrinolytic enzyme from Serratia sp. KG-2-1 using optimized media. 3 Biotech 2017;7:1-5.
- Smitha KV, Pradeep BV. Application of Box-Behnken design for the optimization of culture conditions for novel fibrinolytic enzyme production by Bacillus altitudinis S-CSR 0020. J Pure Appl Microbiol 2017; 11:1447-56.
- Badoei-Dalfard A, Khankari S, Karami Z. One-pot synthesis and biochemical characterization of protease metal organic framework (protease@ MOF) and its application on the hydrolysis of fish protein-waste. Colloids Surf B Biointerfaces 2020; 196:111318.
- Vijayaraghavan P, Rajendran P, Prakash Vincent SG, et al. Novel sequential screening and enhanced production of fibrinolytic enzyme by Bacillus sp. IND12 using response surface methodology in solid-state fermentation. Biomed Res Int 2017.
- Vijayaraghavan P, Vincent SG. Statistical optimization of fibrinolytic enzyme production by Pseudoalteromonas sp. IND11 using cow dung substrate by response surface methodology. SpringerPlus 2014;3:1-0.
- Biji GD, Arun A, Muthulakshmi E, et al. Bio-prospecting of cuttle fish waste and cow dung for the production of fibrinolytic enzyme from Bacillus cereus IND5 in solid state fermentation. 3 Biotech 2016; 6:1-3.
- Vijayaraghavan P, Prakash Vincent SG. Statistical optimization of fibrinolytic enzyme production using agroresidues by Bacillus cereus IND1 and its thrombolytic activity in vitro. Biomed Res Int 2014.
- Verma P, Chatterjee S, Keziah MS, et al. Fibrinolytic protease from marine Streptomyces rubiginosus VITPSS1. Cardiovasc Hematol Agents Med Chem 2018; 16:44-55.
- Kim GM, Lee AR, Lee KW, et al. Characterization of a 27 kDa fibrinolytic enzyme from Bacillus amyloliquefaciens CH51 isolated from cheonggukjang. J Microbiol Biotechn. 2009; 19:997-1004.
- Badoei-Dalfard A, Karami Z. Screening and isolation of an organic solvent tolerant-protease from Bacillus sp. JER02: Activity optimization by response surface methodology. J Mol Catal B Enzym 2013; 89:15-23.
- Pham VH, Kim J, Shim J, et al. Purification and characterization of strong simultaneous enzyme production of protease and α-Amylase from an extremophile-Bacillus sp. FW2 and its possibility in food waste degradation. Ferment 2021; 8:12.
- Chen PT, Chiang CJ, Chao YP. Medium optimization for the production of recombinant nattokinase by Bacillus subtilis using response surface methodology. Biotechnol Prog 2007;23:1327-32.
- Bharathiraja S, Suriya J, Krishnan M, et al. Production of enzymes from agricultural wastes and their potential industrial applications. InAdv Food Nutr Res 2017; 80:125-148.
- Bajaj BK, Singh S, Khullar M, et al. Optimization of fibrinolytic protease production from Bacillus subtilis I-2 using agro-residues. Braz Arch Biol Technol 2014;57:653-62.
- Jeong SJ, Heo K, Park JY, et al. Characterization of AprE176, a fibrinolytic enzyme from Bacillus subtilis HK176. J Microbiol Biotechn 2015; 25:89-97.
- Vijayaraghavan P, Vincent SG. Statistical optimization of fibrinolytic enzyme production by Pseudoalteromonas sp. IND11 using cow dung substrate by response surface methodology. SpringerPlus 2014; 3:1-0.
- Kumar SS, Haridas M, Abdulhameed S. A novel fibrinolytic enzyme from marine Pseudomonas aeruginosa KU1 and its rapid in vivo thrombolysis with little haemolysis. Int J Biol Macromol 2020;162:470-9.
- de Barros PD, e Silva PE, Nascimento TP, et al. Fibrinolytic enzyme from Arthrospira platensis cultivated in medium culture supplemented with corn steep liquor. Int J Biol Macromol 2020; 164:3446-53.
- Al Farraj DA, Kumar TS, Vijayaraghavan P, et al. Enhanced production, purification and biochemical characterization of therapeutic potential fibrinolytic enzyme from a new Bacillus flexus from marine environment. J King Saud Univ Sci 2020; 32:3174-80.
- Park SE, Li MH, Kim JS, et al. Purification and characterization of a fibrinolytic protease from a culture supernatant of Flammulina velutipes mycelia. Biosci Biotechnol Biochem 2007; 71:2214-22.
- Salunke AS, Kharat AS. Data on isolation and purification of fibrinolytic enzyme from Pseudomonas baetica SUHU25. Data Brief 2019; 26:104369.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Author Info
Zahra Mirzaeia, Arastoo Badoei Dalfard* and Zahra Karami
Department of Biology, Shahid Bahonar University, Kerman, IranPublished: 24-Apr-2024
