Research - (2021) Volume 9, Issue 9
In Vitro Color Change of Enamel Surface After Exposed to Acid Beverages Solutions
Aisha A Qasim* and Leqaa H Qibi, Baraa W Alani
*Correspondence: Aisha A Qasim, Department of Pedo Ortho Prevention, College of Dentistry, University of Mosul, Iraq, Email:
Abstract
Background/purpose: The goal of this study was to see how acid beverages, artificial saliva, and toothpaste affected the color of dental enamel (An in vitro study). Materials and Methods: Two hundred healthy human third molar teeth were extracted for surgical purpose reasons randomly divided into 2 main groups (with and without demineralization). Which then subdivided into (8) subgroups. The surface roughness and color change of the enamel surface were measured before and after acid beverage demineralization, as well as after toothpaste remineralization. Results: The mean surface roughness increased substantially with discoloration after demineralizing with acid beverages solutions and decreased after remineralization with artificial saliva and fluoridated dentifrice, according to the results evaluated statistically using one way ANOVA and Duncan test (p ≤ 0.05). Conclusions: Acid beverages solutions increase roughness and cause color change of enamel tooth surface.
Keywords
Acid beverages, Miranda, Demineralization, Roughness, Tooth color
Introduction
Teeth are an important part of the body that aid in chewing, speech together with contributing to appearance thus teeth has a major role in the general appearance of the face which has an effect on the self-confidence of the individuals and social life [1]. Appearance of the teeth depends on their color, shape and alignment in the arch. So, understanding the color of the tooth is essential for many aspects in dental practice, particularly in cosmetic and restorative dentistry [2].
The color of one's teeth can range from white to ceramic yellow, and it's an essential factor in assessing one's facial beauty. Children's teeth are brighter, and as they become older, they grow darker. Discoloration of the teeth occurs when pigments are deposited in the internal structure and on the surface of the teeth. The cause, appearance, and degree of tooth discoloration vary. Extrinsic, intrinsic, or a mix of both factors can induce discoloration [3].
Today's patients have a strong need for white teeth and, as a result, a more attractive smile. A significant variation in the color of the teeth is frequently the first indicator of abnormalities in the human dentition [4].
Dental demineralization is a danger for almost everyone who has natural, undamaged teeth and drinks acidcontaining beverages on a frequent basis [5,6]. Dental demineralization is described as acidic breakdown of the tooth surface that occurs without the presence of bacteria [7] and is produced by acid entering the mouth directly on the tooth surface. It is a multifactorial phenomenon that happens throughout the course of a person's life and is generally irreversible owing to the chemical action of extrinsic or intrinsic acid. The causes of demineralization could be extrinsic, intrinsic or a combination of both. Intrinsic causes include acid reflux, regurgitation and vomiting. Extrinsic causes include exposure to acidic liquids or foods such as beverages, fruit juices, sports drinks, soft drinks and sucking on lemons or other citrus fruits [8]. Acid beverages predominantly contain water, phosphoric acid, citric acid, caffeine, sugar and other chemicals in the form of acid regulators, carbon dioxide, [9]. Acid beverages are prepared by mixing flavoured syrup with carbonated water, both of which are chilled. There are different types of Acid beverages, including colas, Miranda, energy and sports drinks, functional beverages, and low- and mid-calorie beverages [10].
The action of acids with a pH less than 4.5 causes this process. This value is below the critical pH for hydroxyapatite (critical pH of 5.5) and fluorapatite (critical pH of approximately 4.5), leading in the dissolution of both minerals present in the enamel and the formation of a surface lesion [11].
No single component in Acid beverages or other soda drinks causes staining or discoloration of the teeth. The acid in Miranda yields abrasions in the teeth and the brown caramel coloring and sugars will stick to the teeth surface and build up without appropriate care [12].
Saliva flow has a vital function in preventing acid demineralization of the enamel. To understand how saliva counteracts demineralization, researchers looked at several aspects of enamel, including surface shape, hardness, mineral loss, and lesion depth. The buffering ability of saliva, the amount of calcium and phosphate groups, the formation of pellicle on the tooth surface, and other factors are thought to function alone or in combination to prevent tooth demineralization [13].
Different techniques have been tried to prevent and cure tooth wear, which is mostly caused by acid demineralization induced by modern lifestyle food habits, with topical fluoride administration being one of them [14].
Topically fluoride requirement can be well satisfied through toothpaste, mouth-wash and professionally topical application. Fluorides can help in strengthen teeth against cariogenic acids while prevention against erosive acids requires a higher level of protection. The most effective way to protect teeth against the growing danger of dental demineralization and erosive tooth wear is to use toothpaste every day [15].
In clinical practice and dental research a wide range of tooth shade measurement approaches has been done and they can be divided into subjective (visual) and objective (instrumental) methods. The most widely used method in the dental clinical practice is the subjective (visual) method where visual examination of the tooth shade is compared with a specific shade guide. Using colorimeters or spectrophotometers which are instruments that are used to measure the color surface of an object are called the instrumental methods, these methods are more common in dental research because of their simplicity of use, ability to distinguish small changes and unbiased (do not depend on the observer’s judgment). Though, these instruments have several disadvantages; they can only evaluate small areas at a time and they are designed to be used on flat surfaces (teeth surfaces usually are not flat) [16].
Grouping the samples
The total number of samples was (200) samples. Sample divided into 2 main groups (with and without demineralization). This group was subdivided into (8) subgroups, which include:
Group 1: (with demineralization) Exposed to Miranda solution PH=2.9.
Acid group, sample exposed to Miranda solution only 3 time daily for 14 days and then measured.
Artificial saliva group, sample exposed to Miranda solution then immersed in artificial saliva for 9 hours then measured. After being exposed to Miranda solution for 9 hours, the sample was submerged in artificial saliva and quantified.
Toothpaste group 1 (Crest), the teeth samples exposed to Miranda solution only 3 time daily for 14 days then covered with a thin coating of 1450 ppm (NaF) toothpaste before being tested.
Toothpaste group 2 (Signal), the teeth samples exposed to Miranda solution only 3 time daily for 14 days then covered with a thin coating of 1450 ppm (SnF2) toothpaste, which was then analyzed.
Group 2: (without demineralization) Non exposed to Miranda solution.
Acid group, Sample immersed in tap water them measured color test by Easy shade.
Artificial saliva group, Sample immersed in artificial saliva for 9 hours then measured.
Toothpaste group 1 (crest), the teeth samples were covered with a thin layer of 1450 ppm (NaF) toothpaste before being tested.
Toothpaste group 2 (Signal), samples were covered with a thin layer of 1450 ppm (SnF2) toothpaste, which was then analyzed.
The surface roughness and color change of the enamel surface were measured before and after acid beverage demineralization, as well as after toothpaste remineralization.
Materials and Methods
For this research, ethical approval was provided by the Authorized Committee of College of Dentistry, University of Mosul, Mosul, Iraq on 16/3/2021 with number 133.
Preparation of the samples
Two hundred healthy human third molar teeth were extracted for surgical purpose obtained from patient between age 20 and 35 who visited the clinic free from cracks, stains and hypomineralized areas were selected Before the experiment . After extraction, the teeth were preserved in distilled water (pH 7.0) before the experiment. To prepare the enamel surface samples for the experiments, the molar teeth was cross-sectioned longitudinally by using diamond discs were cut into 2 mm thick slices., relatively planar buccal, lingual surfaces, the specimens were polished by sand paper (grit 1200) To achieve a flat and smooth surface. Then, the slice was embedded in self-cured acrylic resin leaving the polished side exposed to the outer surface.
Artificial saliva composition
500 mL of artificial saliva contained: Na2PO4 0.170 gm, sodium ascorbate 0.001 gm, glucose 0.015 gm, NaCl 0.290 gm, CaCl2 0.085 gm, NH4Cl 0.080 gm, KCl 0.635 gm, NaSCN 0.080 gm, KH2PO4 0.165 gm, urea 0.100 gm and the final pH was adjusted to 7 [17].
Measurement of surface roughness
Surface roughness measurements were examined using an optical non-contact profiling system (Berlin, Germany), at a magnification of 70X.
The samples were placed on the stage of the device with a customized Teflon guide so that the tooth surface to be scanned was exposed. Then, the focus was manually adjusted to obtain an image on the monitor. The measurements were obtained using vertical scanning interferometry. Each sample was scanned at three selected points. A mean roughness profile (Ra) was determined for each specimen to describe the overall roughness of the surface (μm) [18].
Microscopic assessment
Initially, the slices were inspected using an electron microscope (moticam 200–2.0 M pixel USB 2.0) .Each specimen's enamel surface was studied under an electron microscope. Photographs were taken at a magnification of 200X.
Measurement of enamel surface color change
Colorimeters or spectrophotometers are used to measure the color surface of teeth .These instruments are more commonly used in dental research because of their simplicity of use, capability of detecting small changes in tooth structure and unbiased Though, Colorimeters measure the difference in the color of objects, color chromaticity values producing from the reflection of light once the light source has passed through a series of filters. The probe with a diameter of 5 mm was placed against a calibrated block within the machine to calibrate the device for each sample. The probe was placed on the previously established region of the tooth and the probe switch was pressed, making sure that the probe was not moved or positioned at a different angle throughout the measurement. Tooth color represented in CIE L*a*b* values and the matching recommended VITA Easy shade (Wilcos, Brazil) according to manufacturer’s instructions. Codes were directly attained for each point along the outer surface of each sample.
Results
The result of this study display the change of color of the enamel tooth surface affected decreased when exposed to artificial saliva and toothpaste and increased in group exposed to demineralization acid beverage but with significant difference existed at p ≤ 0.05. Between them as in (Table 1) . Table 2 and Table 3 demonstrated one way analysis of variance (ANOVA) test which revealed that there was significantly difference at p≤ 0.05 of mean roughness value of all teeth sample groups exposed and not exposed to acidic beverage.
| Solution | Demineralization solution | No. | Mean | SD | t–value | df | p–value |
|---|---|---|---|---|---|---|---|
| Control Negative | With | 25 | 53.744 | 8.16941 | -8.246 | 48 | 0.000* |
| Without | 25 | 68.604 | 3.80115 | ||||
| Control Positive | With | 25 | 81.084 | 2.52185 | 5.165 | 48 | 0.000* |
| Without | 25 | 75.028 | 5.29225 | ||||
| Crest 1450 ppm (NaF) toothpaste | With | 25 | 74.492 | 3.00803 | -9.499 | 48 | 0.000* |
| Without | 25 | 81.624 | 2.24616 | ||||
| Signal 1450 ppm (SnF2) toothpaste | With | 25 | 75.088 | 3.27316 | -9.828 | 48 | 0.000* |
| Without | 25 | 82.232 | 1.58001 |
Table 1: Independent sample t-test of color test for all groups.
| Sum of Squares | df | Mean Square | F | Sig. | |
|---|---|---|---|---|---|
| Between Groups | 10708.02 | 3 | 3569.34 | 153.75 | 0.000* |
| Within Groups | 2228.66 | 96 | 23.215 | ||
| Total | 12936.68 | 99 | |||
Table 2: Mean roughness one-way analysis of variance (Groups with demineralization solution).
| Sum of Squares | df | Mean Square | F | Sig. | |
|---|---|---|---|---|---|
| Between Groups | 3076.782 | 3 | 1025.594 | 82.05 | 0.000* |
| Within Groups | 1199.96 | 96 | 12.5 | ||
| Total | 4276.742 | 99 | |||
Table 3: Mean roughness one-way analysis of variance (Groups without demineralization solution).<14>
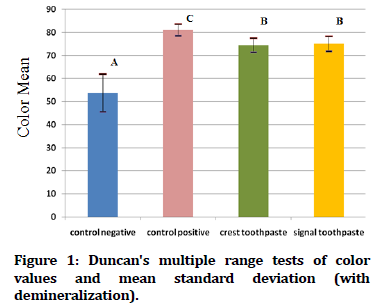
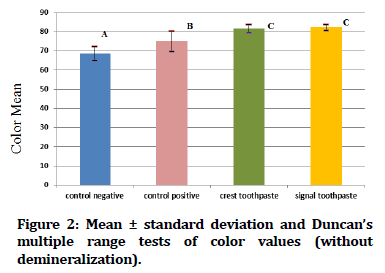
In Figure 1 Duncan's multiple range tests of the color value of tooth samples exposed to demineralization acid beverage reveal that the control positive groups have the greatest mean color value, while the control negative groups have the lowest mean color value. While Figure 2 showed Duncan's multiple range tests of the color value of tooth samples not exposed to acid beverage reveal that the crest and signal toothpaste groups have the greatest mean color value, while the control negative groups have the lowest mean color value.
Figure 1. Duncan's multiple range tests of color values and mean standard deviation (with demineralization).
Figure 2. Mean ± standard deviation and Duncan’s multiple range tests of color values (without demineralization).
Microscopic analysis of test study
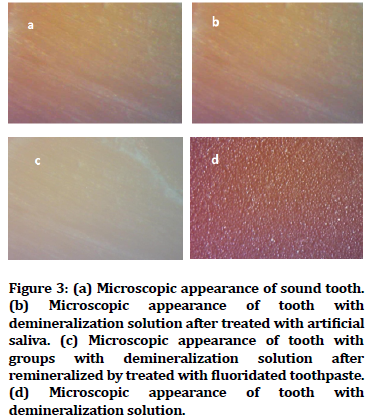
Untreated enamel surface as in Figure 3A, artificial saliva in Figure 3B and fluoridated toothpaste treated surface Figure 3C looked smooth and regular in sterolight microscope micrographs of dental enamel surface samples. While the surface of the Miranda managed surface seemed rough and irregular porosity, as shown in Figure 3D.
Figure 3. (a) Microscopic appearance of sound tooth. (b) Microscopic appearance of tooth with demineralization solution after treated with artificial saliva. (c) Microscopic appearance of tooth with groups with demineralization solution after remineralized by treated with fluoridated toothpaste. (d) Microscopic appearance of tooth with demineralization solution.
Discussion
Tooth discoloration is influenced by a number of variables, including the staining solution's pH. [19] Miranda had the lowest pH in this research that might have caused damage to the samples' surfaces.
The positive demineralized group exhibited tooth discoloration, according to the data. When acid erodes the tooth enamel, which is the tooth's glossy, protective outer coating, tooth demineralization occurs.
Teeth which are not protected by enamel are more susceptible to cavity and more sensitive, cracked and discoloured. This discoloration can be caused by chromogenes which color Miranda. Chromogens stain the surface of the teeth.
Chromogens can simply create a yellow, dingy look of the tooth after the enamel has been damaged by citric acid [20].
Artificial saliva containing minerals ions has an impact that mimics the remineralization of demineralized teeth [21], and saliva is an important element in the prevention of tooth discoloration, according to Monteiro et al. [22].
Nogueira et al. describe the effect of acidic beverages on the color of enamel tooth surfaces. [23], the pace of therapy might be influenced by the use of acidic beverages during demineralization. Côrtes et al. [24] also observed the staining of teeth during and after demineralization when teeth are immersed in Miranda solution. During the treatment, the remineralization of the enamel with artificial saliva was successful in avoiding enamel discoloration during therapy [24]. However, the use of an acidic beverage (Miranda solution) changed the color of the enamel. This finding might be explained by the fact that acid drinks have a higher propensity for demineralization and affect enamel morphology [22,25].
Conclusion
Acidic beverage has a pH low enough to cause tooth demineralization, according to the research. The acidity of Miranda soft drinks is undoubtedly the most important factor in the etiology of dental demineralization and tooth discoloration, which harm persons of all ages. Dentists owe it to their patients to advise them on how to avoid acidic beverages and foods that might harm their dental health. Patients who have poor oral health as a result of consuming too many acidic drinks and foods should be taught about the causes of their dental problems.
Limitations of the Study
The current study was conducted in vitro, which implies that the oral cavity environment could not be duplicated. Natural saliva, on the other hand, has drawbacks and difficulties, such as the time it takes to collect saliva and the fact that saliva decomposes quickly.
Areas for Further Study
The influence of various drinking materials accessible in Mosul city markets on tooth structure should be investigated further.
References
- Boeira GF, Salas MMS, Araújo DC, et al. Factors influencing dental appearance satisfaction in adolescents: a cross-sectional study conducted in Southern Brazil. Braz J Oral Sci 2016; 15:8-15.
- Abdallah MN. Faculty of surface reactivity of tooth enamel with dyes, oxidizing agents and magnesium ions and its effect on tooth color. Master thesis, McGill University 2012.
- Schluetera N, Amaechib BT, Bartlettc D, et al. Terminology of erosive tooth wear: Consensus report of a workshop organized by the ORCA and the cariology research group of the IADR. Caries Res 2020; 54:2-6.
- Armalaite J, Jarutiene M, Vasiliauskas A, et al. Smile aesthetics as perceived by dentalstudents: a cross-sectional study. MC Oral Health 2018; 18:1-7.
- De melo MA, Passos VF, Lima JP, et al. Carbohydrate-electrolyte drinks exhibit risks for human enamel surface loss. Restor Dent Endod 2016; 41:246-254.
- Abd Al-Hussain ZA, Nahidh M. Carbonated soft drinks and orthodontics: Review of literature. Turk J Orthod 2021; 34:136-142.
- Ablal MA, Milosevic A, Preston AJ, et al. A novel approach to study in situ enamel erosion and abrasion lesion. J Dent 2017; 59:78-85.
- Wiegand A, Attin T. Occupational dental erosion from exposure to acids--a review. Occup Med 2007; 57:169-76.
- Kregiel D. Health safety of soft drinks: contents, containers, and microorganisms. Biomed Res Int 2015; 128697:15.
- Abu-Reidah IM. Carbonated Beverages. Galanakis CM. Trends in non-alcoholic beverages. 1st ed. London: Academic press, Elsevier 2020; 1-36.
- Erdemir U, Yildiz E, Saygi G, et al. Effects of energy and sports drinks on tooth structures and restorative materials. World J Stomatol 2016; 5:1-7.
- Lussi A, Jaeggi T, Zero D. The role of diet in the aetiology of dental erosion. Caries Res 2004; 38:34–44.
- Wang X, Mihailova B, Klocke A. Effect of artificial saliva on the apatite structure of eroded enamel. Int J Spectroscopy 2011; 1-9.
- Poggio C, Gulino C, Mirando M, et al. Preventive effects of different protective agents on dentin erosion: An in vitro investigation. J Clin Exp Dent 2017; 9:7-12.
- Noble WH, Faller RV. Protection from dental erosion: All fluorides are not equal. Compend Contin Educ Dent 2018; 39:13-17.
- Brook AH, Smith RN, Lath DJ. The clinical measurement of tooth color and stain. Int Dent J 2007; 57:324-330.
- Fanfoni L, Costantinides F, Berton F, et al. From erosion to remineralization: The possible role of two topic home devices used as combined treatment. Appl Sci 2020; 10:4093.
- Shamrani A. Effects of delayed tooth brushing on enamel surface roughness after home bleaching. Int J Dent Oral Health 2020; 7:1-7.
- Addy M, Prayitno S, Taylor L, et al. An in vitro study of the role of dietary factors in the aetiology of tooth staining associated with the use of chlorhexidine. J Periodontal Res 1979; 14:403-10.
- Watts A, Addy M. Tooth discolouration and staining: A review of the literature. Br Dent J 2001; 190:309-316.
- Batista GR, Torres CRG, Sener B, et al. Artificial saliva formulations versus human saliva pretreatment in dental erosion experiments. Caries Res 2016; 50:78-86.
- Monteiro D, Moreira A, Cornacchia T, et al. Evaluation of the effect of different enamel surface treatments and waiting times on the staining prevention after bleaching. J Clin Exp Dent 2017; 9:e677-81.
- Nogueira JSP, Lins-Filho PC, Dias MF, et al. Does comsumption of staining drinks compromise the result of tooth whitening? J Clin Exp Dent 2019; 11:e1012-7.
- Côrtes G, Pini NP, Lima DANL, et al. Influence of coffee and red wine on tooth color during and after bleaching. Acta Odontol Scand 2013; 71:1475-80.
- Carlos NR, Pinto AVD, Amaral FLB, et al. Influence of staining solutions on color change and enamel surface properties during at-home and in-office dental bleaching: an in situ study. Oper Dent 2019; 44:595-608.
Author Info
Aisha A Qasim* and Leqaa H Qibi, Baraa W Alani
Department of Pedo Ortho Prevention, College of Dentistry, University of Mosul, Mosul, IraqCitation: Aisha A Qasim, Leqaa H Qibi, Baraa W Alani,In Vitro Color Change of Enamel Surface After Exposed to Acid Beverages Solutions, J Res Med Dent Sci, 2021, 9(9): 22-27
Received: 18-Aug-2021 Accepted: 02-Sep-2021