Research Article - (2023) Volume 11, Issue 1
Micronucleus Assay Among A group of Smokers in Relation to Oral Health Status
Umelbaneen J Alalawy1*, Ahlam T Mohammed1 and Layla Sabri Yass2
*Correspondence: Umelbaneen J Alalawy, Department of Pedodontics and Preventive Dentistry, College of Dentistry, University of Baghdad, Baghdad, Iraq, Email:
Abstract
Background: Micronucleus assay is non-invasive, new methods for investigating DNA and chromosomal damage, cytokinetic defect, the regenerative ability of the tissue and cell death in the buccal cells that exfoliated by scraping. The different compounds in cigarette induced DNA strand breakage, gene mutation, chromosomal abnormality and micronuclei, all contribute to the carcinogenic effects of cigarette smoke. The aim of this study was to identify the genotoxic impact of smoking and its relation to oral health status.
Materials and methods: This study was carried on 70 males of (30-35) years of age (35 heavy smokers and 35 non-smokers). A cytobrush was used to obtain the smears and stained by pap stain. The oral health status was evaluated by using the DMFS, plaque and calculus indices, bleeding on probing, pocket depth and clinical attachment loss.
Result: Micronuclei and other abnormalities were significantly different between smokers and nonsmokers (p-value<0.05). The mean of plaque index was significantly different in smokers than that in non-smokers. The mean calculus index, bleeding on probing and DMFS statistically not significant (p-value>0.05). The percentages of pocket depth and clinical attachment loss among smokers were significantly higher than that in nonsmokers.
Conclusion: The smoking has a deteriorated effect on the oral cavity particularly on periodontium. The micronucleus assay is an excellent biomarker for detecting those who are at high risk of oral mutations as a result of smoking's adverse effects.
Keywords
Micronucleus assay, Smoking, Pocket depth, Clinical attachment loss, Bleeding on probing
Introduction
Humans are exposed to a number of genotoxic compounds found in today's polluted environment in the modern world. As a result, tests are needed to determine the level of exposure and the health risks connected with it. Although there are other tests available, the Micronucleus test (MN) is one of the most popular and widely utilized [1]. Micronucleus assay is non-invasive, new methods for investigating DNA and chromosomal damage, cytokinetic defect, the regenerative ability of the tissue and cell death in the buccal cells that exfoliated by scraping [2]. It's a simple, sensitive assay does not need the use of blood or tissue biopsies, nor does it involve the formation of a cell culture [3]. The etiology of these micronuclei can be attributed to environmental pollutants such as drugs, chemicals, food and free radical injuries, as well as occupational exposures to (organic solvents, antineoplastic agents), lead containing paints solvents and arsenic contaminated drinking water, as well as ionizing radiation used to treat neoplasia (smoking, alcohol consumption, diet, vitamin deficiencies) [4-6].
The buccal micronucleus assay is preferred for a variety of reasons, including the fact that buccal tissue serves as a first barrier and absorbent of all inhaled or ingested agents, buccal tissue has limited ability to repair its DNA, allowing it to reflect age related damage and the fact that epithelium is the source of 90% of cancers [7-10].
Tobacco is the only legal medicine that kills large number of its users when used as directed by the manufacturer. According to the World Health Organization (WHO), tobacco use (including smoking and non-smoking) kills approximately six million people globally each year, with many of these deaths happening prematurely. It has been associated to an increased risk of communicable disease related death [11]. Despite its association with ill health, disability and death from non-communicable chronic diseases. Smoking can cause visual alterations in the oral cavity, such as stained teeth, discolored 'tooth-colored' restorations and dentures [12]. Furthermore, smoking is a significant risk factor for periodontal disease, since it promotes the loss of gingival attachment and increases gingival regression, resulting in increased progression periodontal inflammation [13]. Tobacco generates a complex combination of around 7000 chemical compounds in the form of gases, liquid vapours and particulate debris [14]. The mutagenicity of different compounds in cigarette smoke induced DNA strand breakage, oxidative DNA adducts, gene mutation, sister cremated exchange, chromosomal abnormality and micronuclei, all contribute to the carcinogenic effects of cigarette smoke in a wide range of systems [15].
Oral health is a fundamental but often overlooked part of general health and well-being, and oral diseases can have a significant impact on an individual's health and wellbeing by causing pain, morbidity, mortality and a loss of ability to participate in school, social and economic activities [16]. Dental caries, periodontal disease and oral malignancies are significant clinical disorders that are considered global public health issues [17]. Dental caries is a complicated, chronic, multifactorial disease that is one of the most common diseases in both developed and developing countries [18,19]. Periodontal diseases are a type of inflammatory illnesses affecting the supporting components of the teeth (gums, bone and periodontal ligament). Periodontal diseases are a type of inflammatory illnesses affecting the supporting components of the teeth (gums, bone and periodontal ligament). They're produced by a dysbiosis of the commensal oral microbiota (dental plaque), which interacts with the host's immune system, causing inflammation and disease [20]. As available knowledge from previous literatures there is only one Iraqi study about micronucleus assay and its relation to dental caries, oral cleanliness and gingival condition among smokers, but no Iraqi study about its relation to periodontitis among smokers, therefore, this study will be conducted [21].
Materials and Methods
Seventy males’ volunteers aged (30-35) years (35 were heavy smokers those who smoked at least 20 cigarettes per day for at least the past 5 years and had no period of smoking abstinence longer than 3 months in the past years and 35 were non-smokers) attending to the oral diagnosis department of specialized center for dentistry in Al Kut city. They had no systemic disease and were not take any supplements.
Smoking status of the volunteers was assessed by means of self-reported questionnaire which include demographic information about the volunteer, oral health status, smoking status and the cytopathological results.
The oral examinations include the plaque, calculus, DMFS, bleeding on probing, pocket depth and clinical attachment loss indices using CPI modified probe [22-24].
The cytopathological brush should be used to obtain an oral smear from normal buccal mucosa. The stains were laid on a labeled, clean, dry glass slide and spread out. The patient's name and code were written on each slide. The slides fixed at once by 95% ethanol for 20 minutes and then stained by pap stain. The slides were assessed under 40x magnification using a light microscope; 1000 cells per slide were examined in a zig-zag pattern for the presence of micronuclei and other abnormalties (Micronucleated cells (MN), Binucleated cells (BN), Pyknotic cells (PK), Karyorrhectic cells (KR), Karyolysis cells (KL), Nuclear Buds (NB) and Cells with Condensed Chromatin (CC)) [25].
Data analysis was conducted by application of SPSS program (SPSS version 26) using means, standard deviation, percentage, independent sample t-test, ch-isquare test and pearson’s correlation coefficient test.
Results
The participants in the study sample all had various numbers of positive micronucleus expression (Table 1 and Figure 1). There was a statistically significant difference in micronuclei and other abnormalities between smokers and non-smokers, no cells with condensed chromatin were observed in the research group or in the control participants, therefore no statistical difference was found.
| Micronuclei expression | Study group | t-test | P-value | |
|---|---|---|---|---|
| Smoker Mean ± SD | Non smoker Mean ± SD | |||
| Micronucleated cells (MN) | 6.28 ± 2.8 | 2.17 ± 1.2 | 7.908 | 0.001 |
| Binucleated cells (BN) | 0.85 ± 0.84 | 0.45 ± 0.78 | 2.058 | 0.043 |
| Pyknotic cells (PK) | 27.42 ± 9.2 | 16.08 ± 4.1 | 6.655 | 0.001 |
| Karyorrhectic cells (KR) | 0.77 ± 0.87 | 0.2 ± 0.53 | 3.296 | 0.002 |
| Karyolysis cells (KL) | 0.74 ± 0.85 | 0.22 ± 0.42 | 3.194 | 0.002 |
| Nuclear Buds (NB) | 0.4 ± 0.55 | 0.11 ± 0.32 | 2.64 | 0.011 |
| Cells with Condensed Chromatin (CC) | 0 | 0 | - | - |
Table 1: Comparison in micronuclei expression between study and control groups.
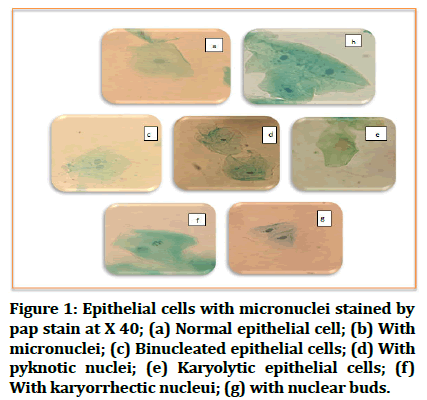
Figure 1: Epithelial cells with micronuclei stained by pap stain at X 40; (a) Normal epithelial cell; (b) With micronuclei; (c) Binucleated epithelial cells; (d) With pyknotic nuclei; (e) Karyolytic epithelial cells; (f) With karyorrhectic nucleui; (g) with nuclear buds.
The mean of Plaque Index (PI) which was significantly higher in smokers than that in non-smokers, while the mean Calculus Index (CI), Bleeding on Probing (BOP) and DMFS were higher among smokers but statistically there were no differences (Table 2).
| Variable | Mean ± SD | Range | t-test | P-value |
|---|---|---|---|---|
| DMFS | ||||
| Smoker | 40.62 ± 22.4 | 14.0-91.0 | 1.868 | 0.066 |
| Non smoker | 30.65 ± 22.2 | 5.0-78.0 | ||
| Plaque index | ||||
| Smoker | 1.07 ± 0.36 | 0.43-1.81 | 3.449 | 0.001 |
| Non smoker | 0.82 ± 0.19 | 0.45-1.27 | ||
| Calculus index | ||||
| Smoker | 0.54 ± 0.27 | 0.17-1.08 | 1.408 | 0.164 |
| Non smoker | 0.46 ± 0.2 | 0.12-0.96 | ||
| Bleeding on Probing (POB) | ||||
| Smoker | 0.29 ± 0.22 | 0-1.0 | 1.651 | 0.103 |
| Non smoker | 0.38 ± 0.22 | 0.125-1.0 | ||
Table 2: PI, CI, BOP and DMFS between study and control group.
The percentages of Pocket Depth (PD) and Clinical Attachment Loss (CAL) scores among smokers were significantly higher than that in non-smokers when using Chi-square test (Table 3).
| Scores | Study groups | Total (%) | X2 | P-value | |
|---|---|---|---|---|---|
| Smoker (%) | Non smoker (%) | ||||
| PD | |||||
| Score 0 | 940 (94.0) | 1017 (96.5) | 1957 (95.3) | 7.307 | 0.025 |
| Score 1 | 48 (4.8) | 28 (2.7) | 76 (3.7) | ||
| Score 2 | 12 (1.2) | 9 (0.8) | 21 (1.0) | ||
| CAL | |||||
| Score 0 | 142 (73.2) | 175 (85.0) | 175 (85.0) | 8.428 | 0.037 |
| Score 1 | 32 (16.5) | 19 (9.2) | 19 (9.2) | ||
| Score 2 | 11 (5.7) | 7 (3.4) | 7 (3.4) | ||
| Score 3 | 9 (4.6) | 5 (2.4) | 5 (2.4) | ||
Table 3: PD and CAL between study and control group.
Statistically significant weak positive correlations were detected between PI and MN, between DMFS and KR, between MN and score 1 of CAL and between NB and sore 1 of PD. Also weak significant negative correlations were detected between MN and score 0 of PD, between Mn and score 0 of CAL, between PK and score 0 of PD and between NB and score 0 of PD. No statistical significant correlations detected (P ≥ 0.05) between other oral health status variables with other micronuclei expression (Tables 4 and 5).
| Variables | DMFS | PI | CI | BOP | |
|---|---|---|---|---|---|
| Micronuclei expression | |||||
| MN | r | 0.235 | 0.317 | -0.002 | -0.125 |
| P-Value | 0.05 | 0.008 | 0.988 | 0.301 | |
| BN | r | 0.107 | 0.101 | -0.094 | -0.092 |
| P-Value | 0.379 | 0.404 | 0.439 | 0.447 | |
| PK | r | 0.154 | 0.115 | 0.187 | -0.192 |
| P-Value | 0.204 | 0.343 | 0.121 | 0.112 | |
| KR | r | 0.248 | 0.174 | -0.006 | 0.1 |
| P-Value | 0.038 | 0.151 | 0.963 | 0.411 | |
| KL | r | 0.118 | 0.184 | 0.15 | 0.053 |
| P-Value | 0.329 | 0.128 | 0.215 | 0.661 | |
| NB | r | 0.021 | 0.11 | -0.046 | 0.052 |
| P-Value | 0.864 | 0.365 | 0.708 | 0.666 | |
Table 4: Correlations between (DMFS, PI, CI and BOP) and micronuclei expression.
| Variables | PD | CAL | ||||||
|---|---|---|---|---|---|---|---|---|
| Micronuclei expression | Score 0 | Score 1 | Score 2 | Score 0 | Score 1 | Score 2 | Score 3 | |
| MN | r | -0.234 | 0.9 | 0.046 | -0.234 | 0.235 | 0.32 | 0.064 |
| P-value | 0.05 | 0.456 | 0.706 | 0.006 | 0.05 | 0.794 | 0.598 | |
| BN | r | -0.077 | -0.054 | 0.006 | -0.069 | -0.011 | -0.191 | 0.008 |
| P-value | 0.529 | 0.655 | 0.958 | 0.572 | 0.93 | 0.113 | 0.948 | |
| PK | r | -0.278 | 0.0062 | -0.014 | -0.139 | 0.108 | 0.179 | -0.64 |
| P-value | 0.02 | 0.612 | 0.907 | 0.25 | 0.374 | 0.139 | 0.598 | |
| KR | r | -0.12 | -0.082 | -0.007 | 0 | 0.005 | 0.96 | -0.12 |
| P-value | 0.323 | 0.502 | 0.955 | 0.998 | 0.966 | 0.43 | 0.324 | |
| KL | r | -0.122 | -0.037 | -0.081 | -0.224 | 0.078 | 0.012 | 0.009 |
| P-value | 0.314 | 0.76 | 0.504 | 0.062 | 0.519 | 0.923 | 0.939 | |
| NB | r | -0.294 | 0.28 | 0.9 | -0.139 | 0.18 | -0.044 | 0.098 |
| P-value | 0.013 | 0.019 | 0.46 | 0.252 | 0.136 | 0.719 | 0.418 | |
Table 5: Correlations between (PD and CAL) and micronuclei expression stained by PAP stain.
Discussion
Tobacco smoking has a well-documented deleterious impact on oral health, which encompasses both common and unusual problems ranging from benign to life threatening diseases [26]. The buccal micronucleus assay is used to detect DNA damage and cell death in exfoliated buccal cells. By evaluating mean frequencies of micronuclei, binucleated cells, nuclear buds, karyolysis, karyorrhexis, pycknosis and condensed chromatin, it provides a powerful tool for detecting genotoxicity.
The current study was originally designated to detect the changes of micronuclei expression in Iraqi male heavy smokers as a biomarker for the oral epithelial cells genomic damage which is the significant cause in oral disease and cancers, the relation of smoking to oral health status variables and the relation of these variables to micronucleus assay since they may participate in micronuclei and other abnormalities formation.
Finding of this study reported that the mean of DMFS was higher in smoker than the non-smokers but statistically there was no significant differences (p>0.05) which agree with al-Deen, but disagree with several studies [27-31]. Many factors that contribute to the development of dental caries should be examined, including age, oral hygiene practices, dietary habits, preventive dental visits, overall health standards and dental care education. As a result, identifying the precise degree of dental caries as a result of smoking is challenging [32].
Significant difference was found in plaque index between smokers and non-smokers group i.e. more plaque accumulation in heavy smokers group than non-smokers group, this agree with Mokeem, et al. and Nanakaly, et al. [33,34]. This may be due to the influence of smoking on both the composition of the biofilm and the host response to this colonization, as reported by a positive correlation between proinflammatory cytokine levels and commensal bacteria in smokers but not in nonsmokers [35].
There is no significant difference in calculus creation between smokers and nonsmokers. Jenkins, et al. studied the link between smoking tobacco and the formation of calculus and found no evidence of a correlation [36]. Oral hygiene practices, availability to professional care, nutrition, age, ethnic origin and interval since last tooth cleaning, systemic disease and prescription drug use all have an impact on the amount of calculus and where it forms in different populations [37].
This study found that control groups had less numbers of sites with bleeding on probing than disease groups but significantly there were no differences. This result disagree Giannopoulou, et al. Jassim and Sura who found that BOP were significantly less in smokers than nonsmokers. Non-significant difference was found in this study might be explained by the high level of education and good oral hygiene in majority of non-smoker group [38-52].
Conclusion
According to results of this study, there was significant difference in Probing Pocket Depth (PPD) and Clinical Attachment Loss (CAL) between non-smokers and smokers groups. This actual increase in PPD and CAL in smokers compared to non-smokers was compatible with several prior studies that indicated smokers had considerably more sites with increased probing depths than non-smokers. Several factors contribute to the deterioration of periodontal health in heavy smokers, including the release of chemical mediators that initiate inflammation, changes in fibroblast proliferation and a suppressed immune response to pathogens that cause periodontitis due to changes in neutrophil function. In the present study, we had significant elevation in Mn and other nuclear anomalies also like BN, NB, PK, KL and KR in the smokers than non-smokers. Cigarette smoking and other forms of tobacco have been found in several studies to increase the frequency of micronuclei in exfoliated buccal epithelial cells. Smoking contains a complex mixture of genotoxic and carcinogenic chemicals that affect oral epithelial cells, such as polycyclic aromatic hydrocarbons, aromatic amines, nitrosamines, heavy metals, noxious gases and pesticide residues. Nitrosamines found in tobacco, such as N-nitrosonor nicotine, are classified carcinogens. In a variety of systems, these materials activated in diverse organs, causing DNA strand breakage, oxidative DNA adducts, gene mutation, sister cremated exchange, chromosomal abnormality and micronuclei. But there are also contrasts, Stich and Rosin noticed an increase in MN frequency in alcoholics who smoked 2-4 packs of cigarettes per day, but those who smoked cigarettes alone did not exhibit any significant increase in MN frequency.
There were positive correlation between the (plaque index, calculus index) and micronucleus assay, when the plaque increased the micronucleus expression increased also, that disagree with Damayanti, et al. found no significant correlation between micronucleus expression and oral hygiene index (plaque and calculus). They concluded that the Genotoxic effect of smoking result in increasing in micronuclei expression and in poor oral health. Also there were positive correlation between the increasing in pocket depth, clinical attachment loss and micronucleus expression and there were negative correlation between micronucleus expression and increasing number of healthy periodontal tissue (PD and CAL less than 4 mm). That agree with Zamora, et al. finding that identified an increase in MN numbers in buccal mucosa cells from patients with periodontal disease and they assumed that the DNA damage is a critical event not only in the initiation of periodontal disease but also in the promotion and progression. So the MN test's efficacy as a cytogenetic marker for the development of a variety of oral diseases.
Ethical Approval
All experimental protocols were approved by the college of dentistry, university of Baghdad. All experiments were carried out following the approved guidelines (Ref no. 274 on 25/3/2021).
Financial Support
There was no financial disclosure.
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
- Sommer S, Buraczewska I, Kruszewski M. Micronucleus assay: The state of art and future directions. Int J Mol Sci 2020; 21:1534. [Crossref][Googlescholar][Indexed]
- Yadav AS, Jaggi S. Buccal micronucleus cytome assay a biomarker of genotoxicity. J Mol Biomark Diagn 2015; 6:1. [Crossref]
- Federico C, Vitale V, La Porta N, et al. Buccal micronucleus assay in human populations from sicily (Italy) exposed to petrochemical industry pollutants. Environ Sci Pollut Res Int 2019; 26:7048-7054. [Crossref][Googlescholar][Indexed]
- Shashikala R, Indira AP, Manjunath GS. Role of micronucleus in oral exfoliative cytology. J Pharm Bioallied Sci 2015; 7:S409. [Crossref][Googlescholar][Indexed]
- Samanta S, Dey P. Micro nucleus and its application. Diagn Cytopathol 2010; 40:84-90. [Crossref][Googlescholar][Indexed]
- Holland N, Bolognesi C, Kirsch-Volders M, et al. The micronucleus assay in human buccal cells as a tool for bio-monitoring DNA damage: The HUMN project perspective on current status and knowledge gaps. Mutat Res 2008; 659:93-108. [Crossref][Googlescholar][Indexed]
- Kashyap B, Reddy PS. Micronuclei assay of exfoliated oral buccal cells: Means to assess the nuclear abnormalities in different diseases. J Cancer Res Ther 2012; 8:184. [Crossref][Googlescholar][Indexed]
- Casartelli G, Bonatti S, De Ferrari M, et al. Micronucleus frequencies in exfoliated buccal cells in normal mucosa, precancerous lesions and squamous cell carcinoma. Anal Quant Cytol Histol 2000; 22:486-492. [Googlescholar][Indexed]
- Fenech M, Holland N, Knasmueller S, et al. Report on the buccal micronucleus assay workshop organized by the international Human Micronucleus (HUMN) project. Mutagenesis 2009; 24:199-201. [Crossref][Googlescholar][Indexed]
- Claudio, Samuel Rangel, Souza A, et al. Genomic instability and cytotoxicity in buccal mucosal cells of workers in banana farming evaluated by micronucleus test. Anticancer Res 2019; 39:1283-1286 [Crossref][Googlescholar][Indexed]
- World Health Organization (WHO). WHO global report on trends in prevalence of tobacco smoking 2015. World Health Organization, 2015. [Googlescholar][Indexed]
- Csikar J, Kang J, Wyborn C, et al. The self-reported oral health status and dental attendance of smokers and non-smokers in England. Plos One 2016; 11:e0148700. [Crossref][Googlescholar][Indexed]
- Tatullo M, Gentile S, Paduano F, et al. Crosstalk between oral and general health status in e-smokers. Medicine 2016; 95:e5589. [Crossref][Googlescholar][Indexed]
- Ampbell MA, Ford C, Winstanley MH. The health effects of secondhand smoke 4.1 What is secondhand smoke? In Greenhalgh, EM, Scollo MM and Winstanley MH. Tobacco in Australia: Facts and issues. Melbourne: Cancer council Victoria. 2021.
- Shafi FA. The effects of smoking on micronucleus frequencies in buccal cells of healthy Iraqi individuals. World J Pharm Res 2014; 4:406-415. [Googlescholar]
- Singh A, Shrestha A, Bhagat TK, et al. Assessment of oral health status and treatment needs among people of foklyan area, Dharan, Nepal. BMC Oral Health 2020; 20:320. [Crossref][Googlescholar][Indexed]
- Peres MA, Daly B, Guarnizo-Herreno CC, et al. Oral diseases: A global public health challenge authors' reply. Lancet 2020; 395:186-187. [Crossref][Googlescholar][Indexed]
- Opal S, Garg S, Jain J, et al. Genetic factors affecting dental caries risk. Aust Dent J 2015; 60:2-11. [Crossref][Googlescholar][Indexed]
- Yildiz G, Ermis RB, Calapoglu NS, et al. Gene environment interactions in the etiology of dental caries. J Dent Res 2016; 95:74-79. [Crossref][Googlescholar][Indexed]
- Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis primers 2017; 3:1-14. [Crossref][Googlescholar][Indexed]
- Saeed SH, Younis WH. A cytopathological study of the effect of smoking on the oral epithelial cells in relation to oral health status by the micronucleus assay. J Bagh Coll Dentistry 2012; 24:67-70. [Googlescholar][Indexed]
- Silness J, Loe H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand 1964; 22:121-135. [Crossref][Googlescholar][Indexed]
- Ramfjord SP. Indices for prevalence and incidence of periodontal disease. J Periodont 1959; 30:51-59. [Crossref][Googlescholar][Indexed]
- World Health Organization (WHO). Oral health surveys: Basic methods. 5th edition, 2013; 125.
- Jadhav, Kiran, Nidhi Gupta, and Mujib BR Ahmed. Micronuclei: An essential biomarker in oral exfoliated cells for grading of oral squamous cell carcinoma. J Cytol 2011; 28:7-12. [Crossref][Googlescholar][Indexed]
- Suchanecka A, Chmielowiec K, Chmielowiec J, et al. Vitamin D receptor gene polymorphisms and cigarette smoking impact on oral health: A case control study. Int J Environ Res Public Health 2020; 17:3192. [Crossref][Googlescholar][Indexed]
- Khair Al-Deen S, Al-Jubouri M, Kamal N. The role of smoking with some salivary parameters, dental caries and gingivitis. Tikrit J Dent Sci 2015; 1:119-123.
- Al-Weheb AM. Smoking and its relation to caries experience and salivary Lactobacilli count. J Coll Dent 2005; 17:92-95. [Googlescholar]
- Tanaka K, Miyake Y, Arakawa M, et al. Household smoking and dental caries in schoolchildren: The ryukyus child health study. BMC Public Health 2010; 10:335. [Crossref][Googlescholar][Indexed]
- Bloom B, Adams PF, Cohen RA, et al. Smoking and oral health in dentate adults aged 18-64. NCHS Data Brief 2012; 85:1-8. [Googlescholar][Indexed]
- Lashkari KP, Shukla A. Prevalence of dental caries among smokeless tobacco chewers in Dakshina Kannada district population: A cross sectional study. OHDM 2016; 15:1-3.
- Smejkalova J, Jacob V, Hodacova L, et al. The influence of smoking on dental and periodontal status. Oral Health Care: Pediatric. Research, Epidemiology and Clinical Practices 2012; 249-270. [Crossref][Googlescholar][Indexed]
- Mokeem SA, Vellappally S, Preethanath RS, et al. Influence of smoking on clinical parameters and gingival crevicular fluid volume in patients with chronic periodontitis. Oral Health Dent Manag 2014; 13:469-473. [Googlescholar][Indexed]
- Nanakaly HT, Ismail AE, Othmn DA. Influence of smoking on salivary interleukin-8 levels in chronic periodontitis. J Baghdad Coll Dent 2020; 32:28-34. [Googlescholar][Indexed]
- Kumar PS, Matthews CR, Joshi V, et al. Tobacco smoking affects bacterial acquisition and colonization in oral biofilms. Infect Immun 2011; 79:4730-4738. [Crossref][Googlescholar][Indexed]
- Jenkins C, Pham XD, Do HN, et al. Tobacco use in vietnam: Prevalence, predictor and the role of the transnational tobacco corporations. JAMA 1997; 277:1726-1731. [Crossref][Googlescholar][Indexed]
- White DJ. Dental calculus: Recent insights into occurrence, formation, prevention, removal and oral health effects of supra gingival and sub gingival deposits. Eur J Oral Sci 1997; 105:508-522. [Crossref][Googlescholar][Indexed]
- Giannopoulou C, Cappuyns I, Mombelli A. Effect of smoking on gingival crevicular fluid cytokine profile during experimental gingivitis. J Clin periodontal 2003; 30:996-1002. [Crossref][Googlescholar][Indexed]
- Jassim SD. Correlation of periodontal health status with salivary matrix metalloproteinase 9 levels and total salivary peroxidase activities in smokers and non-smokers: A comparative study. J Baghdad Coll Dent 2016; 28:128-133.
- Rai B, Kharb S, Anand SC. Salivary enzymes and thiocynate: Salivary markers of periodontitis among smokers and nonsmokers: A pilot study. Adv Med Dent Sci 2007; 1:1-4. [Googlescholar][Indexed]
- Luzzi LIT, Greghi SLA, Passanezi E, et al. Evaluation of clinical periodontal conditions in smokers and non-smokers. J Appl Oral Sci 2007; 15:512-517. [Crossref][Googlescholar][Indexed]
- Khan S, Khalid T, Awan KH. Chronic periodontitis and smoking prevalence and dose response relationship. Saudi Med J 2016; 37:889-894. [Crossref][Googlescholar][Indexed]
- Haveric A, Haveric S, Ibrulj S. Micronuclei frequencies in peripheral blood and buccal exfoliated cells of young smokers and non-smokers. Toxicol Mech Methods 2010; 20:260-2666. [Crossref][Googlescholar][Indexed]
- Joshi MS, Verma Y, Gautam AK, et al. Cytogenetic alterations in buccal mucosa cells of chewers of areca nut and tobacco. Arch Oral Biol 2011; 56:63-67. [Crossref][Googlescholar][Indexed]
- Kamath VV, Anigol P, Setlur K, et al. Micronuclei as prognostic indicators in oral cytological smears: A comparison between smokers and non-smokers. Clin Cancer Investig J 2014; 3:49-54. [Crossref]
- Crispino CC, Fernandes KG, Kamogawa MY, et al. Multivariate classification of cigarettes according to their elemental content determined by inductively coupled plasma optical emission spectrometry. Anal Sci 2007; 23:435-438. [Crossref][Googlescholar][Indexed]
- Kuper H, Adami HO, Boffetta P. Tobacco use, cancer causation and public health impact. J Intern Med 2002; 251:455-466. [Crossref][Googlescholar][Indexed]
- de Marini DM. Genotoxicity of tobacco smoke and tobacco smoke condensate: A review. Mutat Res 2004; 567:447-474. [Crossref][Googlescholar][Indexed]
- Stich HF, Rosin MP. Quantitating the synergistic effect of smoking and alcohol consumption with the micronucleus test on human buccal mucosa cells. Int J Cancer 1983; 31:305–308. [Crossref][Googlescholar][Indexed]
- Damayanti MM, Kharisma Y, Nur IM, et al. Micronucleus assay and oral hygiene index in smokers. In Medical Technology and Environmental Health CRC Press, 2020; 60-64. [Googlescholar]
- Zamora-Perez AL, Ortiz-GarcÃÂ?±a YM, Lazalde-Ramos BP, et al. Increased micronuclei and nuclear abnormalities in buccal mucosa and oxidative damage in saliva from patients with chronic and aggressive periodontal diseases. J Periodont Res 2015; 50:28–36. [Crossref][Googlescholar][Indexed]
- Chatterjee S, Dhar S, Sengupta B, et al. Cytogenetic monitoring in human oral cancers and other oral pathology: The micronucleus test in exfoliated buccal cells. Toxicol Mech Methods 2009; 19:427-433. [Crossref][Googlescholar][Indexed]
Author Info
Umelbaneen J Alalawy1*, Ahlam T Mohammed1 and Layla Sabri Yass2
1Department of Pedodontics and Preventive Dentistry, College of Dentistry, University of Baghdad, Baghdad, Iraq2Department of Oral Diagnosis, College of Dentistry, University of Baghdad, Baghdad, Iraq
Citation: Umelbaneen J Alalawy, Ahlam T Mohammed, Layla Sabri Yass, Micronucleus Assay among A group of Smokers in Relation to Oral Health Status, J Res Med Dent Sci, 2023, 11 (01):201-207
Received: 25-Oct-2022, Manuscript No. JRMDS-22-68367 ; , Pre QC No. JRMDS-22-68367 (PQ); Editor assigned: 28-Oct-2022, Pre QC No. JRMDS-22-68367 (PQ); Reviewed: 11-Nov-2022, QC No. JRMDS-22-68367 ; Revised: 27-Dec-2022, Manuscript No. JRMDS-22-68367 (R); Published: 28-Dec-2022