Research - (2022) Volume 10, Issue 1
In-vitro Anticancer Activity of Cardiospermum Halicacabum against Human A549 Lung Cancer Cell
Harita Ravikumar1, S Raghunandhakumar2*, D Ezhilarasan2 and Lakshmi T2
*Correspondence: S Raghunandhakumar, Department of Pharmacology, Saveetha Dental College and Hospitals, Saveetha Institutes of Medical and Technical Sciences (SIMATS), Saveetha University, India, Email:
Abstract
Introduction: Lung cancer is the major type of cancer that is developing all over the world. Approximately 1.8million new people were affected by lung cancer in 2012 among which 1.6million people were fatal. Cardiospermum halicacabum is also known as balloon vine, climber plant. It belongs to order sapindaceae and it has a high medicinal value. AIM: To assess the anticancer activity of Cardiospermum halicacabum against human A549 lung cancer cell line. Materials and method: The anticancer effect of cardiospermum halicacabum on A549 was measured by MTT assay and the cell morphological evaluation done by inverted phase contrast microscopy. Results and Discussion: From the MTT assay plant extract of Cardiospermum halicacabum showed approximately 50% cell inhibition at 30µl/ mg concentration after 24hrs of treatment. Further it was confirmed with morphological evaluation using phase contrast microscopy. Overall, the MTT result clearly shows that the C.halicacabum has significantly reduced the cell viability in dose dependent manner effect in cell proliferation. Conclusion: From the results, the extracts were cytotoxic to the lung cancer cells at 30µl/ mg concentration and taken as (IC50,µl/ mg) value for further experiments. However more research is needed to understand the mechanisms of cytotoxicity of the plants.
Keywords
Cardiospermum halicacabum, Lung cancer, Anticancer activity, Innovative techniques, Innovative technology
Introduction
Lung cancer is the leading cause of death in the 20th century. In India, the first primary cause of cancer driven death in men is lung cancer whereas it’s second in case of women [1]. Approximately 1.8million new people were affected by lung cancer in 2012 among which 1.6million people were affected. 15% of the smokers who were suffering from lung cancer had traces of genetic susceptibility towards this disorder. There are various occupational hazards which cause the occurrence of lung cancer like asbestos exposure, which increases the rate of malignancy. Presence of general family history with cancer incidence, presence of chronic obstructive lung diseases may also lead to the occurrence of this disorder [2].
There exists a separate category of never makers who use substances other than tobacco like marijuana , e cigarette, etc. which is considered as the seventh most common cause of lung cancer, worldwide. The prevention measures of further invasions and maintenance of these disorders include limited exposure to air pollution, healthy body weight, exercise, healthy diet, protection from unknown carcinogens from inhalation. Despite numerous advances, the mortality rate from lung cancer has been increasing day by day [3]. There are studies which concentrate on various treatment options to this disorder. Stage 1 preoperative chemotherapy, surgery, high dose stereotactic body radiations. If the disorder gets locally advanced, 6 weeks of course thoracic radiotherapy is proceeded [4]. The last advanced lung cancer can be treated with molecular targeted therapy like EGFR mutations, genetic alterations and immunotherapy like immune system action suppression etc. which helps maintain and prevent further progression of the lung cancer [5].
Cardiospermum halicacabum belongs to the family sapindaceae. It grows in tropical and subtropical regions and is commonly called as balloon vines. It is mainly used for cough, nerve illness, arthritis, antioxidants, rubefacient and it contains antifungal properties [6]. Cardiospermum halicacabum is used in chinese medicine, it is a perennial plant where the leaves are compound and leaflets are membranous. It contains pyriform capsules that are wrangled. This plant is used as a traditional medicine and is used to cure many diseases [7].The inhibitor activity occurs through blocking the ion-ion chelating activity by beta-carotene, a fundamental component of the linolelate model system and by the inhibition of the radical DPPH [8].
Based on the previous study done, Cardiospermum and cyclophosphamide dose was injected into mice. The CTX administration caused myelosuppression and it caused decreased WBC count and the bone marrow cellularity [9]. While the hepatoprotective properties of the extract can aid in CCl4-induced liver injury, it also acts as a useful tool for the study of phenolic components [10]. Many researchers reported that evaluating plant derived compounds cytotoxicity, antioxidant activity and antipyretic activity has adjuvant therapy for human diseases as future medicine, [11]. The plant extract had an antihyperglycemic effect on streptozotocin-induced diabetic rats, where plasma and tissue glycoprotein levels were raised, and treatment of the plant extract restored normal levels [11,12]. Further, Rao's investigation discovered the ethanolic extract of the Cardiospermum plant has antidiarrheal properties. [11-13]. Our team has extensive knowledge and research experience that has translate into high quality publications [14-33]. The present study aims to evaluate the in vitro anticancer activity of Cardiospermum halicacabum against human A549 lung cancer cell line.
Materials and Methods
Chemicals
DMEM medium, 0.25% Trypsin-EDTA solution, sodium bicarbonate solution, bovine serum albumin (BSA), low melting agarose, MTT from Sigma Chemicals Co., St.Louis, USA. fetal bovine serum (FBS) and antibiotic/antimycotic solution, DMSO were from Himedia, sodium phosphate monobasic and dibasic, sodium chloride, sodium hydroxide, sodium carbonate, hydrochloric acid and methanol were purchased from Sisco Research Laboratories (SRL) India.
Preparation of the herbal extract
Whole plant powder of Cardiospermum halicacabum commercially purchased from IMPCOPS( Chennai, India) was used for the present study. About 150g of the plant powder was soaked in 500ml of aqueous/ 95% ethanol and kept for 3 days in a static condition at room temperature. The solution was filtered with a whatman filter paper preceded by filtration with crude filter paper. Fine filtrate was subjected to rota evaporation after that 3g of the material was obtained. The total ethanolic extract was concentrated using a vacuum evaporator and immediately stored at 40.
Cell culture reagents
Commercially available Dulbecco's Modified Eagle's medium (DMEM) contains 7.5% sodium bicarbonate solution. To 500ml of DMEM, 5ml of penicillin/ streptomycin solution and 0.5ml of amphotericin B solution was added. Then the medium was sterile filtered (0.22μ) inside the hood. The medium was then dispensed into a sterile container and stored at 4o.
Growth medium (DMEM with 10% FBS)
10ml of FBS was made up to 100ml using sterile DMEM. It was stored in a sterile container in cool and aseptic condition.
Phosphate Buffered Saline (PBS; pH 7.4)
0.63g of sodium phosphate monobasic (NaH2PO4), 0.17g of sodium phosphate dibasic (NaHPO4) and 4.5g of sodium chloride (NaCl) were dissolved in 500ml of double autoclaved milliQ water. The pH was then adjusted to 7.4 using 1N HCl and iN NaOH, sterile filtered (0.22μ) and then stored in a sterile container.
Trypsin-EDTA solution
Trypsin was purchased as 1x with EDTA (0.5% trypsin, 5.3 mM EDTA sodium salt).
(Note: Freeze-thaw process does not affect the enzyme activity. Thawing is done at room temperature).
0.89% Physiological saline
890 mg of sodium chloride was dissolved in 100ml of double autoclaved milliQ water.
Cell line
Human lung adenocarcinoma-A549 cell line was procured from NCCS Pune, India. The cells were grown in T25 culture flasks containing DMEM medium supplemented with 10% FBS. Detachment of the cells using Trypsin-EDTA solution was employed after confluence.
Cell proliferation mtt assay
Extract of Cardiospermum halicacabum on A549 cell line for the anticancer activity was evaluated using the MTT (3-(4, 5-dimethyl thiazol-2 yl)-2, 5-diphenyl tetrazolium bromide) assay for checking the cytotoxic activity according to the method described. In MTT assay cells were placed in the 96 well plates at the density of 5x103/100μl. The incubation period was 24 hours and the cells were treated with the cardiospermum halicacabum extracts in the concentration of 25, 50, 75, 100, 200, 300μg/ml. Wells with serum free medium alone were named as controls. After the incubation period of 24h at 37℃, 10μl of MTT reagent was added to the wells and incubated for 4 hours in the dark. Then, 100μl of sorenson glycine buffer (0.1M glycine, 0.1M NaCl, pH 10.5 with 0.1N NaOH) was added to the wells to solubilise the formazan crystals. The absorbance was measured at 570 nm. The experiment was repeated thrice and each concentration was tested in triplicates. The percentage viability of cells are calculated.
Cell viability (%)=Absorbance of sample/Absorbance of control X 100
Statistical analysis
All data obtained were analyzed by Student's-t-test using MS-Excel, represented as mean ± SD for six animals in each group. Statistical analysis utilizing one way ANOVA (SPSS/10 Software Package; SPSS Inc., Chicago, IL, USA) was performed. For intercomparison including LSD, posthoc testing was recruited. p<0.05 was rooted as the statistically significant value.
Results
The anticancer activity of Cardiospermum halicacabum was determined by MTT assay. From the results of the cell viability assay graph, we observed that the C. halicacabum inhibited the cell proliferation upon done and time dependent manner for 24hrs treatment respectively. Finally the C. halicacabum inhibit 50% of the cell growth via induce apoptosis at 30 μg/ml (IC50) concentration in lung cancer cells as shown in the Figures 1 and 2.
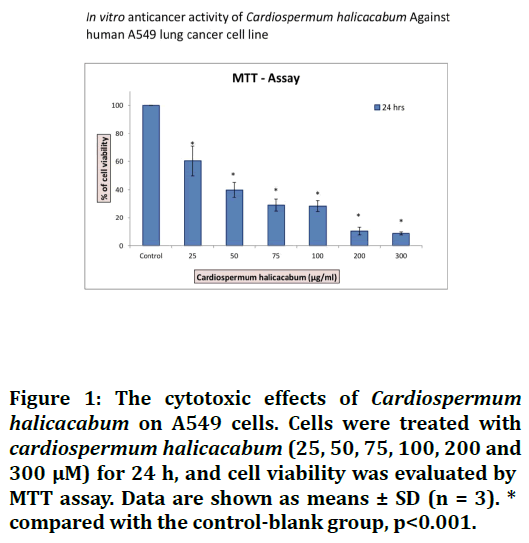
Figure 1. The cytotoxic effects of Cardiospermum halicacabum on A549 cells. Cells were treated with cardiospermum halicacabum (25, 50, 75, 100, 200 and 300 μM) for 24 h, and cell viability was evaluated by MTT assay. Data are shown as means ± SD (n = 3). * compared with the control-blank group, p<0.001.
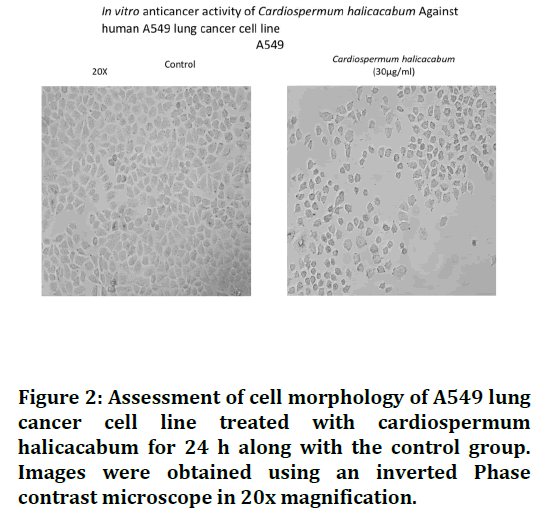
Figure 2. Assessment of cell morphology of A549 lung cancer cell line treated with cardiospermum halicacabum for 24 h along with the control group. Images were obtained using an inverted Phase contrast microscope in 20x magnification.
Discussion
Lung cancer is the most prevalent cancer in general. Mostly, it is related to cigarette smoke and other factors. Pharmacological plant extracts offer several medicinal qualities, such antifungal, antioxidant, and antibacterial ones. The purpose of this research was to determine the anticancer efficacy of the plant extract cardiospermum halicacabum in a lung cancer cell line. According to the results of the study, at a dose of 30 μg/ml, 50% of the cancer cells are killed. In the study conducted by Nyugen, he said that plant extracts had numerous benefits over chemical components, and the plant extract employed by them was Adenosma bracteosum (bonati). Due to the existence of bioactive components such as xanthomicrol and others, it was shown that chlorophyll had substantial efficacy in inhibiting the growth of tumor cells. And he discovered that the plant extract included more cancerfighting medications. [36,37]. The anticancer efficacy of three plant extracts: Urtica membranacea, Artemisia monosperma, and Origanum dayi was compared in the research. It was discovered that all three plants display anticancer action in a dose-dependent manner. Apoptosis causes cell death. additionally, it was shown that Urtica membranacea suppresses breast cancer cell growth directly [38].
Tripathy, reported that the fruit pulp and whole plant extract from L.acidissima and S.cumini where evaluated hemolytic inhibition assay was also done along with the evaluation of anticancer activity and it was found that the plant extract did not undergo any lysis and it hence it was found that they don't damage the erythrocyte since they lack cardiac glycosides, alkaloids, saponins phlobatannins which are responsible for the damage of erythrocyte. It was also found that they had good anticancer activity against the breast cancer cell line (Tripathy G, et al) [39]. In the study conducted by Shridhar C. Ghagane, he used the leaf sample of Leea indica which was subjected to Soxhlet extraction and it tends to increase the polarity of the solvents. It was found that the methanol and ethanolic extract of the leaf was found to have selective anticancer activity in the prostate cell lines and it had no cytotoxic effect on normal embryo fibroblast cells [40]. In the study conducted by Svejda, he used the plant extract of Trailliaedoxa gracilis, and the cell line which was involved in the study was human carcinoid KRJ-1 cell line and it was evaluated using cell counting and WST-1 cell proliferation assay. The apoptosis was found using the DAPI staining technique and the results showed that this plant extract has a good inhibiting activity of the carcinoid [41]
Omanike Ogbole in his study analysed the cytotoxic activity of the medicinal plants from Nigeria. He evaluated the extracts using brine shrimp lethality assay and the MTT cytotoxic assay. It was found in his result that Eleusine indica showed highest cytotoxic activity on the brine shrimp and the plant extract Macaranga barteri Mull. Arg. and Calliandra portoricensis Benth showed significant cytotoxic activity against the RD cell line [42]. Anticancer activity of C.halicacabum in lung cancer cells by inhibiting cell proliferation by inducing biochemically and by disrupting cell morphology upon treatment clearly shows the cytotoxic nature of the compound. Therefore, our finding showed the C.halicacabum proven as a potent anticancer drug in vitro studies.
Conclusion
The whole plant extract of Cardiospermum halicacabum was hence found to be cytotoxic against the A549 lung cancer cell line and it was found that the plant extract induces apoptosis in a dose and time dependent manner against lung cancer cells.. It can be concluded that the plant extract of Cardiospermum halicacabum might be an anticancer agent and further studies need to be done for lung cancer treatment.
Acknowledgement
We thank Saveetha Dental College and Hospitals for providing us the support to conduct the study.
Source of Funding
Saveetha Institute Of Medical and Technical Sciences and Sarkav Health Services.
References
- Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Lung Cancer Personalized Med 2016; 1-9.
- Faria SL. Role of radiotherapy in metastatic non-small cell lung cancer. Frontiers Oncol 2014; 4.
- Cruz CSD, Dela Cruz CS, Tanoue LT, et al. Lung cancer: Epidemiology, etiology, and prevention. Clin Chest Med 2011; 32:605–44.
- Raghunandhakumar S, Paramasivam A, Senthilraja S, et al. Thymoquinone inhibits cell proliferation through regulation of G1/S phase cell cycle transition in N-nitrosodiethylamine-induced experimental rat hepatocellular carcinoma. Toxicol Lett 2013; 223:60–72.
- Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017; 389:299–311.
- Nathiya S, Senthil Kumar B, Devi K, et al. Phytochemical screening and GC-MS analysis of Cardiospermum halicacabum L. leaf extract. Int J Trend Scientific Res Develop 2018; 2:512–6.
- Akbar S. Cardiospermum halicacabum L. (Sapindaceae). Handbook of 200 Medicinal Plants. 2020; 507–14.
- Dixena D, Patel DK. Morphology and Medicinal values of Cardiospermum halicacabum. Flora Fauna 2019; 25.
- Sivakali V, Thangavelu L. Cytotoxic activity of Cardiospermum halicacabum L. against oral cancer cell lines. Asian J Biol Life Sci 2020; 9:88–91.
- Karuppannan SK, Dowlath MJH, Sb MK, et al. Phytochemical and antibacterial activity of Cardiospermum halicacabum against wound pathogens. Pharmaco J 2020; 12:1303–10.
- Devanathan K. Cardiospermum halicacabum L. Sapindaceae. Ethnobotany Mountain Regions 2020; 1–9.
- Veeramani C, Al-Numair KS, Alsaif MA, et al. Protective effect of Cardiospermum halicacabum leaf extract on glycoprotein components on STZ-induced hyperglycemic rats. Asian Pac J Trop Med 2012; 5:939–44.
- Rao TVR, Dave Y. Morpho-histogenic studies in the pericarp of Cardiospermum halicacabum L. (Sapindaceae). Acta Botanica Hungarica 2005; 47:419–24.
- Rajeshkumar S, Kumar SV, Ramaiah A, et al. Biosynthesis of zinc oxide nanoparticles usingMangifera indica leaves and evaluation of their antioxidant and cytotoxic properties in lung cancer (A549) cells. Enzyme Microb Technol 2018; 117:91–5.
- Nandhini NT, Rajeshkumar S, Mythili S. The possible mechanism of eco-friendly synthesized nanoparticles on hazardous dyes degradation. Biocatal Agric Biotechnol 2019; 19:101138.
- Vairavel M, Devaraj E, Shanmugam R. An eco-friendly synthesis of Enterococcus sp.–mediated gold nanoparticle induces cytotoxicity in human colorectal cancer cells. Environ Sci Pollut Res 2020; 27:8166–75.
- Gomathi M, Prakasam A, Rajkumar PV, et al. Green synthesis of silver nanoparticles using Gymnema sylvestre leaf extract and evaluation of its antibacterial activity. South African J Chem Eng 2020; 32:1–4.
- Rajasekaran S, Damodharan D, Gopal K, et al. Collective influence of 1-decanol addition, injection pressure and EGR on diesel engine characteristics fueled with diesel/LDPE oil blends. Fuel 2020; 277:118166.
- Santhoshkumar J, Sowmya B, Venkat Kumar S, et al. Toxicology evaluation and antidermatophytic activity of silver nanoparticles synthesized using leaf extract of Passiflora caerulea. S Afr J Chem Eng 2019; 29:17–23.
- Raj RK. ß-Sitosterol-assisted silver nanoparticles activates Nrf2 and triggers mitochondrial apoptosis via oxidative stress in human hepatocellular cancer cell line. J Biomed Mater Res 2020; 108:1899–908.
- Saravanan M, Arokiyaraj S, Lakshmi T, et al. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb Pathog 2018; 117:68–72.
- Gheena S, Ezhilarasan D. Syringic acid triggers reactive oxygen species–mediated cytotoxicity in HepG2 cells. Hum Exp Toxicol 2019; 38:694–702..
- Ezhilarasan D, Sokal E, Najimi M. Hepatic fibrosis: It is time to go with hepatic stellate cell-specific therapeutic targets. Hepatobiliary Pancreat Dis Int 2018; 17:192–7..
- Ezhilarasan D. Oxidative stress is bane in chronic liver diseases: Clinical and experimental perspective. Arab J Gastroenterol 2018; 19:56–64.
- Gomathi AC, Xavier Rajarathinam SR, Mohammed Sadiq A, et al. Anticancer activity of silver nanoparticles synthesized using aqueous fruit shell extract of Tamarindus indica on MCF-7 human breast cancer cell line. J Drug Deliv Sci Technol 2020; 55:101376.
- Dua K, Wadhwa R, Singhvi G, et al. The potential of siRNA based drug delivery in respiratory disorders: Recent advances and progress. Drug Dev Res 2019; 80:714–30.
- Ramesh A, Varghese S, Jayakumar ND, et al. Comparative estimation of sulfiredoxin levels between chronic periodontitis and healthy patients - A case-control study. J Periodontol 2018; 89:1241–8.
- Arumugam P, George R, Jayaseelan VP. Aberrations of m6A regulators are associated with tumorigenesis and metastasis in head and neck squamous cell carcinoma. Arch Oral Biol 2021; 122:105030.
- Joseph B, Prasanth CS. Is photodynamic therapy a viable antiviral weapon against COVID-19 in dentistry? Oral Surg Oral Med Oral Pathol Oral Radiol 2021; 132:118–9.
- Ezhilarasan D, Apoorva VS, Ashok Vardhan N. Syzygium cumini extract induced reactive oxygen species-mediated apoptosis in human oral squamous carcinoma cells. J Oral Pathol Med 2019; 48:115–21.
- Duraisamy R, Krishnan CS, Ramasubramanian H, et al. Compatibility of nonoriginal abutments with implants: Evaluation of microgap at the implant-abutment interface, with original and nonoriginal abutments. Implant Dent 2019; 28:289–95.
- Gnanavel V, Roopan SM, Rajeshkumar S. Aquaculture: An overview of chemical ecology of seaweeds (food species) in natural products. Aquaculture 2019; 507:1–6.
- Markov A, Thangavelu L, Aravindhan S, et al. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res Ther 2021; 12:192.
- Gaestel M. Molecular chaperones in health and disease. Springer Science Business Media 2006; 442.
- Koka P, Mundre RS, Rangarajan R, et al. Uncoupling Warburg effect and stemness in CD133 cancer stem cells from Saos-2 (osteosarcoma) cell line under hypoxia. Mol Biol Rep 2018; 45:1653–62.
- Nguyen NH, Ta QTH, Pham QT, et al. Anticancer activity of novel plant extracts and compounds from (Bonati) in human lung and liver cancer cells. Molecules 2020; 25.
- Asokkumar S, Naveenkumar C, Raghunandhakumar S, et al. Antiproliferative and antioxidant potential of beta-ionone against benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Mol Cell Biochem 2012; 363:335–45.
- Solowey E, Lichtenstein M, Sallon S, et al. Evaluating medicinal plants for anticancer activity. ScientificWorld J 2014; 2014:721402.
- Paramasivam A, Raghunandhakumar S, Priyadharsini JV, et al. In Vitro Anti-neuroblastoma activity of thymoquinone against neuro-2a cells via cell-cycle arrest. Asian Pac J Cancer Prev 2015; 16:8313–9.
- Ghagane SC, Puranik SI, Kumbar VM, et al. Antioxidant and anticancer activity of leaf extracts on human prostate cancer cell lines. Integr Med Res 2017; 6:79–87.
- Svejda B, Aguiriano-Moser V, Sturm S, et al. Anticancer activity of novel plant extracts from Trailliaedoxa gracilis (W. W. Smith & Forrest) in human carcinoid KRJ-I Cells. Anticancer Res 2010; 30:55–64.
- Ogbole OO, Segun PA, Adeniji AJ. In vitro cytotoxic activity of medicinal plants from Nigeria ethnomedicine on Rhabdomyosarcoma cancer cell line and HPLC analysis of active extracts. BMC Complement Altern Med 2017; 17:494.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Harita Ravikumar1, S Raghunandhakumar2*, D Ezhilarasan2 and Lakshmi T2
1Department of Pathology, Saveetha Dental College, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University, Chennai, India2Department of Pharmacology, Saveetha Dental College and Hospitals, Saveetha Institutes of Medical and Technical Sciences (SIMATS), Saveetha University, Chennai, India
Received: 13-Dec-2021, Manuscript No. Jrmds-21-41707; , Pre QC No. Jrmds-21-41707 (PQ); Editor assigned: 15-Dec-2021, Pre QC No. Jrmds-21-41707 (PQ); Reviewed: 29-Dec-2021, QC No. Jrmds-21-41707; Revised: 03-Jan-2022, Manuscript No. Jrmds-21-41707 (R); Published: 10-Jan-2022
