Research - (2021) Volume 9, Issue 4
Immunohistochemical Expression of Integrin in OSCC in Relation to Tumor Grade
Fatima Ghazi Al-Bayati* and Layla Sabri Yas
*Correspondence: Fatima Ghazi Al-Bayati, Department of Oral and Maxillofacial Pathology, Dental Teaching Hospital, College of Dentistry, University of Baghdad, Iraq, Email:
Abstract
Backgrounds: Squamous cell carcinoma (SCC) is identified as “a malignant epithelial neoplasm account 90% or more of oral lesion. Demonstrating squamous differentiation and have feature of keratin structure and intercellular bridges may be formed, so Integrin is a difficult of noncovalently complicated connected heterodimeric glycoproteins which is serve as adhesion particles of cell, co-operates with extracellular matrices (ECMs) like as laminin, fibronectin, vitronectin, and collagen, and have intercellular attachment function.
Objective: This study is aimed to demonstrate the intra cytoplasmic integrin expression in the tumor cell of (OSCC) in relation to different malignancy grade.
Materials and method: Seventeen case of formalin fixed -paraffin embedded (FFPE) tissue blocks that diagnosed as OSCC will be employed, all these cases were processed histologically and immunohistochemical to evaluate integrin expression.
Results: the positive expression of integrin increase from WDSCC to PDSCC, and statistically there is highly significant appear (P <0.01) in relation of Expression of integrin to grade.
Conclusion: Expression of integrin is increase with the histological grade of OSCC
Keywords
Squamous cell carcinoma, Integrin, Extracellular matrix
Introduction
Squamous cell carcinoma (SCC) comprises more than 90% of all malignancies that occur in the oral cavity. Statistical evaluation for oral cancer so difficult to understand, the Couse related to malignant cancer reporter frequently record oral and pharyngeal cancers collectively. Additionally, perception with intraoral and lip vermilion cancers may not easily made, oral and pharyngeal tumor risk become more with increase in age, especially in males [1]. invasive OSCC is the common cancer that mostly affect the oral cavity that develops from carcinoma in situ or non-invasive dysplastic precursor lesions Also, OSCC can metastasis into regional lymph node and into distant place and organ [2]. The oral stratified Squamous epithelium have intraas well as extra- cellular matrix unit for binding that provide extraordinarily strong intercellular attachment, including cadherins, desmosomes and that locate in matrix layer part and (ECM) which is comprise fibronectin and integrin. There’s different type of study done to understand the role and determined the correlation of attachment between the ECM and tumor cells [3]. Additionally, Malignant transformation is joined by abnormal and irregular protein glycosylation. alike alterations occur in the glycan construction as well as take place in integrin structure, which consider a great family group of the cell surface receptors for the (ECM) [4]. Immunohistochemical studies showed that most type of integrin participate in the cancer invasion process and its advancement and Metastasis of (OSCC), which occur largely and faster when the capacity of cells adherence decreased [3].
Materials and Methods
In this study 17 case of (FFPE) block of OSCC was used with their clinical data are collected from the repository of department of oral pathology laboratory / college of dentistry/Baghdad University, and from alshaheed ghazi hospital in medical city/Baghdad, haematoxylin and eosin (H &E) stain used and another charged slide stained immunohistochemcally by anti integrine B4 antibody.
Histology
One slide was stained with (H &E) for microscopical examination to reevaluate the grades of OSCC.
Immunohistochemistry
Slide was obtained from the each (PEFF) block and stained immunohistochemically by antiintegrin B4 antibody and the cervical cell carcinoma used as positive control (Figure 1).
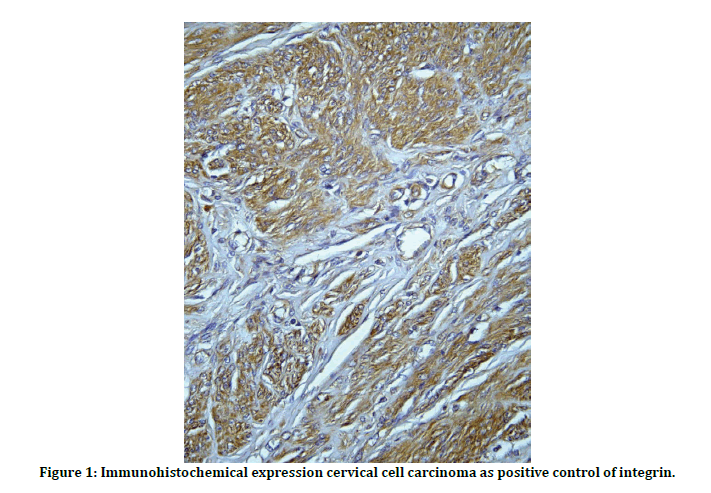
Figure 1: Immunohistochemical expression cervical cell carcinoma as positive control of integrin.
so immunohistochemical staining made according to this step:
✓ Slide backing in the hot air oven at (65 C overnight).
✓ The step deparaffinization and rehydration include that ( at first Xylene jar was deposit in oven for 15 minutes before the use to obtain the same temperature of the incubated slides which were baked for 2 hours at 60˚C ) then (dipped Twice in Xylene for 5 minutes each), (Twice in absolute ethanol for 3 minutes each) ,(95% ethanol for 3 minutes), (75% ethanol for 3 minutes), (50% ethanol for 3 minutes), and in (Distilled water for 3 minutes). Hydrogen peroxide block were applied and incubated the slide in humid chamber for (10 minutes at 37oC), after that immersed in buffer twice (5minutes for each).
✓ Antigen retrieval: Is defined as a Heatinduced epitope retrieval (HIER) which include hot path in which slides was deposit in coupling jar contain citrate buffer solution (PH 6.0) and put the jar in boiling water bath (for 55 minute), left to cool down for (20 minute) at temperature of room, after that all slides were immersed in jar of distilled water for (5 minute) and blotted carefully.
✓ Protein block were applied to then slide incubated at (37oC for10 minutes), then washed (2 times) in buffer (5minutes for each), drained and blotted kindly.
✓ Diluted primary (Ab) added to slides, subsequently incubated for overnight in humid chamber (at 37oC), in the early time following day slides were washed in (buffer 4 times) (5minutes for each), at end drained kindly.
✓ Secondary anti body (link antibody yellow drops) applied to and put in humid chamber for (20 minutes at 37ºC incubated), slides washed (4 times in buffer 5minutes for each), drained and blotted.
✓ Applied Streptavidin-HRP antibodies and incubated for (20 minutes at37ºC incubated), washed for (4 times in buffer 5minutes for each one), drained and blotted.
✓ Diluted DAB was added in (dark room) and in humid chamber for (10 minutes) at (37ºC incubated), washed attentively for (5 minutes) in tap water.
✓ Bathed all slides for (30 seconds) in Hematoxylin stain, flushed for (10 minutes) in the tap water.
✓ At last Dehydration made by (ethanol and Xylene) as this step (50% ethanol for 3 minutes), (75% ethanol for 3 minutes), (95% ethanol for (3 minutes, (Twice in absolute ethanol for 3minutes each) (Xylene for 5 minutes), (Fresh xylene for 5 minutes). Finally DPX mounting medium applying it to Xylene and then covered by using cover slips and remain for overnight to dry.
So Immunohistochemical staining for intra sytoplasmic integrin was evaluated and scored by checking pattern of intensity of staining were scored on a four-point semi-quantitative scale: 3=Strong staining in a normal distribution; 2=Patchy or heterogeneous staining; 1=Weak and fragmented staining; 0=Loss of staining [5].
Statistical analysis
The results were statistically compared using the Monte Carlo test for qualitative variables p values less than 0.05 were taken as significant. All analyses were performed using SPSS® software.
Results
Highest proportion of sample was aged > 50 years (70.59%) in relation to gender, proportion of a male to female ratio of 1.4:1. the most predominant grade was well differentiated OSCC is 9 cases and about (52.94%) of lesions, lasted with poorly differentiated OSCC (11.96%). As show in Table 1. statistically there’s highly significant appear MCP <0.01 , and this show the positive expression of integrin increase with the PDSCC Table 2 and when explain the number and percentage of positive cases show that 7 cases positive from 9 cases and account 77.8 % in WDCC, and positive score account 100 % in MDSCC and PDSCC , this reveal that the expression of integrin increase with progression of grade as in Table 3, immunohistochemicacl expression of integrin in OSCC showed that and the cytoplasmic positive expression of integrin appear as light brown in WDSCC as in (Figure 2) the intensity of staining increase in MDSCC as shown in (Figure 3), and appear as a very dark brown color in PDSCC (Figure 4).
Table 1: Clinicopathological distribution of study sample.
| Variable | No.(n=17) | 100% | |
|---|---|---|---|
| Age(year) | (≤ 50) | 5 | 29.4 |
| (>50) | 12 | 70.6 | |
| Sex | Male | 10 | 58.8 |
| Female | 7 | 41.2 | |
| Site | Antrear part of palate | 1 | 5.9 |
| Buccal mucosa | 4 | 23.5 | |
| Floor of the mouth | 4 | 23.5 | |
| Lower lip | 1 | 5.9 | |
| Mandible &Teeth mass | 1 | 5.9 | |
| Maxilla with Teeth | 1 | 5.9 | |
| Tongue | 5 | 29.4 | |
| Well | 9 | 52.9 | |
| Grade | Moderately | 6 | 35.3 |
| Poorly | 2 | 11.8 |
Table 2: Comparison of integrin intracytoplasmic staining in relation to histopathological grade.
| Grade | Intracytoplasmic integrin expression | Total | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| Well differentiated SCC | No. | 2 | 6 | 1 | 0 | 9 |
| % | 100.00% | 100.00% | 16.70% | 0.00% | 52.90% | |
| Moderately differentiated SCC | No. | 0 | 0 | 4 | 2 | 6 |
| % | 0.00% | 0.00% | 66.70% | 66.70% | 35.30% | |
| Poorly differentiated SCC | No. | 0 | 0 | 1 | 1 | 2 |
| % | 0.00% | 0.00% | 16.70% | 33.30% | 11.80% | |
| Total | No. | 2 | 6 | 6 | 3 | 17 |
| % | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% | |
MCP <0.01
Table 3: The number and percentage of positive and negative cases.
| Inracytoplasmic integrin | ||||||
|---|---|---|---|---|---|---|
| Positive | Negative | Total | ||||
| Grade | No. | % | No. | % | No. | % |
| WDSCC | 7 | 77.8 | 2 | 22.2 | 9 | 100 |
| MDSCC | 6 | 100 | 0 | 0 | 6 | 100 |
| PDSCC | 2 | 100 | 0 | 0 | 2 | 100 |
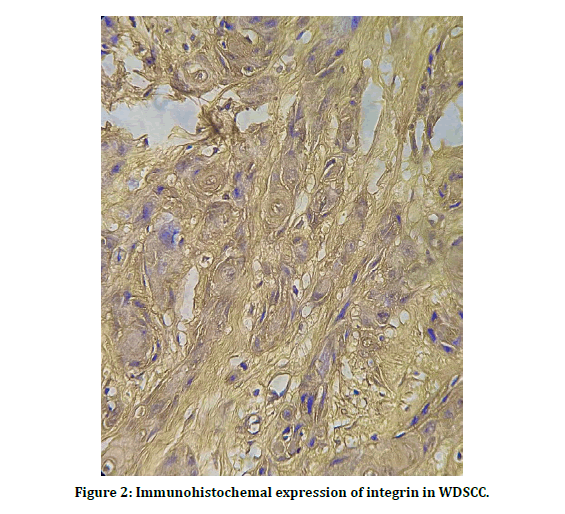
Figure 2: Immunohistochemal expression of integrin in WDSCC.
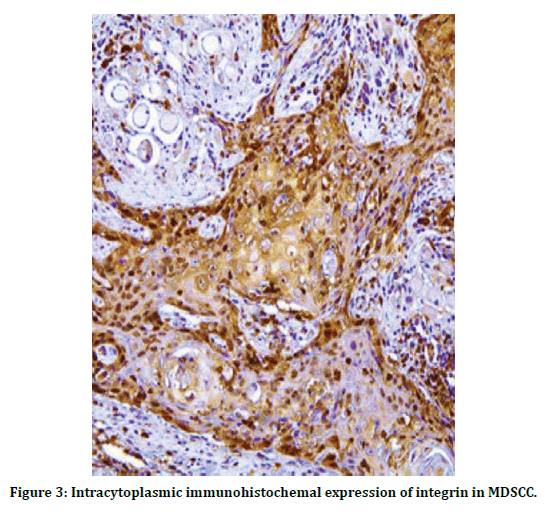
Figure 3: Intracytoplasmic immunohistochemal expression of integrin in MDSCC.
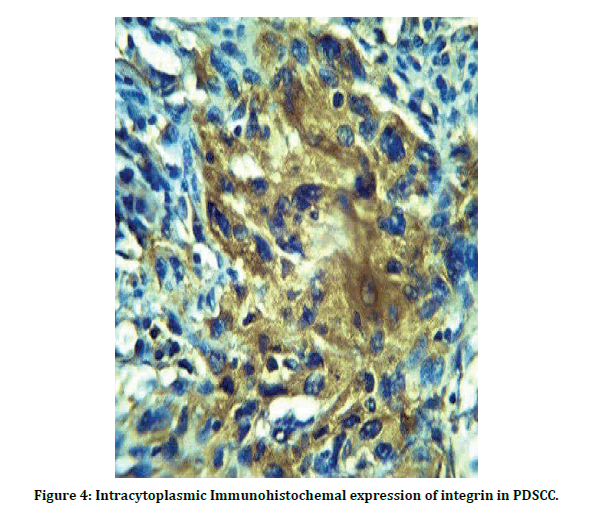
Figure 4: Intracytoplasmic Immunohistochemal expression of integrin in PDSCC.
Discussion
Integrin is a very important (ECM) adhesion molecule act as membrane mediators that found epithelial basal layer as small unit [3]. There is numerous of research have been made that use the integrin as a marker of tumor [6,7].
In the present study statically found there is highly significant correlation between the intra cytoplasmic integrin expression and grade of OSCC , so in this study obtain that with the lesions deterioration the intensity of integrin increase ; as this Finding agree with pervious study which showed that the expression of b4 is correlated with the degree of differentiation , the integrin expression more increase in the SCC and become more intense high grade type , in addition to different kind of solid tumor [8]. almost studies show increases expression with grade, beside to rise expression, alteration in the distribution of β4 additionally related to the grade of cancer [9- 11]. But this finding does not agree with a little number of studies on same kind of cancer that show the expression.
Of integrin in the lesion periphery was decreased as the degree of cancer worsening increased. Therefore, all these studies showed that focal loss of expression of b4 integrin was a feature of the most poorly differentiated tumors [3,5,12,13]. Because participate with cell-to-cell binding instead of with the ECM [3]. Therefore, the conflicting results revealed from Studies examining integrin β4 expression in patientobtained tissue, in many types of cancer. It is unclear whether these contradictory findings may be relating to sample size, cancer subtype examined, or the method of detection [14]. So that Different mode integrin expression has value in prognosis, and the expression of integrin have been indicated a different type of tumor like that and head and neck (HNC) increase expression been associated with poor prognosis [15,16] in skin SCC’ [17], However, in several other cases, poor prognosis is associated with a loss of integrin. There was a significant association between loss of integrin in colon carcinomas [18] and also associated with loss of tumor differentiation in colorectal adenocarcinomas [19] and increase or decrease expression in carcinoma of breast [20] so that in conclusion of the present study , the integrin intensity increase with lesions worsening, and this may explained by that , integrin may assist in the invasion process advancement , so the recommendation for this study is the integrin in OSCCC can be used as malignancy and prognostic Marker.
References
- Neville BW, Damm DD, Chi AC, et al. et al. Oral and maxillofacial pathology. 4th Edn, Elsevier Health Sciences, Canada 2016.
- Zargaran M, Eshghyar N, Vaziri PB, et al. Immunohistochemical evaluation of type IV collagen and laminin‐332 γ2 chain expression in well‐differentiated oral squamous cell carcinoma and oral verrucous carcinoma: a new recommended cut‐off. J Oral Pathol Med 2011; 40:167-73.
- Ohara T, Kawashiri S, Tanaka A, et al. Integrin expression levels correlate with invasion, metastasis and prognosis of oral squamous cell carcinoma. Pathol Oncol Res 2009; 15(3), 429–436.
- Kariya Y, Kariya Y, Gu J. Roles of integrin α6β4 glycosylation in cancer. Cancers 2017; 9:79.
- Bagutti C, Speight PM, Watt FM. Comparison of integrin, cadherin, and catenin expression in squamous cell carcinomas of the oral cavity. J Pathol 1998; 186:8-16.
- Kennel SJ, Foote LJ, Falcioni R, et al. Analysis of the tumor-associated antigen TSP-180. Identity with alpha 6-beta 4 in the integrin superfamily. J Biol Chem 1989; 264:15515–15521.
- Van Waes C, Kozarsky KF, Warren AB, et al. The A9 antigen associated with aggressive human squamous carcinoma is structurally and functionally similar to the newly defined integrin alpha 6 beta 4. Cancer Res 1991; 51:2395–2402.
- Stewart RL, O’connor KL. Clinical significance of the integrin α6β4 in human malignancies, lab. Investig J Tech Methods Pathol 2015; 95:976–986.
- Tennenbaum T, Weiner AK, Belanger AJ, et al. The suprabasal expression of alpha 6 beta 4 integrin is associated with a high risk for malignant progression in mouse skin carcinogenesis. Cancer Res 1993; 53:4803–4810.
- Aplinj D, Dawson S, Seif MW. Abnormal expression of integrin alpha 6 beta 4 in cervical intraepithelial neoplasia. Br J Cancer 1996; 74:240–245.
- Masugi Y, Yamazaki K, Emoto K, et al. Upregulation of integrin β4 promotes epithelial–mesenchymal transition and is a novel prognostic marker in pancreatic ductal adenocarcinoma. Lab Invest 201; 95:308–319.
- Jones J, Sugiyama M, Watt FM, et al. Integrin expression in normal, hyperplastic, dysplastic, and malignant or epithelium. J Pathol 1993; 169:235–243.
- Downer CS, Watt FM, Speight PM. Loss of a 6 and b 4 integrin subunit coincides with the loss of basement membrane component in oral SCC. J Pathol 1993; 17:183- 190.
- Diaz LK, Cristofanilli M, Zhou X, et al. Beta4 integrin subunit gene expression correlates with tumor size and nuclear grade in early breast cancer. Mod Pathol Off J 2005; 18:1165–1175.
- Carey TE, Wolf GT, Hsu S, et al. Expression of A9 antigen and loss of blood group antigens as determi- nants of survival in patients with head and neck squamous carcinoma. Otolaryngd Neck Surg 1987; 9:221-230.
- Wolf GT, Carey TE, Schmaltz SP, et al. Altcred antigen exprebsion predicts outcome in squamous cell carcinoma of the hcad and neck. J Natl Cancer Insi 1990; 82:1566-1572
- Peltonen J, Larjava H, Jaakkola S, et al. Localization of integrin receptors for fibronectin, collagen, and laminin in human skin. Variable expression in basal and squamous cell carcinomas. J Clin Inves 1989; 1916-1923.
- Koretz K, Schlag P, Boumsell L, et al. Expression of VLA-a, VLA-a, and VLA-8, chains in normal mucosa and adenomas of the colon, and in colon carcinomas and their liver metastases. Am J Pathol 1991; 138:741-750
- Pignatelli M, Smith MEF, Bodmer WF. 1990. Low expression of collagen receptors in moderate and poorly differentiated colorectal adenocarcinomas. Br J Cancer 1990; 61:636-638.
- Koukoulis GK, Virtanen I, Korhonen M, et al. Imniunological distribution of hasement membrane in oral SCC. Head Neck 1994; 16:5.
Author Info
Fatima Ghazi Al-Bayati* and Layla Sabri Yas
Department of Oral and Maxillofacial Pathology, Dental Teaching Hospital, College of Dentistry, University of Baghdad, IraqCitation: Fatima Ghazi Al-Bayati, Layla Sabri Yas, Immunohistochemical Expression of Integrin in OSCC in Relation to Tumor Grade, J Res Med Dent Sci, 2021, 9 (4):291-296.
Received: 24-Mar-2021 Accepted: 12-Apr-2021
