Research - (2021) Volume 9, Issue 10
Histological Evaluation Effect of Local Application of Vitamin D3 on Bone Formation in Rabbits
Noor Abdulkareem Razouki1* and Ban A.Ghani Jamil2
*Correspondence: Noor Abdulkareem Razouki, AL-Rafidain University Collage, Iraq, Email:
Abstract
Bone is a highly specialized form of connective tissue that is nature’s provision for an internal support system in all higher vertebrates, its formation and regeneration is still desiring research zone and using the tissue engineering approach that depends on the various types of interactions between the cells, scaffolds, and growth factors. Vit.D3 is a fat-soluble hormone, calcitriol is active form of Vit.D3, can affect osteoblast growth and differentiation stimulating bone formation and mineralization, no consensus exists regarding the applicable benefit of local application in bone defect healing which still controversy. Twenty-four adult male New Zealand rabbits weighting an average of (1.5–2 kg) used in this study. One intra bony holes created in both tibias (right tibiae for experimental groups and left for control group) of each animal which will be divided as follows: Group I(C): Bone defect left to heal spontaneously as control. Group II (Vit.D3): Bone defect filled with Vit.D3. Animal scarification was done for the healing durations (7 and 21days). Routine processing and sectioning technique was performed for histological evaluation. The histological finding in the bone defect area filled with Vit.D3 shows fine separated bone spicules rimmed by osteoblast and some embedded osteocytes. VIT.D3 group after 21 days shows defect area with many bone trabeculae surround bone marrow, and primary harversian canals start to organized. At 7 day duration of healing period histomorphometrical study shows highly significant difference between (C) and (Vit.D3) for all measured parameters. Regarding 21days duration high significant differences were estimated in all bone parameters in between groups, except trabecular number there was no significant difference between C and Vit.D3 experimental groups. Conclusion: local Vit.D3 application may accelerate new bone formation.
Keywords
Vitamin D3, Bone formation
Introduction
Osteogenesis is highly dependent on the substrate carrier used, which has to provide a favourable environment into which bone cells can migrate before proliferating, differentiating, and depositing bone matrix [1]. Biomaterials have different groups but we focus here on the naturally derived types that present already in the body from natural origin like Vit.D3 [2]. It is the only vitamin supposed to have a behaviourally hormonal function, since its pathway of molecular alteration produces active metabolites and its mechanism of action is analogous to that of steroid hormones [3]. Calcitriol is active form of Vit.D3, can affect osteoblast growth and differentiation stimulating bone formation and mineralization [4]. Calcitriol significantly accelerated the production of mature matrix vesicles in the premineralization. It’s role on osteoblast via increased production of mature matrix vesicles in the period proceeding to mineralization. The augmented fabrications of mature matrix vesicles lead to an earlier onset and advanced rate of mineralization. These effects are independent of alterations in extracellular matrix protein composition [5]. The action of Vit.D3 is initiated through link to Vit.D3 receptors VDRs, which are nuclear and which have a greater affinity for calcitriol than other Vit.D3 Metabolites.
These receptors activate the formation of a complex with the retinoic acid X RECEPTOR (RXR), resulting in a heterodimeric binding to calcitriol, which will finally bind to DNA.
This interaction enables transcription, which is the synthesis of messenger RNA that involves certain proteins such as alkaline phosphatase in the osteoblasts, osteocalcin [3,6]. Studies didn’t found the corresponding clinical or microscopic side effects after local calcitriol application to concur the previous evidence [7]. The aim of this study was histological evaluation of effectiveness local application of active form of Vit.D3 in bone defect in rabbit’s tibia.
Materials and Methods
Twenty-four adult male New Zealand rabbits weighting (1.5–2 kg) were used in this study. The general anesthesia by intramuscular injection of xylazine 20mg (Arendonk, Belgium) (0.2 ml/kg-B.W.) plus ketamine HCL 50 mg (Holland) (20 mg/kg B.W.) [8,9]. The operation site was the proximal tibia metaphysis of the right limb(2) . Expose tibia and initial intermittent drilling was done by small round bur (no.010) and fissure bur (no. 010) with speed 1500 rpm and vigorous irrigation by normal saline, the drilling accomplished by round and fissure bur (no.012) to diameter of about 3mm and depth 4 mm. Four intra bony holes were created in both tibias of each animal divided as follows:
• Group C: Bone defect left to heal spontaneously as control.
• Group Vit.D3: Bone defect filled with active form Vit.D3 (Calcitriol) (Turkey).
Animal scarification done by over dose of general anesthesia and specimens immediately stored in 10% freshly prepared formalin (Tedia, U.S.A) for fixation ,bone decalcification was performed by using 10% formic acid then processed to paraffin blocks as a bone defect faced outside of block toward microtome knife. Tissue sections of 5 μm thickness were performed followed by Hematoxylin and Eosin stain procedure, histological and histomorphometrical evaluation using light microscope (OpticaB-350, Italy). The following microarchitectures were measured.
Bone cells number counting
• Osteoblast cell number (OB/ mm2).
• Osteocyte cell number (OC/ mm2).
• Osteoclast cell number (OCL/ mm2).
Trabecular and bone marrow area
• Trabecular area (mm2).
• Bone marrow area (mm2).
• Trabecular number (mm2).
Measurement of trabecular and bone marrow area was performed by software program (ImageJ.exe). Statistical analysis of recorded data was done by (ANOVA) test.
Results
After surgical procedure, all animals monitored and no signs of any negative tissue reaction as infection or fractures were recorded at surgical sites throughout the healing intervals. One rabbit only passes away and was substituted by other.
Histological and histomorphometrical findings
Histological findings at (7 days) duration of defect area in control group shows bone marrow with many inflammatory cells and bone marrows stromal cells Figure 1. The histological finding in the bone defect area filled with Vit.D3 shows fine separated bone spicules rimmed by osteoblast and some embedded osteocytes Figure 2.
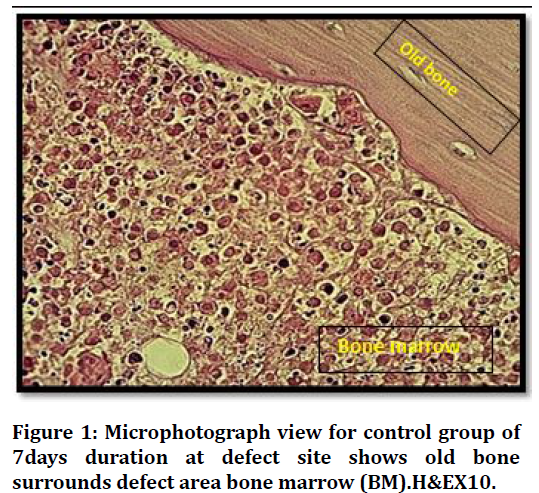
Figure 1. Microphotograph view for control group of 7days duration at defect site shows old bone surrounds defect area bone marrow (BM).H&EX10.
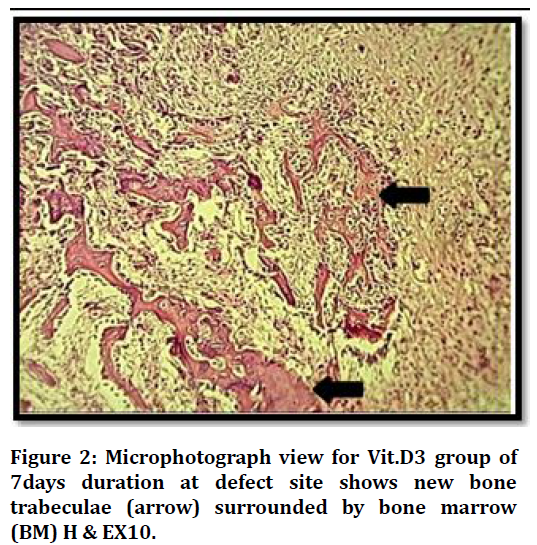
Figure 2. Microphotograph view for Vit.D3 group of 7days duration at defect site shows new bone trabeculae (arrow) surrounded by bone marrow (BM) H & EX10.
Histological View at (21 days) duration in control group shows bone marrow with progenitor cells, osteoblasts rimming bone and osteocytes embedded Figure 3.
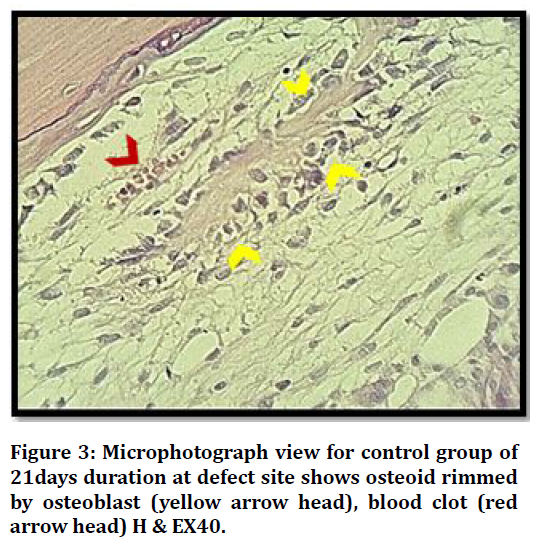
Figure 3. Microphotograph view for control group of 21days duration at defect site shows osteoid rimmed by osteoblast (yellow arrow head), blood clot (red arrow head) H & EX40.
Histological results of VIT.D3 group after 21 days shows defect area with many bone trabeculae surround bone marrow, and primary harversian canals start to organize Figure 4.
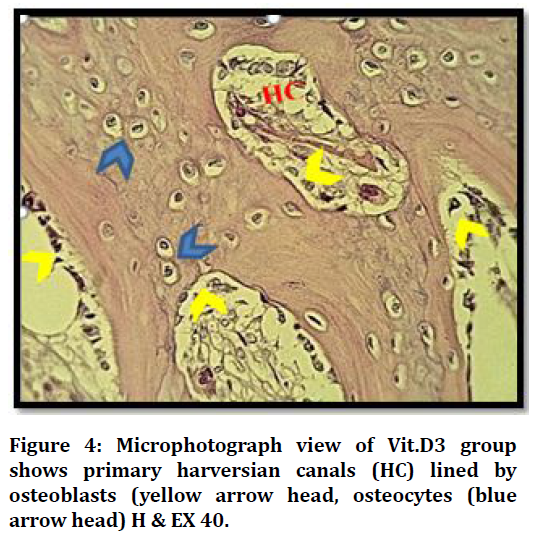
Figure 4. Microphotograph view of Vit.D3 group shows primary harversian canals (HC) lined by osteoblasts (yellow arrow head, osteocytes (blue arrow head) H & EX 40.
Concerning histomorphometrical analysis, the mean values of trabecular number, bone marrow area, trabecular area, osteoblasts, osteocytes and osteoclasts showed highly significant difference in all groups between both durations (7days and 21days). All measured parameters in both durations had increased mean value with time except for bone marrow area and osteoblasts decreased in all groups, the highest mean value of all measured parameters were recorded in (Vit.D3) group except bone marrow area higher in (C) group.
At 7 day duration of healing period histomorphometrical study shows highly significant difference between (C) and (Vit.D3) for all measured parameters.
Regarding 21days duration high significant differences were estimated in all bone parameters in between groups, except trabecular number there was no significant difference between C and Vit.D3 experimental groups (Figure 5 to Figure 6).
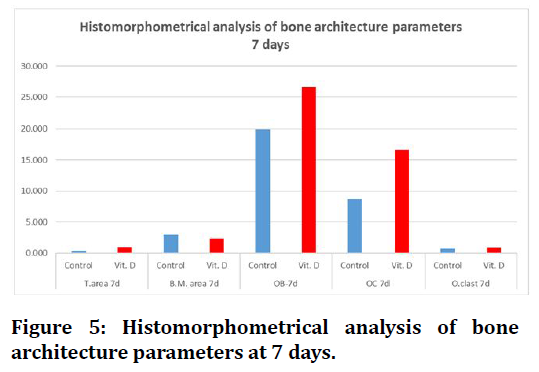
Figure 5. Histomorphometrical analysis of bone architecture parameters at 7 days.
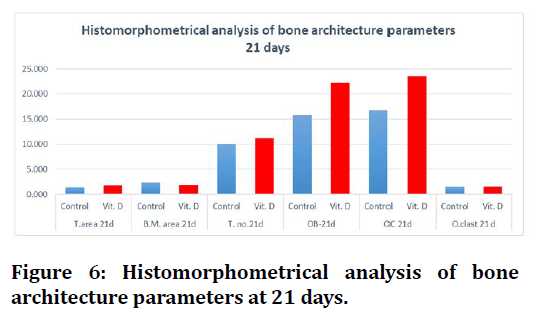
Figure 6. Histomorphometrical analysis of bone architecture parameters at 21 days.
Discussion
Rabbits are one of the most generally appropriate animal models, and it orders first among all the animals chose for skeletal research. Rabbits are in practice, adaptable, not ignored species for bone therapeutic trials [10].
The choose of Vit.D3 in this study was for the following reasons:
• In vitro studies and systemic administration, many articles indicate role of Vit.D3 on bone cells and bone healing but in vivo little researches stated role of direct application on induced bone defect healing.
• Use of endogenous and affordable biomaterial to induce bone formation is still demanded.
Histological findings of this study showed bone deposition almost filling defect site in almost all examined sections could be explained according to previous vitro studies which revealed that the vitamin D receptor is present in osteoblasts and that calcitriol directly affects the ability of osteoblasts to regulate the expression of several genes that affect osteogenesis. Subsequently, the proliferation of osteoblasts and the production of type I collagen, alkaline phosphatase and osteocalcin intensify; these verifications provide further support for the regulatory function of the vitamin D3 in bone formation and mineralization [11,12].
The Vit.D3 receptor (VDR) are principally involved in mineral metabolism, is expressed in osteoblasts (12). Vit. D3 suppresses bone resorption through VDR in osteoblast-lineage cells and that the role of Vit.D3 in stimulating osteoblast development through exerting anti-apoptotic effects ,Vit.D3 increased the expression of key osteogenic signalling proteins; specifically Runx2 [13] which may support results of this study regarding Vit.D3 group as compared to control group in 7 and 21 days healing intervals.
In controversy to this study, Fügl et al. stated a single local calcitriol application in rats femurs’ does not enhance healing [14] this may related to differences in species and site of operation.
Conclusion
Within the restrictions of this study the observed histological results indicated that local Vit.D3 application may accelerate new bone formation.
References
- Ono I, Tateshita T, Kuboki Y. Prostaglandin E1 and recombinant bone morphogenetic protein effect on strength of hydroxyapatite implants. J Biomed Materials Res 1999; 45:337-44.
- Mahmood RAJ, Razouki NA. Comparative study between the effect of melatonin and hyaluronic acid on induced bone defect healing in rabbit. Medico Legal Update 2020; 20:1271-1276.
- de Freitas RP, Nunes FP, dos Santos LM, et al. Influence of vitamin D in bone healing. J Oral Diagnosis 2017; 2:1-8.
- Posa F, Di Benedetto A, Colaianni G, et al. Vitamin D effects on osteoblastic differentiation of mesenchymal stem cells from dental tissues. Stem Cells Int 2016; 2016.
- Woeckel VJ, Alves R, Swagemakers S, et al. 1Alpha,25-(OH)2D3 acts in the early phase of osteoblast differentiation to enhance mineralization via accelerated production of mature matrix vesicles. J Cellular Physiol 2010; 225:593-600.
- Al-Suhaimi EA, Al-Khater KM, Aljafary MA, et al. Vitamin D as therapeutic agent acting against cancers caused by proteases. Cancer-leading proteases. Elsevier 2020; 417-48.
- Hong HH, Yen TH, Hong A, et al. Association of vitamin D3 with alveolar bone regeneration in dogs. J Cell Mol Med 2015; 19:1208-17.
- Razouki NA, Ghani BA. Histological evaluation of effect of beta-tricalcium phosphate on bone healing in alloxan-induced diabetes. J Baghdad College Dent 2016; 325:1-7.
- Razouki NA, Mahmood RAJ, Mahdi HA, et al. Histological evaluation the effectiveness of aloe vera oral gel application on gingiva subjected to dental lightâ??emitting diodes. Indian J Forensic Med Toxicol 2020; 14:822-826.
- Li Y, Chen SK, Li L, et al. Bone defect animal models for testing efficacy of bone substitute biomaterials. J Orthop Translation 2015; 3:95-104.
- Van Leeuwen J, Van Driel M, Van Den Bemd G, et al. Vitamin D control of osteoblast function and bone extracellular matrix mineralization. Critical Rev Eukaryotic Gene Exp 2001; 11.
- Zarei A, Morovat A, Javaid K, et al. Vitamin D receptor expression in human bone tissue and dose-dependent activation in resorbing osteoclasts. Bone Res 2016; 4:16030.
- Al Saedi A, Myers D, Stupka N, et al. 1, 25 (OH) 2D3 ameliorates palmitate-induced lipotoxicity in human primary osteoblasts leading to improved viability and function. Bone 2020; 115672.
- Fügl A, Gruber R, Agis H, et al. Alveolar bone regeneration in response to local application of calcitriol in vitamin D deficient rats. J Clin Periodontol 2015; 42:96-103.
Author Info
Noor Abdulkareem Razouki1* and Ban A.Ghani Jamil2
1AL-Rafidain University Collage, Baghdad, Iraq2Department of Oral Diagnosis, College of Dentistry, University of Baghdad, Baghdad, Iraq
Citation: Noor Abdulkareem Razouki, Ban A.Ghani Jamil,Histological Evaluation Effect of Local Application of Vitamin D3 on Bone Formation in Rabbits, J Res Med Dent Sci, 2021, 9(10): 148-151
Received: 07-Jul-2021 Accepted: 05-Oct-2021
