Research Article - (2022) Volume 10, Issue 5
Interest of Botulinum Toxin in the Treatment of Temporomandibular Disorder
Saida El Khayati1*, Jalal Hamama1, Karim El Khatib1 and Amal El yamani2
*Correspondence: Saida El Khayati, Department of Medicine, Mohammed V University, Morocco, South Africa, Email:
Abstract
Temporomandibular Disorders (TMD) refer to a group of debilitating masticatory conditions that are often associated with considerable morbidity and a reduction in a person’s quality of life. Approximately 44% of the population are affected, but only a quarter of them seek professional help.
The aim of this review was to critically investigate and assess the evidence relating to the use and efficacy of Botulinum Toxin (BTX) in the management of temporomandibular joint disorders and masticatory myofascial pain.
A comprehensive search was conducted of PubMed, Scopus, Embase, and Cochrane central, to find relevant studies from the last 30 years up to the end of April 2021, using the following Mesh terms: Botulinum Toxin, Temporomandibular Joint Disorders.
Despite the demonstrated benefits, a consensus on the therapeutic benefit of BTX in the management of TMD, bruxism and masticatory myofascial pain is lacking. Further randomized controlled trials with larger sample sizes, minimal bias and longer follow-up periods are now needed.
Keywords
Temporomandibular disorder, Botulinum toxin, Myofascial pain, Randomized controlled trialsIntroduction
Temporomandibular Disorders (TMD) refer to a group of debilitating masticatory conditions that are often associated with considerable morbidity and a reduction in a person’s quality of life. Approximately 44% of the population are affected, but only a quarter of them seek professional help [1].
Patients can often present with a combination of specific and non-specific signs and symptoms, such as pain in and around the jaw on movement, neck pain, reduced jaw excursion, crepitus, trismus, tinnitus, earache, periorbital pain, and headache [2].
The management of TMD ranges from nonpharmacological conservative treatment to invasive surgical procedures. Initial management includes the avoidance of triggers, jaw rest through a soft diet, physiotherapy, warm compresses, dental review for an occlusal splint, and simple analgesia [3].
Literature Review
Objectives
The aim of this review was to critically investigate and assess the evidence relating to the use and efficacy of Botulinum Toxin (BTX) in the management of Temporo Mandibularjoint Disorders (TMD) and masticatory myofascial pain.
Methods
A comprehensive search was conducted of PubMed, Scopus, Embase, and Cochrane CENTRAL, to find relevant studies from the last 30 years up to the end of April 2021, using the following Mesh terms: Botulinum Toxins, Temporomandibular Joint Disorders.
In manual search, the bibliographic references of the original journals and articles were crossed to identify additional essays.
In this systematic review we have attempted to answer the following PICO question: Compared with other conservative treatments or placebo, does intramuscular injection of BTX reduce pain in adult patients with TMD.
The Participants (P) were adult patients (over 16 years of age) with clinically diagnosed TMD, which includes masticatory myofascial pain. The intervention (I) was intramuscular injection of BTX, irrespective of dose, timing, or subtype (A-G), into the muscles of mastication, irrespective of which ones were injected. The comparator/control (C) was any alternative conservative treatment such as physiotherapy, use of an occlusal splint, or placebo alone.
The primary Outcome (O) was subjective assessment based on a Visual Analogue Scale (VAS) for pain, or questionnaires assessed by the patients. Secondary outcomes were maximal mouth opening (assessed by maximal incisal opening) and adverse events that were associated with BTX.
This study was done according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [4]. The Cochrane risk-of-bias tool for randomised controlled trials was used to assess each study. Based on the results of each element in the tool “low risk”, “high-risk”, or “unclear 23+”, the overall risk was assessed.
Results
Search outcome
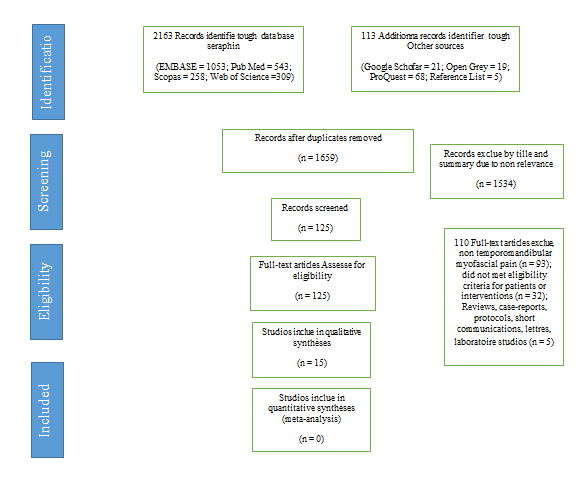
Of 125 articles identified for full-text review, 15 articles met the eligibility criteria and were included in the review. The inter-examiner agreement (Kappa) was 0.99 in the first stage (title and abstract screening stage) and 1.00 in the second stage (full-text reading stage). The selection process is shown in Figure 1 (Table 1, 2).
Figure 1: Flow chart of the article search and selection.
Study characteristics
Table 1: Characteristics of the reviewed studies based on PICO-like structured reading.
| Study first author, year | Population (P) | Intervention (I) | Comparison (C) | Outcomes (O) |
|---|---|---|---|---|
| Patel 2017 [3]Double-blinded, placebo-controlled RCT with selective crossover |
10T/10C(includesone dropout)TMD | Group 1: normal saline injectionGroup 2: BoNT -A injection (Xeomin)50 U to each masseter, 25 U to each temporalis, 10 U to each lateral pterygoid in a Bilateral. |
Pain scale (1–10) | Baseline:C=5.43, T=5.41 month:C=4.5 reduction,T=1.7 reduction |
| Chaurand J 2017 [5]Double-blinded, placebo-controlled |
11T/11C (sameparticipantsacting ascontrol andtest)TMD (myofacial pain) (RDC/TMD) |
T=BoNT C=conservative treatment |
Pain (VAS)Maximum mouth opening |
Pain (VAS)Baseline:C and T=8.48 1 month:C=5.2% reduction,T=19.2% reductionMaximum mouth openingBaseline: C and T=42.3 mm 1 month: C=42.3 mm, T=43.4 mm |
| De Carli B M 2016 [6] | 7T/8C (this excludes the three drop outs)Myofacial pain |
T=BoNT C=low level laser |
Pain (VAS)Mouth opening | Pain (VAS)Baseline: C=7,T=7Day 30: C=3.5,T=just under 3.5Mouth opening Baseline=C=42, T=38Day 30=C=42, T=36 |
| Zhang L D 2016 [7] | 10/10/10 (two controls used)TMD and bruxisme or daytime clenching for>2/12 |
T=BoNT C1=saline C2=no treatment |
Occlusal force | Occlusal forceChanges in mean (SEM) maximum bite force(kg) from baseline to 1/3/6 months:C1: 7.97/13.33/22.52C2: 0.94/8.63/3.77T: 41.97/48.17/39.79 |
| Shim YJ 2014 [8]RCT, parallel | 24 participants 10 M, 14 F, age range 20.2-38.7 years sleep bruxism, morning jaw stiffness with or without orofacial pain |
Group A: 10 subjects receiving bilateral BoNT-A injections (25 U per muscle) into the masseter muscles onlyGroup B: 10 subjects receiving the injections (25 U per muscle) into both the masseter and temporalis muscles |
EMG of both masseter and temporalis muscles=Bruxism events during sleep |
The injection decreased thepeak amplitude of EMG burstof repetitive masticatory muscle activity episodes in the injected muscles in both group |
| Guarda NL 2012 [9]RCT, parallel | 30 patients (22 F and 8 M, aged 23-69 y)TMD (myofascial pain) | Group A: n=15;BoNT 150 U per treated side in a single session in the temporalis and masseter muscles.Group B: n=15; each patient underwent three ( ± 1) 50-minsessions of fascial manipulation on a weekly basis,Over a 2- to 4-week span.No placebo group | Pain (VAS) (0-10)Maximum mouth opening |
Pain (VAS) Pain levels decreased to 5.2 at immediate post-injection and 4.8 at 3 mosMaximum mouth openingIncrease from baseline to 3 months: C=0.44 mm T=2.7 mm |
| Ernberg M 2011[2]RCT, placebocontrolled, double-blind, crossover (washout of at least 4 wks) |
21 patients (19 F and 2 M and aged 26-50 y)TMD (myofascial pain) |
• BoNT group: n=12; 0.2 mL of BT 50 U per muscle (and a maximum dose of 100 U to the patient if both muscles) diluted in 1 mL of Saline solution • Control group: n=9; 1 mL Saline solution |
Pain intensity (RDC/TMD)maximal incisal opening (mm) | Pain intensity (RDC/TMD)BT decreased Pain intensity by 33% after 1 mo and 30% after 3 mosMaximal incisal opening (mm)Increase from baseline to 1/3 months: C=0.9 mm/0.1 mm T=1.6 mm/1.6 mm |
| Redaelli A 2011 [10]. | N=120 (14 M, 106 F) with nocturnal bruxism |
BoNT-A injection into masseter muscles in three sites. 100 patients received 14 U in each side+6 U in five patients; 20 patients received 8 U in each side+6 U in 18 of those patientsNo control group |
Pain VAS (0-10) | 94.1% of the patients declared a fairly good to excellent result after BoNT-A injection |
| Lee S J 2010 [11] | N=12 subjects (7 M, ma. 25 ± 2.3 years; 5 F, ma. 24.8 ± 0.8 years) with nocturnal bruxism unspecified criteria |
Test group (3 M, 3 F; 25.0 ± 2.2 years): BoNT -A into each subject’s masseter muscles at three sites-80 U of BoNT -A 12-week observation EMG of both masseter and temporalis muscles for three consecutive nights at home for an average of 6 h per night Four observation points (baseline, 4, 8, 12 weeks) |
EMG of both masseter and temporalis muscles=Bruxism events during sleep |
The injection of BoNT in the masseter muscle reduces the number of bruxism events during sleep for up to 12 weeks |
| Venancio Rde A2009 [12]RCT, parallel | • 45 patients (40 F and 5 M, aged 18-65 years) | • Group 1: n=15; dry needling • Group 2: n=15; Lidocaine at 0.25% without VC • Group 3: n=15; BT 25-50 U |
• Palpation of trigger point; • pain diary; • pain questionnaire |
They reported positive outcomes for BTX use; however, its use was recommended for refractory cases only due to attached higher cost. |
| Guarda-Nardini L 2008 [1]Single-centre, placebo-controlled, double-blinded RCT |
10 M and 10 FAge range 25-45Myofascial pain and clinical diagnosis of bruxism |
Group 1: BoNT -A injectionGroup 2: placebo (normal saline) injectionBoth groups: four intramuscular injections within the masseter (30 U) and three intramuscular injections within the anterior temporalis (20 U) (single session, bilaterally) |
• Pain VAS (0-10)• maximum mouth opening | Pain VAS (0-10)Pain at rest and on chewing had lessened in the BoNT -A group but had remained constant in the placebogroup.Maximum mouth openingslight increase in the maximum non-assisted mouth opening in the BoNT group but no change in the placebo group |
| Kurtoglu C 2008 [13]Single-centre, placebo-controlled, double-blinded RCT |
20 F and 4 M (equally assigned to both groups)Myofascial pain with or without disc displacement |
Group 1: BTX-A injectionGroup 2: placebo (normal saline) injectionBoth groups: three injections within the masseter (30 U) and two injections within the temporalis (20 U) (single session, bilaterally |
RDC/TMD axis II biobehavioural questionnaire Q7-9 (relates to pain)EMG readings at rest and maximal clenching, of the anterior temporal muscles and masseters bilaterally |
Pain and psychological status BoNT group showed improvement in pain and psychological statusThe EMG of the temporalis and masseter muscles both showed greater reduction during clenching, having been administered with BoNT in relation to the control group, implicating force reduction by these muscle groups in the test subjects. |
| Bolayir G 2005 [14] | N=12 subjects (5 M, 7 F, age range 18-35 years), with nocturnal bruxism, who had not responded to splint and medication treatment |
BoNT-A injection into the masseter muscles-50 U of BoNT-A in 3 sites; VAS (baseline, 1 and 3 months)No control group |
• Pain VAS | The injection of BoNT-A in the masseter muscle reduces pain degree up to 3 months |
| von Lindern JJ 2003 [15]Multicentre, placebo-controlled, single-blinded RCT |
• 90 patientsTMD (myofascial pain) and bruxism |
• BoNT group: n=60; 35 MU BT liquidated in 0.7 mL saline • Control group: =30; 0.7 mL Saline solution |
• Pain VAS | BoNT patients showed a significant mean reduction of 3.2 on VAS |
| Nixdorf DR 2002 [16]Single-centre, placebo-controlled, double-blinded, crossover RCT |
15 patients (all F, aged 18-45 y)TMD (RDC/TMD) myofascial pain without or with limited mouth opening |
Maximum mouth opening (mm) | Maximum opening with/without pain increase from baseline to 8 weeks |
|
| T: test/C: Control/TMD: Temporomandibular Disorder/VAS: Visual analogy scaleRDC: Recommended Diagnostic Criteria/M: Male/F: FemaleRCT: Randomised Control Trial/EMG: Electromyography/Bont: Botulinum Toxin | ||||
Table 2: Quality assessment of rcts based on the cochrane handbook of systematic reviews of interventions.
| Study | Selection bias | Selection bias | Reporting bias | Performance | Detection bias | Attrition bias | Overall bias |
|---|---|---|---|---|---|---|---|
| (random | (allocation | (selective | bias (blinding of | (blinding of | (incomplete | ||
| sequence | concealment) | reporting) | participants and | outcome | outcome data) | ||
| generation) | personnel) | assessment) | |||||
| Patel 2017 | Low | Low | Unclear | Low | High | Low | Moderate |
| Chaurand J 2017 | High | High | Unclear | High | High | High | High |
| De Carli 2016 | low | High | Unclear | High | High | High | Moderate |
| Zhang L D 2016 | Unclear | Unclear | Low | Unclear | Unclear | low | |
| Shim YJ 2014 | Unclear | Unclear | Low | Unclear | Unclear | low | Unclear (no control |
| group with saline | |||||||
| placebo injection) | |||||||
| Guarda NL 2012 | High | High | High | High | High | low | High |
| Ernberg M 2011 | low | low | High | Low | low | low | Moderate |
| Redaelli A 2011 | Unclear | Unclear | Low | Unclear | Unclear | low | Unclear (no control |
| group with saline | |||||||
| placebo injection) | |||||||
| Lee S J 2010 | unclear | unclear | Low | low | unclear | low | Unclear (no PSG, |
| only EMG diagnosis) | |||||||
| Venancio Rde A | High | unclear | unclear | unclear | unclear | High | Unclear |
| 2009 | |||||||
| Guarda-Nardini L 2008 | High | High | High | High | High | High | High |
| Kurtoglu C 2008 | low | unclear | Low | low | Low | low | Low |
| Bolayir G 2005 | Unclear | Unclear | Low | Unclear | Unclear | low | Unclear (no control |
| group with saline | |||||||
| placebo injection) | |||||||
| von Lindern JJ 2003 | Unclear | Unclear | Low | low | High | low | Low |
| Nixdorf DR 2002 | low | low | High | Unclear | Unclear | High | Moderate |
Discussion
Pain
The results from this systematic review seem to indicate that botulinum toxin (Bont) helps to lessen pain levels in those suffering from TMD.
A number of the trials that did not meet the inclusion criteria also demonstrated encouraging results for the effectiveness of Bont when treating patients with TMD [17-18].
There is consensus that mechanisms of the peripheral and central nervous systems are responsible for the pain in TMD, and it has been postulated that injection of Bont leads to the direct attenuation of muscle contractions through chemical denervation.
The Bont has a direct analgesic effect on sensory nociceptive symptoms, as it partially antagonises the release of substance P, glutamate, and calcitonin gene-regulated peptide. This reduction in pain typically occurs a few days after injection [19].
All the adverse effects experienced by the patients were temporary and included localised pain, difficulty chewing, and focal muscle weakness. Paralysis of the zygomaticus major that resulted in an asymmetrical smile was common and is thought to be the result of local diffusion of the Bont-A from the masseter [20].
It is also important to point out the contraindications to Bont to know, inflammation at the proposed injection site, breast-feeding, pregnancy, chronic degenerative neuromuscular disorders, and treatment with aminoglycoside antibiotics.
The high costs of treatment with Bont compared with other conservative measures also needs consideration.
Mouth opening
The efficacy of Bont-An injection on maximal mouth opening was assessed.
Limitation of mouth opening is a symptom that is often painful and affects food, interferes with oral hygiene and restricts access to care preservatives. It can also affect speech and facial appearance.
Injection of botulinum toxin into a spasmed muscle, mainly the masseter or the temporal, can be considered to give way to a contracture. This process is used in patients with trismus related to temporomandibular dysfunction, on bruxism ground [21].
The effect of botulinum toxin on mandibular kinetics is positive with improvement in the quality of the opening buccal, symmetrisation of the kinetics of the mandibular condyles. On average, there is an 8 mm increase in the amplitude of the mouth opening after botulinum toxin injections [22]. According to Harding, the mouth opening amplitude after treatment increased by an average of 9.29 mm, or 43% of its initial value [23].
According to Luc's study, the impact of botulinum toxin on mandibular kinetics comes down to an average increase of 8 mm in the mouth opening and a symmetrisation of the kinetics of the two mandibular condyles allowing them a better synergy [ 24].
Bruxism
Within the limitations of this review, botulinum toxin represents a possible management option for purported SB consequences, minimizing symptoms and reducing the intensity of contractions for Repetitive Masticatory Muscle Activity (RMMA), rather than for SB itself.
Studies reported reduced jaw stiffness and pain after injection of type a botulinum toxin Bont-A in both groups a (masseter) and B (masseter and temporalis). Other research looking at the use of BTX in the management of bruxism that did not meet the inclusion criteria, also presented some encouraging results [17].
Bont-An injection may influence just the last phase of an SB episode, by reducing the intensity of the contraction. It may only have effects on morning jaw stiffness and pain. The available data do not support its usefulness to actually reduce the number RMMA [15].
Sleep Bruxism (SB) follows a sequence of physiological activations in relation to micro-arousals. The RMMA are under the influence of the brainstem arousal–reticular ascending system [28]. First, there is a rise in sympathetic cardiac activity around 4 min before RMMA. Then, there is a rise in the frequency of electroencephalographic activity 4 s before RMMA, followed by a tachycardia starting 1 s before RMMA with an increase in jaw-opener suprahyoid muscle activity 0.8 s before RMMA. Finally, RMMA episodes occur on masseter muscles, with or without tooth grinding sounds [25].
Some case reports showed that Bont-An injection in patients with severe AB episodes may be an alternative option to wearing oral appliances. In addition, it has been also used to manage secondary bruxism triggered by medication intake or neurological and/or psychiatric disorders [26,27].
Conclusion
Despite the demonstrated benefits, a consensus on the therapeutic benefit of Bont in the management of TMD, bruxism and masticatory myofascial pain is lacking. Further randomized controlled trials with larger sample sizes, minimal bias and longer follow-up periods are now needed.
What is known about this subject
- Temporomandibular Disorder (TMD) sometimes causes disability as well as physical and psychological suffering that have a real impact on the quality of life of patients and, more generally, on public health.
- TMD support remains largely insufficient.
- The efficacy of botilium toxin in the management of TMD is not widely known.
What is new in our paper
- In the management of TMD, botulinum toxin is a treatment full of promise and future that should be desecrated.
- Injections of botulinum toxin into masticatory muscles appear to be an effective therapeutic solution for TMD.
- Draw attention to the need to develop a consensus on the dose of botulinum toxin to inject, the dilution, and the number of injections to be given per muscle.
Conflicts of Interest
Authors do not declare any conflict of interest.
References
- Guarda-Nardini L, Manfredini D, Salamone M, et al. Efficacy of botulinum toxin in treating myofascial pain in bruxers: a controlled placebo pilot study. Cranio 2008; 26:126-35
- Ernberg M, Hedenberg-Magnusson B, List T, et al. Efficacy of botulinum toxin type A for treatment of persistent myofascial TMD pain: a randomized, controlled, double-blindmulticenter study. Pain 2011; 152:1988-1996
- Patel AA, Lerner MZ, Blitzer A. Incobotulinumtoxin A injection for temporomandibular joint disorder. Ann Otol Rhinol Laryngol 2017; 126:328-33.
- Nixdorf DR, Heo G, Major PW. Randomized controlled trial of botulinum toxin A for chronic myogenous orofacial pain. Pain 2002; 99:465-73
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339:b2535.
[crossreff][Google Scholar][Indexed]
- Chaurand J, Pacheco-Ruiz L, Orozco-Saldivar H, et al. Effcacy of botulinum toxin therapy in treatment of myofascial pain. J Oral Sci 2017; 59:351-356.
[crossreff][Google Scholar][Indexed]
- De Carli BM, Magro AK, Souza-Silva BN, et al. The effect of laser and botulinum toxin in the treatment of myofascial pain and mouth opening: A randomized clinical trial. J Photochem Photobiol B. 2016;159:120-123.
- Zhang LD, Liu Q, Zou DR, et al. Occlusal force characteristics of masseteric muscles after intramuscularinjection of Botulinum Toxin A (BTX A) for treatment of temporomandibular disorder. Br J Oral Maxillofac Surg. 2016;54:736-740.
- Shim YJ, Lee MK, Kato T, et al. Effects of botulinum toxin on jaw motor events during sleep in sleep bruxism patients: a polysomnographic evaluation. J Clin Sleep Med 2014; 10:291-298.
- Guarda-Nardini L, Stecco A, Stecco C, et al. Myofascial pain of the jaw muscles: comparison of short-term effectiveness of botulinum toxin injections and fascial manipulation technique. Cranio 2012; 30:95-102.
- Redaelli A. Botulinum toxin A in bruxers: one year experience. Saudi Med J. 2011; 32:156-158. [crossreff]
- Lee SJ, Mc Call WD Jr, Kim Y K, et al. Effect of botulinum toxin injection on nocturnal bruxism: a randomized controlled trial. Am J Phys Med Rehabil 2010; 89:16-23. [crossreff]
[Google Scholar][Indexed]
- Venancio Rde A, Alencar FG Jr, Zamperini C. Botulinum toxin, lidocaine, and dry-needling injections in patients with myofascial pain and headaches. Cranio. 2009;27:46-53.
- Kurtoglu C, Gur OH, Kurkcu M, et al. Effect of botulinum toxin-An in myofascial pain patients with or without functional disc displacement. J Oral Maxillofac Surg. 2008;66:1644-1651.
- Bolayir G, Bolayir E, Coskun A, et al. Botulinum toxin type-A practice in bruxism cases. Neurol Psychiatry Brain Res. 2005;12:43-46. [crossreff]
[Google Scholar][Indexed]
- von Lindern JJ, Niederhagen B, Berge S, et al. Type A botulinum toxin in the treatment of chronic facial pain associated with masticatory hyperactivity. J Oral Maxillofac Surg. 2003;61:774-8.
- Nixdorf DR, Heo G, Major PW. Randomized controlled trial of botulinum toxin A for chronic myogenous orofacial pain. Pain. 2002;99:465-73.
- Lee K M, Chow J, Hui E, et al. Botulinum Toxin Type an Injection for the Management of Myofascial Temporomandibular Pain Disorder. Asian J Oral Maxillofac Surg. 2005; 17:100-103.
[crossreff][Google Scholar][Indexed]
- Sidebottom AJ, Patel A A, Amin J. Botulinum injection for the management of myofascial pain in the masticatory muscles. A prospective outcome study. Br J Oral Maxillofac Surg 2013; 51:199-205.
[crossreff][Google Scholar][Indexed]
- Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology 2005; 26:785-93.
- Mor N, Tang C, Blitzer A. Temporomandibular myofacial pain treated with botulinum toxin injection. Toxins (Basel) 2015; 7:2791-800.
- Sangla S. Current therapeutic aspects of botulinum toxin in neurology. EMC, Physiotherapy-Physical Medicine-Rehabilitation. 2007;26-455[crossreff][Google Scholar][Indexed]
- Chikhani L, Dichamp J. Bruxism, functional pain syndrome of the temporomandibular joints and botulinum toxin. Ann Phys Rehabil Med 2003;333-337[crossreff][Google Scholar][Indexed]
- Harding MB, Batifol D. Botulinum toxin versus gutter in the treatment of bruxism and dysfunctions of the masticatory system. Rev Col Odonto-Stomatol Afr Chir Maxillo-fac 2015;22:41-45[crossreff][Google Scholar][Indexed]
- Luc Chikhani, Jacques Dichamp. Treatment of algo-dysfunctional syndromes of the temporomandibular joints by botulinum toxin. Study of 3,200 patients from 1994 to 2009. Odonto-Stomatological News-n° 250. 2010;11-14[crossreff][Google Scholar][Indexed]
- Macaluso GM, Guerra P, Di Giovanni G, et al. Terzano MG Sleep bruxism is a disorder related to periodic arousals during sleep. J Dent Res 1998; 77:565-573.
- Lavigne GJ, Huynh N, Kato T, et al. Genesis of sleep bruxism: motor and autonomic-cardiac interactions. Arch Oral Biol 2007;52:381-384.
Author Info
Saida El Khayati1*, Jalal Hamama1, Karim El Khatib1 and Amal El yamani2
1Department of Medicine, Mohammed V University, Morocco, South Africa2Department of Dental Clinics, Mohammed V University, Morocco, South Africa
Citation: Saida El Khayati, Jalal Hamama, Karim El Khatib, Amal El yamani. Interest of Botulinum Toxin in the Treatment of Temporomandibular Disorder, J Res Med Dent Sci, 2022, 10(5):262-275.
Received: 21-Feb-2022, Manuscript No. 50012; , Pre QC No. 50012; Editor assigned: 23-Feb-2022, Pre QC No. 50012; Reviewed: 09-Mar-2022, QC No. 50012; Revised: 22-Apr-2022, Manuscript No. 50012; Published: 06-May-2022