Research - (2021) Volume 9, Issue 11
Isolation of Cellulose Producing Marine Streptomyces Sp. from Sediment Samples and their Antioxidants Properties
Arunn Jaikumarr Ram, Sivaperumal Pitchiah*, Anitha Roy and Lakshmi Thangavelu
*Correspondence: Sivaperumal Pitchiah, Department of Pharmacology, Saveetha Institute of Medical and Technical Sciences, Saveetha University, India, Email:
Abstract
Cellulase generated by cellulolytic bacteria helps to degrade the cellulose. This enzyme is considered a major industrial enzyme group and has several industrial applications. The bioconversion of renewable cellulose biomass to commodity chemicals is a potential challenge area where cellulase plays a central role. Marine Streptomyces species are drawing more and more attention as a promising source of new natural products. Oxidation causes Endothelial stress which is ultimately involved in endothelial dysfunction, which is obvious in adults with different cardiovascular conditions, including thalassemia. The sediment sample was collected from the Thondi area, Tamilnadu. The collected sample was sun dried for 48 hrs. and turned into fine powder by mortar and pestle. The actinobacteria was isolated and identified the marine actinobacteria with the help of aerial mass colour, melanoid pigments, reverse side pigments, soluble pigments, and spore chain morphology. Chemotaxonomic characteristics and scanning of cellulase production were done and showed potential antioxidant properties. The effect of pH and temperature on cellulase enzyme production were analysed and their potential antioxidant properties also done.
Keywords
Marine Streptomyces, Enzyme, Cellulase, Antioxidant activity
Introduction
The most important agricultural waste in the world is cellulose and is an abundant natural biopolymer. This cellulosic biomass is a rich, renewable resource with a high bioconversion potential to value-added products [1]. Cellulase generated by cellulolytic bacteria helps to degrade the cellulose. This enzyme is considered a major industrial enzyme group and has several industrial applications [2]. Moreover, in the textile industry, the most important industrial application of cellulases is biopolishing textiles and stoned denims as well as laundry detergents for home laundry in order to improve fabric softness and shine [3]. In addition, they are used in animal feed for improved nutritional quality and digestibility, fruit juice processing as well as in the baking process [4]. The bioconversion of renewable cellulose biomass to commodity chemicals is a potential challenge area where cellulase plays a central role [5].
Marine Streptomyces species are drawing more and more attention as a promising source of new natural products [6]. Actinomycetes bacteria of genus Streptomyces are one of the most promising biological sources for new natural products and will continue, at least for the near future [7]. The intensive investigation of terrestrial actinomycetes in 1950-1970 did lead to the frequent reconstruction of bioactive compounds, attracting attention to new, ecological niches that could have been sources of new actinomycetes [8].
As the sea accounts for more than 70 percent of the surface of the world and hosts about 87 percent of the world's biodiversity, the findings of new microorganisms, including actinomycetes, appear largely undeveloped. Given the never seen diversity of marine organisms and the relatively little work done to date, more than 25000 new marine natural products have now been identified as astounding [9]. Many such compounds come from deep sea sediments, coral reefs, aquatic invertebrates and plants isolated from marine actinomycetes [10,11].
Various marine actinomycetes compounds have a significant potential to become pharmaceutical drugs. Diazepinomicin, a marine micromonospora dibenzodiazepine alkaloid, has had antibacterial and antitumor activities, and had glioblastoma treatment clinical studies in Phase II [12,13].
Some important papers which were done by the researchers helped in this study. Some of them are regarding the antioxidant [14,15], anti-inflammatory [16,17] and antidiabetic properties [18-20]. Papers about anticancer were also [21,22] helpful.
As toxic and beneficial compounds, free radicals and oxidants can play a dual role, since they can be damaging or helpful for the body. The development of many human diseases has been involved. Among them, arthritis, inflammatory diseases, kidney diseases, cataracts, inflammatory bowel disease, colitis, lung dysfunction; pancreatitis are the most common [23]. Oxidation causes endothelial stress which is ultimately involved in endothelial dysfunction, which is obvious in adults with different cardiovascular conditions, including thalassemia [24]. Red blood cells are protected against oxidant damage by antioxidants and other supportive treatments [25]. Further, our team has extensive knowledge and research experience that has translated into high quality publications [26-46]. This study aims at isolation of cellulase producing marine Streptomyces sp. from sediment samples and their antioxidant properties.
Material and Method
Sample collection and preparation
The sediment sample was collected from the Thondi area, Tamilnadu. The collected sample was sun dried for 48 hrs. and turned into fine powder by mortar and pestle.
Isolation of actinobacteria
Aerial mass colour:
The colour of the mature sporulating aerial mycelium was recorded in naked eye. When the aerial mass colour fell between two colours series, both the colours were recorded. If the aerial mass colour of a strain to be studied showed intermediate tints, then also, both the colour series were noted. The media used were Yeast Extract-Malt Extract Agar and Inorganic-Salt Starch Agar.
Melanoid pigments:
The grouping was made on the production of melanoid pigments (i.e. Greenish brown, brownish black or distinct brown, pigment modified by other colours) on the medium. The strains were grouped as melanoid pigment produced (+) and not produced (-). In a few cases, the production of melanoid pigments was delayed or weak, and therefore, it was not distinguishable. This is indicated as variable (V). This test was carried out on the media ISP-1 and ISP-7 (Appendix I), as recommended by the International Streptomyces Project (Shirling and Gottlieb, 1966).
Reverse side pigments:
Reverse side pigment production of the isolate was determined on ISP7 medium. The pigment production was noted as distinctive (+) and not distinctive or none (-). In case, a colour with low Chroma such as pale yellow, olive or yellowish brown occured, it was included in the latter group (-).
Soluble pigments:
Soluble pigment production of isolate was observed on ISP7 medium. The diffusible pigment production other than melanin was considered positive (+) and not produced (-). The colour was recorded (red, orange, green, yellow, blue and violet).
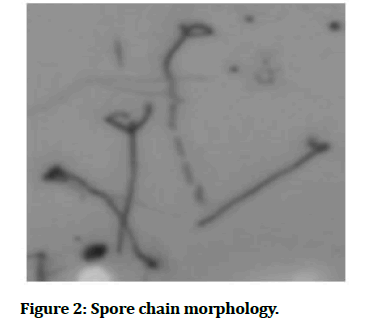
Spore chain morphology:
Spore morphological characters of the strains were studied by inoculating a loopful of one week old cultures into solidified agar medium contained sterile glass slide. The cultures were incubated at 28 ± 20oC and examined periodically for the formation of aerial mycelium, sporophore structure and spore morphology.
Chemo taxonomical characteristics
Hydrolysis
Hydrolysis was done for releasing amino acids. Harvested cells of each strain weighing 20 mg (fresh) were placed in an ampo bottle and 1 ml of 6 N HCl was added and sealed with an alcohol blast burner. The samples were kept at 1210 C for 20 h in a sand bath. The bottles were cooled by keeping them at a room temperature of 28+20C. Hydrolysis was also done for releasing sugars. Harvested cells of each strain weighing 50 mg (fresh) were placed in an ampo bottle and 1 ml of 0.5N HCl was added and sealed with alcohol blast burner. The samples were kept at 1100oC for 2h. The bottles were then cooled by keeping them at a room temperature of 28±20°C.
Thin Layer Chromatography (TLC)
Spotting of the whole cell hydro lysates was made carefully on TLC plate using a microliter pipette. Spots were 5-10 mm in diameter. This was done by multiple applications on the same spot of very small portions of the sample, which were dried by a hand dryer.
Amino acids
Each sample (3 μl) was applied on the baselines of the TLC plate (20 cm x 20 cm). Adjacent to this, 1μl of DLdiaminopimelic acid (an authentic material mixture of DAP isomers) and 1μl of amino acetic acid (glycine) were spotted as standards. TLC plate was developed with the solvent system containing methanol: pyridine: glacial acetic acid: H2O (5: 0.5: 0.125: 2.5 v/v). It took approximately more than 4 h for development. The spots were visualized by spraying with 0.4% ninhydrin solution in water-saturated n-butanol, followed by heating at 1000 C for 5 min. Spots of amino acids ran faster than DAP. The sample spots were immediately compared with the spots of the standards since spots gradually disappeared in a few hours.
Whole-Cell sugars
On a cellulose TLC plate (20 cm x 20 cm), 5μl of samples was spotted along with 3 μl of sugar solutions as standards on the same plates. Galactose, arabinose, xylose and madurose were the sugars, which were used as standards. TLC plate was developed with the solvent mixture containing ethyl acetate: pyridine: acetic acid: distilled water (8: 5: 1: 1.5 v/v). The developing time was more than 4 h. Spots were visualized by spraying with aniline phthalate reagent (3.25 g of phthalic acid dissolved in 2 ml of aniline and made upto 100 ml with water saturated n-butanol). The sprayed plate was heated at 1000 C for 4 min. Hexoses appeared as yellowish brown spots and pentoses, as maroon coloured spots.
Assimilation of carbon source
The ability of the actinobacterial strain in utilizing various carbon compounds as source of energy was studied, following the method recommended by International Streptomyces Project (Shirling and Gottlieb, 1966). Chemically pure carbon source certified to be free of admixture with other carbohydrates and contaminating materials were used for this purpose. Carbon sources for this test were Arabinose, Xylose, Inositol, Mannitol, Fructose, Rhamnose, Sucrose and Raffinose. These carbon sources were sterilized by ether sterilization without heating. The media and plates were prepared and inoculated according to the convention of ISP project (Shirling and Gottlieb, 1966). For each of the carbon sources, utilization is expressed as positive (+), negative (-), or doubtful ( ± ). In the 'doubtful ' strains, only a trace of growth slightly greater than that of the control was noticed.
Screening of Cellulase Production
Cellulase activity of the strains was screened qualitatively in CMC (Carboxymethyl Cellulose) agar medium. After inoculation, the plates were incubated at 37oC for 5 days. To visualize the hydrolysis zone, the plates were flooded with an aqueous solution of 0.1% Congo red for 15 min and washed with 1 M NaCl. To indicate the cellulase activity of the organisms, diameters of clear zones around colonies on CMC agar were measured.
Determination of enzyme activity
The medium was inoculated with 1 ml of spore suspension of a 7 days old culture and incubated in rotary shaker (150rpm) at ambient temperature for three days. The cell free supernatant was collected by centrifugation at 12,000rpm for 15 min. The supernatant was the enzyme source. The substrate 2% of carboxymethyl cellulose solution (CMC) was prepared with a 50mM phosphate buffer (pH 7). 1ml of crude enzyme was added with 1ml of CMC solution which incubated for 60min at (50ºC) desired temperature. After incubation 2ml of 3, 5-dinitrosalicylic (DNS) reagent was added to terminate the enzyme reaction. After termination of enzyme activity all samples should be incubated for 5min at boiling temperature then the samples transferred to an ice cold water bath. After pulp settlement, the aqueous layer was e by centrifuge (12000 rpm/min for 5min at 4ºC) and the optical density was measured at 540nm. One International Unit (IU) of enzyme activity for cellulase was defined as the amount of enzyme releasing 1 μmol reducing sugar from CMC per minute using glucose as standard.
Effect of pH on enzyme production
The isolates were inoculated in CMC broth and the medium pH was adjusted from 6 – 8. The confirmation of growth was observed at 600nm. The enzyme production was quantified by the method described before.
Effect of Temperature on enzyme production
Effect of temperature on the cellulase enzyme production was analyzed by adjusting from 25, 30, 35, 40 and 50ºC. The isolate was inoculated in CMC broth and incubated at various ranges of temperature. The cell growth was confirmed by the absorbance at 600nm. The enzyme production was quantified by the method described before.
Results and Discussion
The rise of widespread antibiotic- resistant bacteria heightened the need to discover new antimicrobial agents. Actinomycetes, especially Streptomyces sp., have attracted a lot of attention because they produce a lot of useful bioactive metabolites. Isolating these species from less -explored environments may improve the chances of discovering new microbial species. Isolation of cellulase producing Streptomyces from marine sediments sample and effect of physical factors on enzyme production (Tables 1-5). This study isolated marine actinobacteria from a sediment sample and identified Streptomyces genus from the isolate using specific characteristics of the bacteria. Then the production of enzyme was confirmed by screening for cellulase and enzyme assay was carried out. The enzyme assay revealed that the Carboxymethyl cellulase agar flooded with iodine, the total activity of the enzyme was 127.41 IU/mg. The effect of temperature and pH enzyme production was studied on CMC broth. It was found that the optimum pH and temperature for maximum enzymatic activity was and respectively. It was also observed that as the temperature increased, the enzymatic activity increased. However at the highest temperature, the rate decreased again which might probably be due to enzyme degeneration. Finally, antioxidant testing was performed which showed positive results (Figures 1-2).
| Color of aerial mycelium | White |
|---|---|
| Melanoid pigment | - |
| Reverse side pigment | + |
| Soluble pigment | - |
| Spore chain | RA |
| Assimilation of carbon source | |
| Arabinose | + |
| Xylose | + |
| Inositol | + |
| Mannitol | + |
| Fructose | + |
| Rhamnose | + |
| Sucrose | + |
| Raffinose | + |
Table 1: Conventional Identification of marine Streptomyces.
| Cell wall amino acids | Cell wall sugar | Cell wall type | Index | |||
|---|---|---|---|---|---|---|
| LL-DAP | Meso DAP | Glycine | Arabinose | Galactose | I | Streptomyces |
Table 2: Chemo taxonomical characteristics of marine Streptomyces sp.
| Effect of Temperature | IU/ml | Effect of pH | IU/ml |
|---|---|---|---|
| 25 | 11.79 ± 2.3 | 6 | 5.29 ± 2.2 |
| 30 | 13.52 ± 2.7 | 6.5 | 7.24 ± 2.5 |
| 35 | 16.47 ± 2.5 | 7 | 9.51 ± 2.1 |
| 40 | 12.18 ± 2.9 | 7.5 | 12.62 ± 2.9 |
| 50 | 10.25 ± 2.4 | 8 | 13.58 ± 2.3 |
Table 3: Depicts the effect of pH and effect of temperature.
| Concentration (µg/ml) | DPPH Scavenging | Standard |
|---|---|---|
| 25 | 11.07 ± 1.5 | 37.3 ± 1.27 |
| 50 | 18.24 ± 1.9 | 62.7 ± 1.31 |
| 75 | 31.35 ± 1.4 | 78.52 ± 1.28 |
| 100 | 42.18 ± 1.8 | 83.59 ± 0.78 |
| 125 | 55.46 ± 1.4 | 92.4 ± 1.26 |
| 150 | 68.09 ± 1.7 | 98.6 ± 1.24 |
Table 4: Depicts the DPPH scavenging at different concentrations.
| μg/ml | Nitrous oxide scavenging | Std |
|---|---|---|
| 25 | 12.37 ± 1.4 | 30.38 ± 1.127 |
| 50 | 25.64 ± 1.8 | 51.92 ± 1.164 |
| 75 | 32.91 ± 1.4 | 70.34 ± 1.152 |
| 100 | 47.64 ± 1.9 | 82.17 ± 1.231 |
| 125 | 58.27 ± 1.7 | 90.53 ± 1.204 |
| 150 | 73.59 ± 1.4 | 95.68 ± 1.168 |
Table 5: Depicts the Nitrous oxide scavenging at different concentrations.
Figure 1:Streptomyces sp.
Figure 2:Spore chain morphology.
Conclusion
The present study was isolated and identified the marine actinobacteria of Streptomyces sp. From marine sediment samples. The conventional identification of chemotaxonomic characteristics was done and further scanning of cellulase production was also done. The marine actinobacterial cellulase showed potential antioxidant properties. The effect of pH and temperature on cellulase enzyme production was also analysed. The outcome of the present work concluded that marine actinobacterial enzymes can act as natural products and it could be useful in the biomedical sector.
Acknowledgement
This research was supported by the Department of research of Saveetha Dental College. We thank our colleagues who provided insight and expertise that greatly assisted the research.
Conflict of Interest
There are no conflicts of interest.
Source of Interest
Self.
Ethical Clearance
Not Required.
References
- Dhaliwal M, More S. Optimization of cellulase production by soil bacteria using statistical design.Int J Biol Chem Sci 2016; 1–7.
- Sadhu S, Maiti TK. Cellulase production by bacteria: A review. Microbiol Res J Int 2013; 13:235-58.
- Hill J, Nelson E, Tilman D, et al. Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc Natl Acad Sci 2006; 103:11206-11210.
- Lynd LR, Van Zyl WH, McBride JE, et al. Consolidated bioprocessing of cellulosic biomass: an update. Curr Opinion Biotechnol 2005; 16:577-83.
- Lynd LR, Currie D, Ciazza N, et al. Consolidated bioprocessing of cellulosic biomass to ethanol using thermophilic bacteria. Bioenergy 2008; 55-74.
- Paulus C, Rebets Y, Tokovenko B, et al. New natural products identified by combined genomics-metabolomics profiling of marine Streptomyces sp. MP131-18. Scientific Reports 2017; 7:1-1.
- Solecka J, Zajko J, Postek M, et al. Biologically active secondary metabolites from Actinomycetes. Open Life Sci 2012; 7:373-90.
- Monciardini P, Iorio M, Maffioli S, et al. Discovering new bioactive molecules from microbial sources. Microbiol. Biotechnol 2014; 7:209-220.
- Hu GP, Yuan J, Sun L, et al. Statistical research on marine natural products based on data obtained between 1985 and 2008. Marine Drugs 2011; 9:514-25.
- Valli S, Suvathi SS, Aysha OS, et al. Antimicrobial potential of Actinomycetes species isolated from marine environment. Asian Pacific J Tropical Biomed 2012; 2:469-473.
- Valliappan K, Sun W, Li Z. Marine actinobacteria associated with marine organisms and their potentials in producing pharmaceutical natural products. Appl Microbiol Biotechnol 2014; 98:7365-77.
- Charan RD, Schlingmann G, Janso J, et al. Diazepinomicin, a new antimicrobial alkaloid from a marine Micromonospora sp. J Nat Prod 2004; 67:1431-3.
- Mason WP, Belanger K, Nicholas G, et al. A phase II study of the Ras-MAPK signaling pathway inhibitor TLN-4601 in patients with glioblastoma at first progression. J Neurooncol 2012; 107:343-349.
- Meenapriya M, Anitha R, Lakshmi T. Effect of lutein on cytochrome P450 (Isoform CYP3A4)-An in vitro Study. Pharmacogn J 2018; 10.
- Devaraj E, Roy A, Veeraragavan GR, et al. ß-Sitosterol attenuates carbon tetrachloride–induced oxidative stress and chronic liver injury in rats. Naunyn Schmiedebergs Arch Pharmacol 2020; 1-9.
- Prathoshni SM, Anitha R, Lakshmi T. The effect of Capsicum oleoresin on nitric oxide production and nitric oxide synthase gene expression in macrophage cell line. Pharmacogn Res 2018; 10.
- Cinthura C, Thangavelu L, Rajeshkumar S, et al. COX2 Inhibitory activity of Abutilon indicum--An Invitro Study. Indian J Public Health Res Develop 2019; 10.
- Ashwini S, Anitha R. Antihyperglycemic activity of Caralluma fimbriata: An In vitro approach. Pharmacogn Mag 2017; 13:S499.
- Leya MM, Anitha R. Anti-inflammatory effect of the aqueous fruit pulp extract of tamarindus indica linn in lipopolysaccharide-stimulated macrophages. Pharmacogn J 2019; 11.
- Roy A, Rajagopal P, Thangavelu L. Molecular docking analysis of compounds from Lycopersicon esculentum with the insulin receptor to combat type 2 diabetes. Bioinformation 2020; 16:748-752.
- Ashwini S, Ezhilarasan D, Anitha R. Cytotoxic effect of Caralluma fimbriata against human colon cancer cells. Pharmacogn. J 2017; 9.
- Roy A, Rasheed A, Sleeba AV, et al. Molecular docking analysis of capsaicin with apoptotic proteins. Bioinformation 2020; 16:555.
- Bendich A. Role of antioxidants in the maintenance of immune functions. Natural antioxidants in human health and disease. 1994; 447-67.
- Hebbel RP, Leung A, Mohandas N. Oxidation-induced changes in microrheologic properties of the red blood cell membrane. Blood 1990; 76:1015–1020.
- Shinar E, Rachmilewitz EA. Oxidative denaturation of red blood cells in thalassemia’, Seminars in hematology 1990; 27:70–82.
- Rajeshkumar S, Kumar SV, Ramaiah A, et al. Biosynthesis of zinc oxide nanoparticles usingMangifera indica leaves and evaluation of their antioxidant and cytotoxic properties in lung cancer (A549) cells. Enzyme Microb Technol 2018; 117:91-95.
- Nandhini NT, Rajeshkumar S, Mythili S. ‘The possible mechanism of eco-friendly synthesized nanoparticles on hazardous dyes degradation’. Biocatal. Agric. Biotechnol 2019; 19:101138.
- Rajkumar PV, Prakasam A, Rajeshkumar S, et al. Green synthesis of silver nanoparticles using Gymnema sylvestre leaf extract and evaluation of its antibacterial activity. S Afr J Chem Eng 2020; 32:1-4.
- Rajasekaran S, Damodharan D, Gopal K, et al. Collective influence of 1-decanol addition, injection pressure and EGR on diesel engine characteristics fueled with diesel/LDPE oil blends. Fuel 2020; 277:118166.
- Vairavel M, Devaraj E, Shanmugam R. An eco-friendly synthesis of Enterococcus sp.–mediated gold nanoparticle induces cytotoxicity in human colorectal cancer cells. Environ Sci Pollut Res 2020; 27:8166-75.
- Santhoshkumar J, Sowmya B, Kumar SV, et al. Toxicology evaluation and antidermatophytic activity of silver nanoparticles synthesized using leaf extract of Passiflora caerulea S. Afr J Chem Eng 2019; 29:17-23.
- Raj RK. ß-Sitosterol-assisted silver nanoparticles activates Nrf2 and triggers mitochondrial apoptosis via oxidative stress in human hepatocellular cancer cell line. J Biomed Mater Res 2020; 108:1899-908.
- Saravanan M, Arokiyaraj S, Lakshmi T, et al. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb Pathog 2018; 117:68-72.
- Gheena S, Ezhilarasan D. Syringic acid triggers reactive oxygen species–mediated cytotoxicity in HepG2 cells. Hum Exp Toxicol 2019; 38:694-702.
- Ezhilarasan D, Sokal E, Najimi M. Hepatic fibrosis: It is time to go with hepatic stellate cell-specific therapeutic targets. Hepatobiliary Pancreat Dis Int 2018; 17:192-197.
- Ezhilarasan D. Oxidative stress is bane in chronic liver diseases: Clinical and experimental perspective. Arab J Gastroenterol 2018; 19:56-64.
- Dua K, Wadhwa R, Singhvi G, et al. The potential of siRNA based drug delivery in respiratory disorders: Recent advances and progress. Drug Dev Res 2019; 80:714-30.
- Gomathi AC.Anticancer activity of silver nanoparticles synthesized using aqueous fruit shell extract of Tamarindus indica on MCF-7 human breast cancer cell line. J Drug Deliv Sci Technol 2020; 55:101376.
- Vairavel M, Devaraj E, Shanmugam R. An eco-friendly synthesis of Enterococcus sp.–mediated gold nanoparticle induces cytotoxicity in human colorectal cancer cells. Environ Sci Pollut Res 2020; 27:8166–8175.
- Ramesh A, Varghese S, Jayakumar ND, et al. Comparative estimation of sulfiredoxin levels between chronic periodontitis and healthy patients–A case-control study. J Periodontol 2018; 89:1241-1248.
- Duraisamy R, Krishnan CS, Ramasubramanian H, et al. Compatibility of nonoriginal abutments with implants: Evaluation of Microgap at the implant–abutment interface, With Original Nonoriginal Abutments Implant Dent. 2019; 28:289-95.
- Ezhilarasan D, Apoorva VS, Ashok Vardhan N. Syzygium cumini extract induced reactive oxygen species-mediated apoptosis in human oral squamous carcinoma cells. J Oral Pathol Med 2019; 48:115-21.
- Arumugam P, George R, Jayaseelan VP. Aberrations of m6A regulators are associated with tumorigenesis and metastasis in head and neck squamous cell carcinoma. Arch Oral Biol 2021; 122:105030.
- Joseph B, Prasanth CS. Is photodynamic therapy a viable antiviral weapon against COVID-19 in dentistry?. Oral Surg Oral Med Oral Pathol Oral Radiol 2021.
- Gnanavel V, Roopan SM, Rajeshkumar S. Aquaculture: An overview of chemical ecology of seaweeds (food species) in natural products. Aquaculture. 2019;507:1-6.
- Markov A, Thangavelu L, Aravindhan S et al. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res Ther 2021; 12:1-30.
Author Info
Arunn Jaikumarr Ram, Sivaperumal Pitchiah*, Anitha Roy and Lakshmi Thangavelu
Department of Pharmacology, Saveetha Institute of Medical and Technical Sciences, Saveetha University, IndiaCitation: Arunn Jaikumarr Ram, Sivaperumal Pitchiah, Anitha Roy, Lakshmi Thangavelu, Isolation of Cellulose Producing Marine Streptomyces Sp. from Sediment Samples and their Antioxidants Properties , J Res Med Dent Sci, 2021, 9(11): 223-229
Received: 09-Sep-2021 Accepted: 08-Nov-2021