Research - (2021) Volume 9, Issue 7
Mechanical Evaluation of Fluor Apatite and Nano-Alumina Composite Coating on Commercially Pure Titanium Implants
Nagham B Kamil1, Sabreen W Ibrahim1* and Intisar Kadhum2
*Correspondence: Sabreen W Ibrahim, Department of prosthodontics, Collage of Dentistry, Mustansiriyah University, Iraq, Email:
Abstract
Dental implant treatment is widely used with long-term effective results and be taken into consideration “more predictable” than other treatments to replace missing teeth and to support a prosthesis. This study was aimed to assess the effect of screw-shaped commercially pure titanium coated with Fluorapatite and Nano-Alumina composite at the bone-implant contact by torque removal test in rabbit tibia. 80 screws were prepared from commercially pure titanium rods and were surgically implanted in 14 healthy New Zeeland rabbits. Screws were categorized into 4 groups, 1st group (20% flouroapitate-80% nano Al2O3), 2nd group (80% flouroapitate-20% nano Al2O3), 3rd group fluorapatite coating (FA) and 4th nano-Al2O3. The electrophoretic deposition process (EPD) was utilized for producing a homogenous coating film. Torque meter was used to determine the highest torque value required to unscrew the coated implants from tibia bone at different healing periods. The data obtained then analysed with IBM SPSS software (ver. 23, SPSS Inc., IL, USA) utilizing descriptive statistics and t-test for each time. When p-values <0.05, differences had been taken into consideration statistically significant. The results showed that the mean removal torque value for the 2nd group (80% flouroapitate-20% nano Al2O3) were significantly greater when compared with other groups after 2 and 6 weeks. With time, there was a significant increase in torque removal values. It was concluded that (80% flouroapitate-20% nano Al2O3) was more efficient through the rapid bone formation.
Keywords
Electrophoretic deposition, Fluorapatite, Nano-alumina, Torque, Implants
Introduction
Implants are considered as one of the successful options for Prosthodontic rehabilitation. Titanium and its alloys have excellent corrosion resistance, high strength, and light weight. The elastic modulus of titanium is nearer to the human bone than other metal materials like (stainless steel and CoCrMo alloys). Titanium and its alloys are bioinert material; so, do no longer effectively form chemical bonds with the bone. After long-term implantation, the implants generally tend to become detached from the tissue. Coating the implant with a bioactive material could improve the biological activities and mechanical properties of implants, such as TiO2, chitosan, and hydroxyapatite (HA, Ca10 (PO4) 6OH2) [1].
Even though the titanium has excellent biocompatibility and biomechanics, but it isn't bioactive, so, to beat the restricted bioactivity of titanium and to enhance the new bone development around these implants, research became focused on preparing coatings on titanium and its alloys. It has been well set up that the coated implants favour the bone response in comparison with the uncoated titanium [2,3].
Fluorapatite (FA) is biocompatible, has been used as an alternative biomedical material, it became recognized as the most thermally stable bioactive material [4]. Fluorapatite has additionally been taken into consideration as an attractive material for its comparability in structure and composition to the bone with the additional benefit of fluoride release at a controlled rate [5]
In recent years, the addition of fortifying materials (zirconia (ZrO2) and alumina (Al2O3)) to HA and FA coatings has attracted growing attention, they could decrease the thermal expansion coefficient mismatch between the coating and the substrate, and to enhance the biological activities and the mechanical properties [6,7].
Because of high wear resistance, good wear resistance, excellent biocompatibility, good corrosion resistance, and high strength, Alumina (α-Al2O3) is one of the extensively utilized biomaterials for orthopaedic applications.
Additionally, Al2O3 is thermally stable and chemically inert ceramic material [8,9].
Electrophoretic deposition EPD is a unique colloidal forming technique. It is considered a simple, low cost, a fairly rapid method for the deposition of coating materials, it based totally at the mechanism of electrophoresis which reasons the charged small particles to give in the colloidal suspension to deposit under the impact of an electrical area on opposite charge substrate to result in thin or thick coating layer depending on different factors [10].
This study aims to investigate the effect of nano-alumina and fluorapatite composite coating on commercially pure titanium compared to machined (uncoated) CpTi implants.
Materials and Methods
Rod-shaped commercially pure titanium grade II (supplied by Orotig Srl EU company Italy), with 6mm diameter, were machined using Lathe machine into 56 screw-shaped implants, diameter of these implants was 3.0 mm and 8mm in length(divided into smooth part (3 mm) in length and the threaded part (5 mm )with a distance between each thread was (0.6 mm)).
Before coating preparation, all screws were cleaned by acetone and alcohol. They were washed with distilled water, ultrasonic cleaning with ethanol was performed and then left to dry at 45°C.
For the preparation of the fluorapatite powder by a wetchemical method, NH4F Ca (NO3)2·4H2O and (NH4)2HPO4. Ca(NO3)2 solution is slowly introduced using a boiling solution containing 28% NH4OH; (NH4)2HPO4 solution was poured to the mixture and adjusting the pH at 9. The precipitate became aged with stirring at 80˚C for 1h, and then filtered, washed, dried at 70˚C for 12 h and sintering at 500˚C, with elimination undesirable synthesized powder [11].
α- Al2O3 nanoparticles (Sky Spring Nanomaterials, USA) particle size 40 nm were utilized in this study. Screw shaped implants were divided into 4 groups (the first group was coated with 20% fluorapatite and 80 %nano-alumina, the second group was coated with 80% fluorapatite and 20 %nano-alumina, the third group was coated with fluorapatite and fourth group coated with alumina)
Electrophoretic deposition (EPD) of first electrolyte solution was prepared by adding 20% fluorapatite with 80% nano-alumina to liter of ethanol with 3g Polyvinyl butyral (act as a binder) (PVB)), second electrolyte solution was prepared by adding 80% fluorapatite with 20% nano-alumina to liter of ethanol with 3g Polyvinyl butyral (PVB)), while third electrolyte solution was prepared by adding fluorapatite powder to ethanol (l00g/ 1 liter) in a container over a stirrer for 10 min. dispersant agent( phosphate ester (3.5g/l liter)) with 3g Polyvinyl butyral were added, and the fourth electrolyte solution was prepared by adding alumina powder to solvent (ethanol) (l00g/ 1 liter) in a container over a stirrer for 10 min. dispersant agent( phosphate ester (3.5g/l liter)) with 3g Polyvinyl butyral were added, after 10 min. The stirring continued until a colloidal suspension was obtained.
Selection of desirable parameters for electrophoretic deposition (EPD) process, different durations and different voltages for coating were used. The coating procedure was repeated with different applied voltage (10, 20, 30, 40, 50&60V) for 5 &10 min for coating a mixture of fluorapatite and nano- alumina and fluorapatite alone, while the specimens were coated with Al2O 3 for 5 min at 60V. Carbolite furnace (Carbolite Type MTF 12/38A.BAMFORD England) was used for Sintering of the coated samples; the treatment temperature is 850°C for coated samples. To avoid oxidation of the samples, the treatment is done with inert gas (argon).
Optical microscope ((Nikon Type 120, Japan) supplied with a digital camera type DXM 1200 F) was used to analyse the coated layer at different times and different applied voltages. The micrographs were analyzed through Nikon ACT- version 2.62, 2000 software.
The phase analysis of the powders was diagnosed by XRD (3121 powders X-ray Diffractometer using Cu Ka radiation), The2Өangles were ranged from 20 to 70° of one-degree increment. IBM SPSS software (version 23, SPSS Inc, IL, USA) was used to analyze the data obtained in this study by using descriptive statistics, Testing equality of mean values by T-test for each time with analysis of variance (one-way ANOVA) and LSD were used for comparing the differences between the groups. At p-values˂0.05, the differences were considered statistically significant.
Fourteen healthy New Zealand adult male rabbits were used weighing 2- 2.5 kg and about 11 months of age(this study was approved by the Animal Care and Use Committee at the College of Veterinary Medicine, University of Baghdad. Baghdad\Iraq. #1832 in 25 Feb. 2018). Generally, rabbits were categorized into 2groups depending on healing intervals (2&6 weeks), 7 rabbits for each healing period. Before the surgical operation, Rabbits were left for 2 weeks in the same environment with antibiotic cover by oxytetracycline intramuscular injection, which was given to prevent any infection.
The sterilized surgical instruments, gowns, surgical masks, and sterile gloves were used. Intramuscular injection of ketamine hydrochloride (1ml/kg Bodyweight) and xylocaine 2% (1ml/kg B.W) were used to anesthetized the rabbits.
Shaving by shaving spray and cleaning with chlorhexidine alcohol of both tibia were done before the operation. A longitudinal incision was done along the medial aspect of each rabbit tibia. Bone penetration was performed at a rotary speed of 1500 rpm with a round guide drill of 2.0mm in diameter under irrigation to avoid bone necrosis. The distance between the holes was 10 mm. The screw was inserted with a torque meter.
Implantation of 2 screws was done in the right tibia and 1 screw in the left tibia.
Absorbable catgut suture was used for muscle suturing and silk suture for skin suturing. The surgical site of each rabbit was sprayed with oxytetracycline spray (local antibiotics), and oxytetracycline 0.5ml/kg B.W. (longacting systemic antibiotics). Postoperative antibiotic care became executed by giving oxytetracycline antibiotics (local and systemic) for three days after the operation.
At the termination of each healing period (2&6 weeks), The rabbits have been anesthetized and an incision became made to expose the screws to measure the maximum torque values require to unscrew the implant from its site by torque-meter( STURTEVANT RICHMOND TORQUE PRODUCT, MODEL F 80-1-0. the USA).
Results
Figure 1 illustrates a set of micrographs for the microstructure of a.20% flouroapitate-80% nano Al2O3 at 60V for 3 min. b. 80% flouroapitate-20% nano Al2O3 3 at 60V for 3 min. c. fluorapatite coating at 30V for 5min. d. nano- Al2O3 at 60V for 5min.
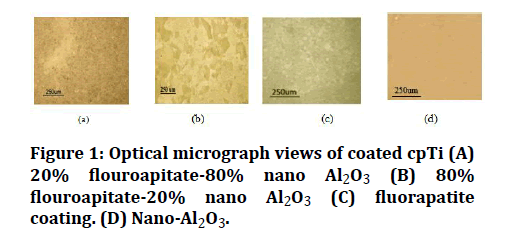
Figure 1: Optical micrograph views of coated cpTi (A) 20% flouroapitate-80% nano Al2O3 (B) 80% flouroapitate-20% nano Al2O3 (C) fluorapatite coating. (D) Nano-Al2O3.
The XRD pattern of coated specimens is offered in Figures 2 and 3. It was evident that the coated samples show a perfect match to ICDD file # 44.1294 for titanium, # 15-0876 for FA, and #43-1484 for Al2O3.
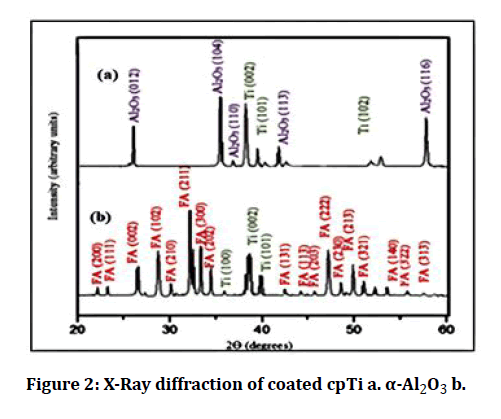
Figure 2: X-Ray diffraction of coated cpTi a. α-Al2O3 b.
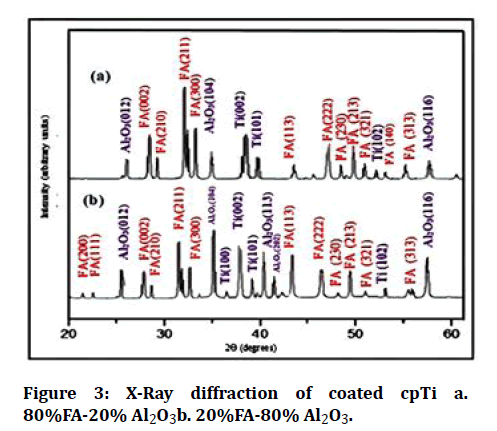
Figure 3: X-Ray diffraction of coated cpTi a. 80%FA-20% Al2O3b. 20%FA-80% Al2O3.
In this study, the removal torque test was used for evaluating the amount of force required to unscrew coated implants after a different period of healing. The torque means the value of the 2nd group (80%FA-20% Al2O3) was the highest for both 2 and 6 weeks intervals when compared with other groups (Table 1). The results of the paired sample t-test showed a highly significant difference at P ≤ 0.01 in the 2nd group (80%FA-20% Al2O3), as seen in Table 2.
Table 1: Descriptive statistical analysis for torque removal test after 2 & 6weeks interval.
| Time | Groups | No. | Min. | Max. | Mean | Std.Dev. |
|---|---|---|---|---|---|---|
| 2 weeks | 1st group (80% Al2O3+20%FA) | 7 | 14.12 | 28.18 | 20.05 | 4.9 |
| 2nd group (80% FA +20% Al2O3) | 7 | 19.56 | 33.24 | 25.7 | 4.7 | |
| 3rd group (100% FA) | 7 | 10 | 24.71 | 17.02 | 5.1 | |
| 4th group (100% Al2O3 ) | 7 | 12 | 21 | 15.7 | 4.07 | |
| 6weeks | 1st group (80% Al2O3+20%FA) | 7 | 17.71 | 38 | 28.7 | 7.6 |
| 2nd group (80% FA +20% Al2O3) | 7 | 24.8 | 40.7 | 30.4 | 5.2 | |
| 3rd group (100% FA) | 7 | 17.12 | 31.24 | 23.04 | 6.1 | |
| 4th group (100% Al2O3 ) | 7 | 12 | 31.24 | 20.7 | 7.3 |
Table 2: Paired sample t-test comparison for torque removal test of each group at 2 and 6 week intervals.
| Groups | Time | t | df | Sig.(2- tailed) |
|---|---|---|---|---|
| 1st group (80% Al2O3+20%FA) | 2 × 6 weeks | -2.8 | 6 | 0.03 |
| 2nd group (80% FA +20% Al2O3) | 2 × 6 weeks | -5.4 | 6 | 0.002 |
| 3rd group (100% FA) | 2 × 6 weeks | -2.1 | 6 | 0.07 |
| 4th group (100% Al2O3 ) | 2 × 6 weeks | 3.7 | 6 | 0.05 |
The ANOVA test showed a significant difference between the experimental groups for 2 weeks and a highly significant difference among the experimental groups for 6 weeks of healing intervals (Tables 3 and 4). Multiple comparison test (LSD) for 2 weeks healing interval showed that there was highly significant difference between the 1st group (20%FA-80% Al2O3) and the 2ndgroup(80%FA-20% Al2O3) and between the 1stgroup (20%FA-80% Al2O3) and the FA group, also a highly significant difference when comparing the 2nd group(80%FA-20% Al2O3) with Al2O3 group. A significant difference between the 1st group (20%FA-80% Al2O3) and Al2O3 group and between the 2nd group (80%FA-20% Al2O3) with the FA group, but it was non-significantly different between the Al2O3 group and FA group. The results for the 6 weeks healing interval showed a non-significant difference between the 1st group (20%FA-80% Al2O3) and the 2nd group(80%FA-20% Al2O3) and between the 1st group (20%FA-80% Al2O3) and the FA group, also a nonsignificant difference between the Al2O3 group and FA group. A significant difference between the 1st group (20%FA-80% Al2O3) and Al2O3 group and between the 2nd group (80%FA-20% Al2O3) with the FA group. While there was a highly significant difference between the 2nd group (80%FA-20% Al2O3) with the Al2O3 group (Table 5).
Table 3: ANOVA table for torque removal test after 2 week interval.
| Sum of squares | df | Mean square | f. | Sig. | |
|---|---|---|---|---|---|
| Between groups | 418.9 | 3 | 139.64 | 6.2 | 0.003 |
| Within groups | 540.93 | 24 | 22.5 | ||
| total | 959.87 | 27 |
Table 4: ANOVA table for torque removal test after 6 weeks interval.
| Sum of squares | df | Mean square | f. | Sig. | |
|---|---|---|---|---|---|
| Between groups | 441.5 | 3 | 147.18 | 3.3 | 0.037 |
| Within groups | 1067.9 | 24 | 44.49 | ||
| total | 1509.47 | 27 |
Table 5: Multiple comparison test (LSD Least Significant Differences) for torque removal test after 2&6 weeks interval.
| Groups | Mean difference | Sig. | |
|---|---|---|---|
| 1st group (80% Al2O3+20%FA) (2 weeks) | 2nd group (80% FA +20% Al2O3) (2weeks) | -13.7 | 0 |
| 3rd group (100% FA) (2weeks) | -8.05 | 0 | |
| 4th group (100% Al2O3 ) (2weeks) | -5.02 | 0.03 | |
| 2nd group (80% FA +20% Al2O3) (2weeks) | 3rd group (100% FA) (2weeks) | 5.7 | 0.02 |
| 4th group (100% Al2O3 ) (2weeks) | 8.7 | 0 | |
| 3rd group (100% FA) (2weeks) | 4th group (100% Al2O3 ) (2weeks) | 3.03 | 0.19 |
| 1st group (80% Al2O3+20%FA) (6 weeks) | 2nd group (80% FA +20% Al2O3) (6 weeks) | -1.7 | 0.6 |
| 3rd group (100% FA) (6 weeks) | 5.6 | 0.12 | |
| 4th group (100% Al2O3 ) (6 weeks) | 7.9 | 0.03 | |
| 2nd group (80% FA +20% Al2O3) (6 weeks) | 3rd group (100% FA) (6 weeks) | 7.3 | 0.05 |
| 4th group (100% Al2O3 ) (6 weeks) | 9.6 | 0.01 | |
| 3rd group (100% FA) (6 weeks) | 4th group (100% Al2O3 ) (6 weeks) | 2.3 | 0.5 |
Discussion
The optical microscopic examination for the coated samples confirmed uniform, homogenous, crack-free; this can suggest that there has been no shrinkage within the coating and the homogeneity of the coating film, confirming that the electrophoretic deposition method produces constant-thickness deposits on substrates, irrespective of the substrate shape. thermal expansion coefficient of Al2O3 (8.3×10 -6 /K) and while it mixed with FA, could result in decrease the thermal expansion coefficient difference among the coating film and the substrate (titanium), which may want to conquer problems resulting from firing shrinkage during sintering that’s may additionally result in the formation of cracks and enhances the interfacial bonding between coating layer and substrate, These results agreed with Ghiban et al in 2006 [12].
The X-ray diffraction pattern of coated samples showed that the surface of the sample is well coated because most of the diffraction peaks could belong to FA, Al2O3, and titanium, corresponding to JCPDS file # 44.1294, # 15-0876 and # 43-1484 respectively.
Permeation of X- rays behind the coated layers is the result of the existence of Ti peaks in the XRD pattern following the coating process.
In the X-ray diffraction pattern, the narrower peaks are representative of the coating layer consists of a high level of a crystalline form, this agrees with Kweh et al. [13].
The removal torque test was used as an indicator for the existence of osseointegration and considered as a useful parameter when studying and comparing screw-shaped implants [14].
One week after implant placement, all animals recovered well and demonstrated normal movement which indicates that they tolerated the implantation. An allergy defined as a hypersensitivity reaction. It is a disorder of the immune system, an over-reaction to something that is usually harmless, but triggers a reaction in anyone sensitive to the substance concerned, manifestations of hypersensitivity include chronic inflammation and pain which take 48 to 72 hours to develop. At sacrifice, no sign of infection, tissue reaction, or any other negative clinical observation was noted at implant-tissue contact in any of the animals. Regardless of the type of coating material and the duration of the implantation the results obtained showed that no inflammatory reaction had happened during the experimental periods, this is in agreement with the results of Mano et al. and Lins et al. [15,16].
The result of this study reveal increase in retention of the coated screws in the bone for all groups at different periods of healing as a result of a new bone formation around the implant during healing period, which generally improves the osseointegration, agreed with the study of Hammad, et al. and Al-Mudarris, et al. [14,17] they stated that there is an increase in the removal torque with time, after 2 weeks of implantation, all groups reveals a less torque mean value after 2 weeks, and high torque mean values after 6 weeks of the healing period. The maturation of woven bone to lamellar bone results in an increase in torque mean values [18].
After 2 weeks, implants coated with (80%FA-20%Al2O3) recorded a highly significant increase in removal torque value than other groups, this indicated increased bond strength at the bone-implant interface which may be due to the effect of FA on bone formation. FA has the capacity to the liberation fluoride ions which may be incorporated in a bone lattice and could increase the proliferation and differentiation of osteoprogenitor cells. Because of its affinity for calcium, fluoride can accumulate in bone and replace the hydroxyl ions within the hydroxyapatite crystals, so the structure becomes more stable, more bioactive and less soluble [19].
Enhanced osteoblast numbers within the bone at a faster rate, and inhibit the maturation of osteoclasts and suppress phagocyte activity so improving earlier implant osseointegration [20]. Liu et al. observed a suitable cellular response in osteoblast-like cells on FA coatings in vitro and demonstrated improved and rapid mineralized tissue formation integrated with FA coatings in vivo [21].
Al2O3 coating of the cpTi surface (20-100 nm) can improve the mesenchymal stem cell differentiation into osteoblast [22].
Pure alumina has been widely used as femoral heads because of its high wear resistance, due to high inertness of alumina but no application requiring osseointegration has been implemented to date [23]. EPD process of mixing of bioactive (FA) and bioinert (alumina) ceramic materials increased the activity of the coated layer which could enhance bone maturation in bone-implant contact, this finding was in agreement with the finding of Juna et al, 2003 [24].
Conclusion
After one week of implantation, all rabbits can normally tolerate the coating materials that exhibited by normal movement with the absence of any infection. At both implantation periods, Higher torque removal mean values for the 2nd group (80% FA-20% Al2O3) composite coating compared to other groups of coated implants and these values increased with time for all coating groups.
References
- Orlovskii VP, Komlev VS, Barinov SM. Hydroxyapatite and hydroxyapatite-based ceramics. Inorg Mater 2002; 38:973-984.
- Yu-Liang C, Lew D, Park JB, et al. Biomechanical and morphometric analysis of hydroxyapatite-coated implants with varying crystallinity. J Oral Maxillofac Surg 1999; 57:1096-1108.
- Wheeler SL. Eight-year clinical retrospective study of titanium plasma-sprayed and hydroxyapatite-coated cylinder implants. Int J Oral Maxillofac Implants 1996; 11.
- Barinov SM, Shvorneva LI, Ferro D, et al. Solid solution formation at the sintering of hydroxyapatite–fluorapatite ceramics. Sci Technol Adv Mater 2004; 5:537-541.
- Larsen MJ, Thorsen A. A comparison of some effects of fluoride on apatite formationin vitro andin vivo. Calcif Tissue Int 1984; 36:690.
- Viswanath B, Ravishankar N. Interfacial reactions in hydroxyapatite/alumina nanocomposites. Scr Mater. 2006; 55:863-866.
- Li J, Fartash B, Hermansson L. Hydroxyapatite—alumina composites and bone-bonding. Biomaterials 1995; 16:417-422.
- Yoon BH, Kim HW, Lee SH, et al. Stability and cellular responses to fluorapatite–collagen composites. Biomaterials 2005; 26:2957-2963.
- Guidara A, Chaari K, Bouaziz J. Elaboration and characterization of alumina-fluorapatite composites. J Biomater Nanobiotechnol 2011; 2:103.
- Boccaccini AR, Zhitomirsky I. Application of electrophoretic and electrolytic deposition techniques in ceramics processing. Curr Opin Solid State Mater Sci 2002; 6:251-260.
- Ghorbel HF, Guidara A, Danlos Y, et al. Synthesis and characterization of alumina-fluorapatite coatings deposited by atmospheric plasma spraying. Mater Lett 2016; 185:268-271.
- Ghorbel H, Guidara A, Guidara R, et al. Assessment of the addition of fluorapatite–alumina coating for a durable adhesion of the interface prosthesis/bone cells: Implementation in vivo. J Med Biol Eng 2019; 1-11.
- Ayed F Ben, Bouaziz J, Bouzouita K. Calcination and sintering of fluorapatite under argon atmosphere. J Alloys Compd. 2001; 322:238-245.
- Ghiban B, Jicmon G, Cosmeleata G. Structural Investigation of Electrodeposited Hydroxyapatite on titanium supports. Rom J Phys 2006; 51:187.
- Chow LC, Sun L, Hockey B. Properties of nanostructured hydroxyapatite prepared by a spray drying technique. J Res Natl Inst Stand Technol 2004; 109:543.
- Sennerby L, Meredith N. Implant stability measurements using resonance frequency analysis: biological and biomechanical aspects and clinical implications. Periodontol 2008; 47:51-66.
- Whitford GM. Fluoride metabolism and excretion in children. J Public Health Dent. 1999; 59:224-228.
- Hammad TI, Al-Ameer SS, Al-Zubaydi T. Histological and mechanical evaluation of electrophoretic bioceramic deposition on Ti 6AL-7Nb dental implants. A PhD thesis, Coll Dent Univ Baghdad 2007.
- Al-Mudarris BA, Salem SA, Al-Zubaydi TL. The significance of biomimetic calcium phosphate coating on commercially pure titanium and Ti-6Al-7Nb alloy. A PhD thesis, Coll Dent Univ Baghdad 2006.
- Alhilou A, Do T, Mizban L, et al. Physicochemical and antibacterial characterization of a novel fluorapatite coating. ACS Omega 2016; 1:264-276.
- Liu J, Jin T, Chang S, et al. The effect of novel fluorapatite surfaces on osteoblast-like cell adhesion, growth, and mineralization. Tissue Eng 2010; 16:2977-2986.
- Mendonca G, Baccelli Silveira Mendonça D, Gustavo Pagotto Simões L, et al. Nanostructured alumina-coated implant surface: Effect on osteoblast-related gene expression and bone-to-implant contact in vivo. Int J Oral Maxillofac Implants 2009; 24.
- Schierano G, Mussano F, Faga MG, et al. An alumina toughened zirconia composite for dental implant application: In vivo animal results. Biomed Res Int 2015; 2015.
- Jun YK, Kim WH, Kweon OK, et al. The fabrication and biochemical evaluation of alumina reinforced calcium phosphate porous implants. Biomaterials 2003; 24:3731-3739.
Author Info
Nagham B Kamil1, Sabreen W Ibrahim1* and Intisar Kadhum2
1Department of prosthodontics, Collage of Dentistry, Mustansiriyah University, Iraq2Department of Prosthodontics, College of Dentistry, Uruk Private University, Iraq
Citation: Nagham B Kamil, Sabreen W Ibrahim, Intisar Kadhum, Mechanical Evaluation of Fluor Apatite and Nano-Alumina Composite Coating on Commercially Pure Titanium Implants, J Res Med Dent Sci, 2021, 9(7): 257-262
Received: 08-Jun-2021 Accepted: 14-Jul-2021
