Research - (2023) Volume 11, Issue 1
Healing effects of agomelatine on methotrexate-induced Kidney damage in rats
Nurhan Gumral1, Ozlem Ozmen2, Rahime Aslankoc1, Arzu Yalcin1* and Oguzhan Kavrik1
*Correspondence: Arzu Yalcin, Department of Physiology, Faculty of Medicine, Süleyman Demirel University, Turkey, Email:
Abstract
MTX is a chemotherapeutic agent widely used in the treatment of some types of cancer (as in acute lymphoblastic leukemia and lymphoma) as well as inflammatory disorders as rheumatoid arthritis. Agomelatonin is a molecule synthesized and released in the pineal gland as a synthetic anologue of the hormone melatonin. Agomelatonin has a strong agonist effect on the melatonin hormone receptors MT1 and MT2. Twenty-four 3-4 months old male Wistar albino rats were randomly divided into three groups of eight rats. MTX group was administered a single dose of 20 mg/kg MTX i.p. on the second day of the experiment. Ago group a single dose of 40 mg/kg AGO was given by oral gavage for 7 days. AGO group, BUN and creatine levels decreased statistically significantly compared to the MTX group. There was a statistically significant increase in TAS levels in the AGO group compared to the MTX group (p=0.001). There was a statistically significant decrease in the TOS levels of the AGO group compared to the MTX group (p=0.046). Immunohistochemically increase in iNOS, HSP-70, OPN and CGSF immunoreactions in both epithelial and mesenchymal cells of the kidneys were observed in the MTX group. Treatment of rats with AGO significantly reduced MTX-induced renal toxicity. Finally, the results suggest that AGO has a protective effect against MTX-induced oxidative stress renal toxicity.
Keywords
Methotrexate, Agomelatine, Kidney damage, Renal toxicity
Introduction
Methotrexate (MTX) is an antimetabolite compound that binds to the enzyme dihydrofolate reductase (DHFR) and inhibits the enzyme, preventing the conversion of dihydrofolate to tetrahydrofolate and blocking DNA synthesis [1]. MTX is a chemotherapeutic agent widely used in the treatment of some types of cancer (as in acute lymphoblastic leukemia and lymphoma) as well as inflammatory disorders as rheumatoid arthritis [2]. These drugs can cause side effects such as immunosuppression, cardiological (arrhythmia), and gastrointestinal disorders (ie nausea-vomiting), psychological disorders such as depression [3]. Agomelatonin (AGO) was synthesized at the Servier Institute during studies to develop new and safe antidepressant drugs. It is a molecule synthesized and released in the pineal gland as a synthetic anologue of the hormone melatonin. Agomelatonin has a strong agonist effect on the melatonin hormone receptors MT1 and MT2. It is also thought to antagonize the serotonin receptor 5-HT2C receptor. In addition, agomelatonin has no interaction with adrenergic, cholinergic and histaminergic receptors. Studies have shown that agomelatonin shows antioxidant effect and prevents oxidative stress [4].
The aim of this study was to develop a new treatment model that prevents toxicity in kidney tissue after MTX administration. In particular, we aimed to determine whether AGO decreases oxidative damage and increases antioxidant activity in the experimental MTX model.
Material-Method
Chemicals
MTX (50 mg/mL flk; Koçak, Turkey) and Valdoxan tablets containing 25 mg AGO (Servier Industries, Wicklow Arklow/Ireland) were obtained from the local pharmacy. AGO was dissolved in saline (0.9% NaCl) solution. Saline solution was given to the control group. Serum BUN, creatine, uric acid were analyzed using an enzyme-linked immunosorbent assay (ELISA) kit (LOT 187 861-03, Germany). All other chemicals for MDA, SOD, CAT and GPx were obtained from Sigma-Aldrich (Germany).
Animal groups and drug protocols
Twenty-four 3-4 months old male Wistar albino rats weighing 200-250 g were obtained from Mehmet Akif Ersoy University Experimental Research Unit (Burdur, Turkey). The experimental procedure was approved by Mehmet Akif Ersoy University Animal Experiments Local Ethics Committee (Ethics No: 303, June 2017/07). National and international laws on the care and use of laboratory animals were followed in the treatment and care protocols of all rats. Rats were kept under standard laboratory conditions (temperature 21 ± 2°C, humidity 60 ± 5%, 12/12 h light-dark cycle). All animals were fed standard commercial feed (Korkuteli Feed) containing 88% dry matter (mostly oat hulls), 23% protein, 7% cellulose, 2% ash insoluble in 2% HCl, 11.8% Ca 2%, 0.9% PO-4, 0.5-0.8% Na, 1% NaCl, 0.3% methionine and 1% lysine and had free access to food pellets and water.
The animals were randomly divided into three groups of eight rats in each group;
Grup I (Control): Each rat was injected intraperitoneally (i.p) with saline solution on the second day of the experiment. For 7 days, 0.1 ml of saline (SF) was given by oral gavage as a single dose.
Grup II (MTX): Rats were administered a single dose of 20 mg/kg MTX i.p. on the second day of the experiment. For 7 days, a single dose of 0.1 ml SF was given by oral gavage [5].
Grup III (MTX+AGO): Each rat received a single dose of 20 mg/kg MTX i.p. on the second day. A single dose of 40 mg/kg AGO was given by oral gavage for 7 days [6].
All rats were weighed after the experimental procedure. Rats were anesthetized i.p. with 90 mg/kg ketamine (Alfamine, Alfasan, IBV) and 10 mg/kg xylazine (Alfamine, Alfasan, IBV). The rats were decapitated and bled following standard ethical procedures. Afterwards, the kidney tissue was removed bilaterally and weighed. One kidney tissue was placed in 10% formaldehyde solution for histopathologic and immunohistochemical analysis. The other kidney tissue and blood samples were taken for biochemical analysis. Blood samples were centrifuged at 4000 rpm and the supernatant obtained was stored at -80° C for biochemical analysis.
Biochemical analyzes
Oxidant and antioxidant status
Kidney tissue was homogenized in ice-cold phosphate buffer using a homogenizer (IKA Ultra-Turrax T25 Basic; Labortechnic, Staufen, Germany) and sonicator (UW- 2070 Bandelin Electronic, Germany). Protein levels in the samples obtained after homogenization were determined using the Bradford method.
Total oxidant status (TOS) was determined by a calorimetric method developed by Erel. Rel Assay commercial kits and hydrogen peroxide as calibrator were used. In an acidic environment, the oxidizing agent present in the samples can oxidize Fe+2 to Fe+3, which binds with high affinity with xylenol orange to form a blue-violet molecular complex. When the pH of this solution is in the range 2-3, it can be measured on a spectrophotometer by setting the wavelength to 590 nm. In order to indirectly calculate the depth of the color, it was proportioned to the content of oxidizing agents at a given time and concentration. The color formed in the samples can be measured with a spectrophotometer. The unit was expressed as μmol H2O2 Eqv/L.
Total antioxidant status (TAS) was determined by calorimetric method developed by Erel. Trolox was used as a reference substance. Trolox has an antioxidant status similar to vitamin E and is an analog of vitamin E. ABTS is reduced to colorless ABTS in the presence of antioxidants and to green ABTS in the presence of oxidants. The color at which antioxidants in samples convert ABTS can be measured at 660 nm in a spectrophotometer. The unit of the measured substance was expressed as Trolox equivalent/L.
Serum BUN, creatine, uric acid level
Serum samples were collected for determination of serum BUN, creatine, uric acid. Serum BUN, keratin, uric acid levels were analyzed by ELISA kit (Elabscience, Shanghai, China) according to the manufacturer's instructions. The sensitivity of this test was 18.75 pg/ml. The intra- and inter-assay CV was less than 10%. 50 μl was used for this assay.
Histopathological analyses
During the necropsy, kidney samples were fixed in 10% buffered formalin, routinely processed by an automatic tissue processor equipment (Leica ASP300S, Wetzlar, Germany), paraffin embedded, cut 5μm by a Leica 2155 rotary microtome (Leica, Wetzlar, Germany) and then stained with hematoxylin and eosin (HE) to be interpreted by light microscopy. Histopathological changes were graded in a blinded manner. Hyperemia, edema, inflammatory reaction, degeneration, necrosis at tubular epithelium and proteinous material in tubular lumen were evaluated according the severity of lesions using a 0 – 3 scoring system where 0, normal; 1, slight hyperemia, and slight degeneration in tubular epithelial cells; 2, mild to severe degeneration and inflammatory reaction; 3, necrosis at tubular epithelium, proteinous material in lumen and severe inflammatory reactions.
Immunohistochemical analyses
Kidney samples were immunostained with primary antibodies. All antibodies purchase from the Abcam, Cambridge, UK and used in 1/100 dilution. Selected tissue sections were immunostained by granulocyte colony stimulating factor [Anti-G-CSF antibody (ab9691)], heat shock protein-70 [Anti-Hsp70 antibody [5A5] (ab2787)], iNOS [Anti-iNOS antibody (ab15323), Osteopontin [Anti-Osteopontin antibody (ab8448), Abcam, UK] antibodies according the manufacturer’s instructions. All the slides were analyzed for immunopositivity and a semiquantitative analysis was carried out. For to evaluate percentage of immune-positive cells for each marker 100 cells counted in 10 different fields for every section at a magnification under X40 objective for all groups. Statistical analyses were subjected of the results obtained from the image analyzer. Morphometric analyses were performed using the Database Manual Cell Sens Life Science Imaging Software System (Olympus Co., Tokyo, Japan).
Statistical analysis
Variables were presented as mean ± standard deviations. ANOVA and Bonferroni Dunn tests were used to compare histopathological and immunohistochemical scores between the groups. Calculations were made using the SPSS 15.0 program pack. p<0.05 was set as the value for significance.
Results
Effects of AGO on MTX-induced changes in serum, BUN, creatine and uric acid levels
The protective effect of AGO on BUN, keratin and uric acid levels is shown in Table 1. BUN, creatine, uric acid levels in all groups were analyzed respectively and statistically significant difference was found between the groups (p=0.003, p=0.003). In the MTX group, BUN level increased statistically significantly compared to the control group (p=0.012). In the AGO group, BUN levels decreased statistically significantly compared to the MTX group (p=0.005). Creatine levels increased statistically significantly in the MTX group compared to the control group (p=0.003). Creatine levels in the AGO group decreased statistically significantly compared to the MTX group (p=0.022). There was no statistically significant difference in uric acid levels in the MTX group compared to the control group. There was no statistically significant difference in uric acid levels in the AGO group compared to the MTX group.
| Control | MTX | AGO | |
|---|---|---|---|
| BUN | 23.5 ± 1.19 | 27.7 ± 2.49a | 23 ± 3.74b |
| Uric acid | 0.7 ± 0.26 | 0.58 ± 0.11c | 0.62 ± 0.34d |
| Creatine | 0.35 ± 0.03 | 0.41 ± 0.03 | 0.36 ± 0.02 |
| *Values are presented as means ± SD. The relationships between groups and results of biochemical analysis are assessed by one-way ANOVA. | |||
| **NS: Statistically non-significant. | |||
| aStatistically significant compared the Control group (p < 0.05). | |||
| bStatistically significant compared the MTX group (p < 0.05). | |||
| cStatistically significant compared the Control group (p < 0.05). | |||
| dStatistically significant compared the MTX group (p<0.05). | |||
Table 1: Statistical analysis results of biochemical analysis.
Changes induced by methoteraxate in TAS and TOS levels in renal tissue
TAS and TOS levels are shown in Table 2. A significant difference was found between the groups in TAS and TOS levels, respectively (p=0.001, p=0.035). There was no statistically significant difference in TAS levels of the MTX group compared to the control group. There was a statistically significant increase in TAS levels in the AGO group compared to the MTX group (p=0.001). There was also a statistically significant difference in TAS levels of the AGO group compared to the control group (p=0.003). There was no statistically significant difference in TOS levels of the MTX group compared to the control group. There was a statistically significant decrease in the TOS levels of the AGO group compared to the MTX group (p=0.046).
| Control | MTX | AGO | |
|---|---|---|---|
| TAS | 2.7 ± 0.35a | 0.48 ± 0.39 | 3.72 ± 0.65b |
| TOS | 8.52 ± 0.95 | 9.59 ± 0.73 | 8.39 ± 0.87c |
| *Values are presented as means ± SD. The relationships between groups and results of biochemical analysis are assessed by one-way ANOVA. | |||
| **NS: Statistically non-significant. | |||
| aStatistically significant compared the AGO group (p < 0.05). | |||
| bStatistically significant compared the MTX group (p < 0.05). | |||
| cStatistically significant compared the MTX group (p < 0.05). | |||
Table 2: Statistical analysis results of biochemical analysis.
Histopathologic Results
At necropsy, kidneys were slight swollen and pale in MTX group. No pathological findings observed in kidneys belonging Control and AGO treated groups. Microscopically, normal kidney architecture was observed in control and AGO groups. At the histopathological examination of the kidneys of rats in the MTX group showed marked hyperemia, slight hemorrhages, tubular cell necrosis and enlargement of Bowman spaces (Figure 1). Because of the no pathological findings in groups except MTX group, lesions attributed the MTX toxicity.
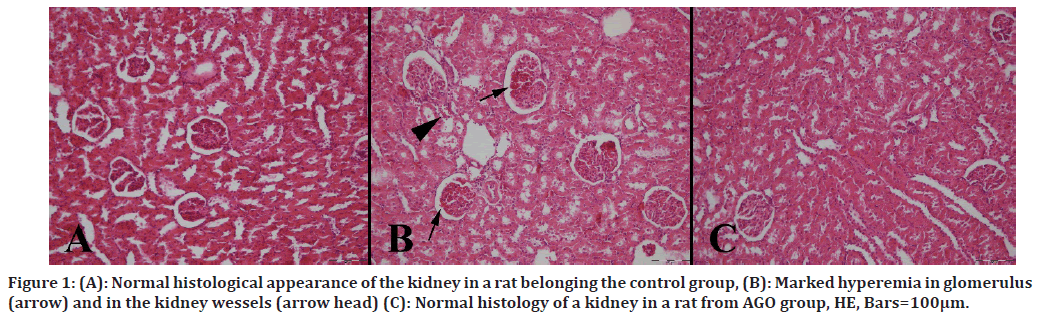
Figure 1. (A): Normal histological appearance of the kidney in a rat belonging the control group, (B): Marked hyperemia in glomerulus (arrow) and in the kidney wessels (arrow head) (C): Normal histology of a kidney in a rat from AGO group, HE, Bars=100μm.
Immunohistochemical Results
Immunohistochemical results are shown in Table 3. Immuno-histochemically increase in iNOS, HSP-70, OPN and CGSF immunoreactions in both epithelial and mesenchymal cells of the kidneys were observed in the MTX group (Figures 2 to Figure 5).
| Control | MTX | AGO | P | |
|---|---|---|---|---|
| Histopathology | 0.00 ± 0.00 | 0.75 ± 0.25a | 0.25 ± 0.16b | CON-MTX (0.05) |
| CON-AGO (NS) | ||||
| MTX-AGO (0.05) | ||||
| CGSF | 0.00 ± 0.00 | 0.87 ± 0.22c | 0.25 ± 0.16d | CON-MTX (0.01) |
| CON-AGO (NS) | ||||
| MTX-AGO (0.05) | ||||
| HSP-70 | 0.00 ± 0.00 | 0.25 ± 0.16 | 0.12 ± 0.01 | CON-MTX (NS) |
| CON-AGO (NS) | ||||
| MTX-AGO (NS) | ||||
| iNOS | 0.00 ± 0.00 | 0.50 ± 0.18e | 0.00 ± 0.00f | CON-MTX (0.05) |
| CON-AGO (NS) | ||||
| MTX-AGO (0.05) | ||||
| OPN | 0.00 ± 0.00 | 1.00 ± 0.26g | 0.25 ± 0.16h | CON-MTX (0.05) |
| CON-AGO (NS) | ||||
| MTX-AGO (0.05) | ||||
| *Values are presented as means ± SD. The relationships between groups and results of histopathological and immunohistochemical scores are assessed by one-way ANOVA. | ||||
| **NS: Statistically non-significant. | ||||
| aStatistically significant compared the Control group (p < 0.05). | ||||
| bStatistically significant compared the MTX group (p < 0.05). | ||||
| cStatistically significant compared the Control group (p < 0.05). | ||||
| dStatistically significant compared the MTX group (p<0.05). | ||||
| eStatistically significant compared the Control group (p < 0.05). | ||||
| fStatistically significant compared the MTX group (p < 0.05). | ||||
| gStatistically significant compared the Control group (p < 0.05). | ||||
| hStatistically significant compared the MTX group (p<0.05). | ||||
Table 3: Statistical analysis results of histopathological and immunohistochemical scoring results of kidney.
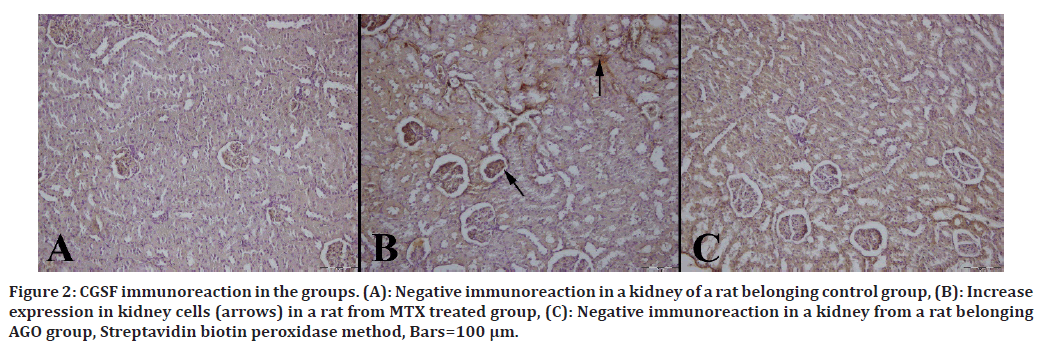
Figure 2. CGSF immunoreaction in the groups. (A): Negative immunoreaction in a kidney of a rat belonging control group, (B): Increase expression in kidney cells (arrows) in a rat from MTX treated group, (C): Negative immunoreaction in a kidney from a rat belonging AGO group, Streptavidin biotin peroxidase method, Bars=100 μm.
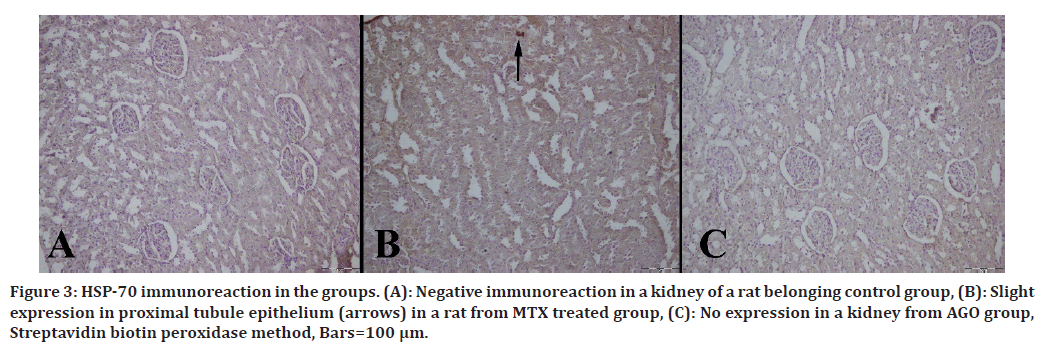
Figure 3. HSP-70 immunoreaction in the groups. (A): Negative immunoreaction in a kidney of a rat belonging control group, (B): Slight expression in proximal tubule epithelium (arrows) in a rat from MTX treated group, (C): No expression in a kidney from AGO group, Streptavidin biotin peroxidase method, Bars=100 μm.
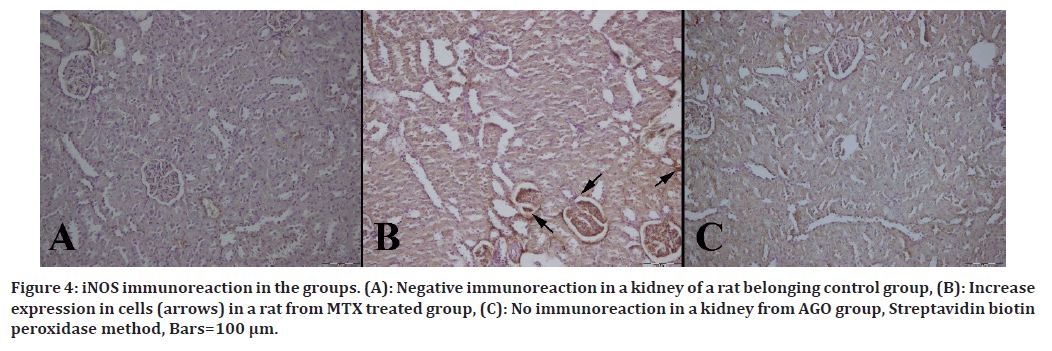
Figure 4. iNOS immunoreaction in the groups. (A): Negative immunoreaction in a kidney of a rat belonging control group, (B): Increase expression in cells (arrows) in a rat from MTX treated group, (C): No immunoreaction in a kidney from AGO group, Streptavidin biotin peroxidase method, Bars=100 μm.
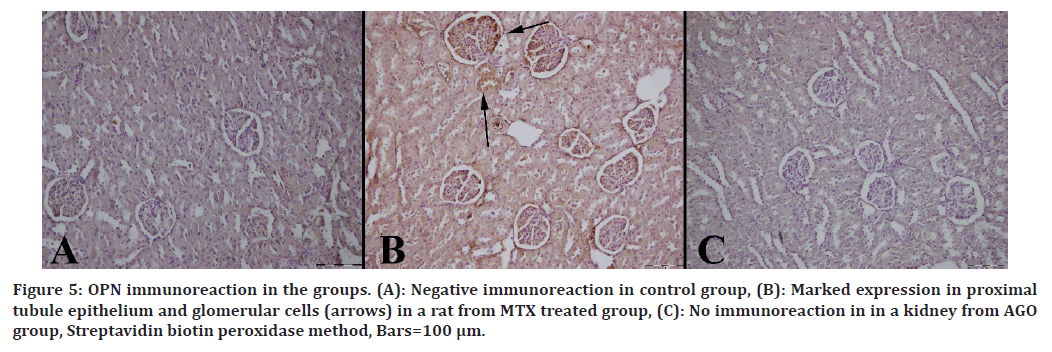
Figure 5. OPN immunoreaction in the groups. (A): Negative immunoreaction in control group, (B): Marked expression in proximal tubule epithelium and glomerular cells (arrows) in a rat from MTX treated group, (C): No immunoreaction in in a kidney from AGO group, Streptavidin biotin peroxidase method, Bars=100 μm.
Discussion
Chemotherapeutic agents such as MTX show unwanted side effects in body tissues. In the literature, there are not enough studies on MTX-induced kidney damage with AGO. We suggest that the potential side effects of MTX may be ameliorated by AGO treatment. In our study, we suggest that MTX induces kidney damage and AGO has a curative effect against this damage.
The most important indicators of kidney damage in our body are BUN, creatine and uric acid levels in serum. An increase in these molecules above the reference values in serum is an indication of kidney damage. In a study, a statistically significant increase in serum creatine levels was determined in MTX-treated rats. Similarly, in our study, a statistically significant increase in creatine levels was observed.
Moreover, a statistically significant increase was found in serum BUN levels. However, no statistically significant difference was found in uric acid levels. In another study, statistically significant decreases in serum creatinine levels were observed in the AGO group. Similarly, in our study, statistically significant decreases in serum creatinine levels were determined in the AGO group. Statistically significant decreases were also found in serum BUN levels. However, no statistically significant difference was found in serum uric acid levels. Therefore, it was determined that AGO prevented the toxicity caused by MTX in kidney tissue. Based on our results, we think that AGO can be used as a therapeutic agent to prevent the toxicity of MTX in kidney tissue [7].
Oxidative stress is one of the important factors in the formation of kidney damage. TOS is an important indicator of oxidative stress. In this study, it was observed that MTX increased MDA levels in kidney tissue [8]. In contrast to this study, no statistical difference was found in the TOS values of the MTX group compared to the control group in our study. Under normal conditions, the increase in intracellular oxidative stress is reduced by the antioxidant mechanisms within the cell. Weakening of this antioxidant mechanism in the cell causes an increase in intracellular oxidative stress. Studies have shown that MTX weakens the intracellular antioxidant mechanism. On the contrary, in our study, no statistical difference was found in TAS values of the MTX group compared to the control group.
Antioxidant mechanisms react with reactive oxygen species (ROS) in the cell and convert them into harmless compounds for the cell. If the balance of ROS and antioxidant enzyme system in the cell is disrupted, intracellular oxidative stress increases. In a study, it was determined that agomelatine decreased oxidative stress and increased the levels of antioxidant system enzymes [9].
Similarly, in our study, we found that TOS levels decreased statistically significantly in the AGO-treated group, while TAS levels increased significantly. These results suggest that AGO prevents the damage caused by oxidative stress in renal tissue.
In an MTX-administered study, kidney sections from rats showed atrophy of some glomeruli, dark eosinophilic hypertrophy glomeruli and brush border disintegration. Leukocyte inflammatory cell infiltration was also focal. In other tubules, cystic luminal dilatation and degeneration area of epithelial cells lining the renal tubules were found. In our study, the kidneys were slightly swollen and pale in necroscopy performed in the MTX group. Histopathologic examination revealed marked hyperemia, mild hemorrhages, tubular cell necrosis and dilatation of Bowman's cavities [10].
In the AGO-treated group, no pathologic findings were observed in the kidneys. Microscopically, normal renal structure was observed in control and AGO groups. The lesions were attributed to MTX toxicity since there were no pathologic findings in the groups other than the MTX group. These results suggest that AGO ameliorates MTXinduced renal damage.
iNOS has an effective role in kidney injury because it can produce free radicals. The NO it produces causes lipid peroxidation in the cell. It has been shown in a study that excess NO decreases intracellular GSH levels and increases oxidative stress [11].
Similarly, in our study, iNOS expression was found to be statistically significantly increased in the MTX-treated group compared to the control group. On the contrary, iNOS expression was found to be statistically significantly decreased in the AGO-treated group compared to the MTX group. Based on this result, we suggest that AGO may prevent oxidative stress and reduce renal damage by decreasing iNOS levels.
HSP70 is a chaperone molecule and ensures that proteins fold correctly. An increase in molecular chaperones may be a marker that there is damage in the cell. In a study, HSP70 and 90 were found to be increased in kidney tissue and were associated with kidney damage [12].
In another study, increased HSP70 expression was observed in MTX-treated rats [13]. In our study, we found similarly high HSP70 expressions in the MTX-treated group. On the contrary, we found a decrease in HSP70 expressions in the AGO-treated group. We think that this supports the therapeutic effect of AGO on kidney injury.
OPN is synthesized in many tissues, particularly bone and epithelial tissue. In contrast to these tissues, it is expressed in low amounts in the distal nephron of the kidney [14,15].
In studies, OPN has been reported to increase kidney damage [16,17].
In our study, OPN expression increased in the MTXtreated group, whereas it decreased in the AGO-treated group. These findings suggest that AGO reduces kidney damage. G-CSF is a cytokine that activates stem cells in bone. In a study, it was determined that G-CSF expression increased in the kidney tissue of rats in the MTX-treated group. On the contrary, it was reported that G-CSF expression decreased in the kidney tissue of rats given apigenin as treatment. Similarly, in our study, G-CSF expression increased in the MTX-treated group and decreased in the AGO-treated group.
Conclusion
The findings of our study showed that MTX caused severe biochemical and histopathologic changes and oxidative damage in kidney tissues by increasing TOS levels and decreasing TAS levels. MTX increased the expression of OPN, HSP70, G-CSF and iNOS. It may have restored the antioxidant defense system and down-regulated OPN, HSP70, G-CSF. Treatment of rats with AGO significantly reduced MTX-induced renal toxicity. Finally, the results suggest that AGO has a protective effect against MTXinduced oxidative stress renal toxicity.
References
- Vazi EPG, Holanda F, Santos NA, et al. Short-term systemic methotrexate administration in rats induces astrogliosis and microgliosis. Res Vet Sci 2021; 138:39-48.
- Ahmed ZSO, Galal MK, Drweesh EA, et al. Protective effect of starch-stabilized selenium nanoparticles against melamine-induced hepato-renal toxicity in male albino rats. Int J Biol Macromol 2021; 191:792-802.
- Yang M, Kim JS, Kim J, et al. Neurotoxicity of methotrexate to hippocampal cells in vivo and in vitro. Biochem Pharmacol 2011; 82:72-80.
- Aslankoc R, Ozmen O, Ellidag HY. Ameliorating effects of agomelatine on testicular and epididymal damage induced by methotrexate in rats. J Biochem Mol Toxicol 2020; 34:e22445.
- Güvenç M, Aksakal M. Ameliorating effect of kisspeptin‐10 on methotrexate‐induced sperm damages and testicular oxidative stress in rats. Andrologia 2018; 50:e13057.
- Inanir S, Copoglu US, Kokacya H, et al. Agomelatine protection in an LPS-induced psychosis-relevant behavior model. Med Sci Monit 2015; 21:3834.
- Cherngwelling R, Pengrattanachot N, Swe MT, et al. Agomelatine protects against obesity-induced renal injury by inhibiting endoplasmic reticulum stress/apoptosis pathway in rats. Toxicol Appl Pharmacol 2021; 425:115601.
- Ozturk E, Karabulut D, Akin AT, et al. Evaluation by different mechanisms of the protective effects of vitamin B12 on methotrexate nephrotoxicity. J Mol Histol 2022; 53:133-143.
- Karaman A, Diyarbakir B, Durur-Subasi I, et al. A novel approach to contrast-induced nephrotoxicity: The melatonergic agent agomelatine. Br J Radiol 2016; 89:20150716.
- Abdel-Raheem IT, Khedr NF. Renoprotective effects of montelukast, a cysteinyl leukotriene receptor antagonist, against methotrexate-induced kidney damage in rats. Arch Pharmacol 2014; 387:341-353.
- Goligorsky MS, Brodsky SV, Noiri E. Akut böbrek yetmezliğinde nitrik oksit: NOS'a karşı NOS. Böbrek Uluslararası 2022; 61:855–861.
- Zhang PL, Lun M, Schworer CM, Blasick TM, Masker KK, Jones JB ve diğerleri. Isı şoku protein ekspresyonu, sıçan böbreklerinde iskemi-reperfüzyon hasarına karşı oldukça hassastır. Ann Clin Laboratuvar Bilimi 2008; 38:57-64
- Ulusoy HB, Öztürk İ, Sönmez MF. Protective effect of propolis on methotrexate-induced kidney injury in the rat. Renal Failure 2016; 38:744-750.
- Kahles F, Findeisen HM, Bruemmer D. Osteopontin: A novel regulator at the cross roads of inflammation, obesity and diabetes. Mol Metabol 2014; 3:384-393.
- Hudkins KL, Giachelli CM, Cui Y, et al. Osteopontin expression in fetal and mature human kidney. J Am Soc Nephrol 1999; 10:444-457.
- Irita J, Okura T, Jotoku M, et al. Osteopontin deficiency protects against aldosterone-induced inflammation, oxidative stress, and interstitial fibrosis in the kidney. Am J Physiol Renal Physiol 2011; 301:F833-F844.
- Lorenzen J, Shah R, Biser A, et al. The role of osteopontin in the development of albuminuria. J Am Soc Nephrol 2008; 19:884-890.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Nurhan Gumral1, Ozlem Ozmen2, Rahime Aslankoc1, Arzu Yalcin1* and Oguzhan Kavrik1
1Department of Physiology, Faculty of Medicine, Süleyman Demirel University, Isparta, Turkey2Department of Pathology, Faculty of Veterinary, Burdur Mehmet Akif Ersoy University, Burdur, Turkey
Received: 16-Dec-2022, Manuscript No. JRMDS-22-87198; , Pre QC No. JRMDS-22-87198(PQ); Editor assigned: 19-Dec-2022, Pre QC No. JRMDS-22-87198(PQ); Reviewed: 03-Jan-2023, QC No. JRMDS-22-87198 (QC); Revised: 09-Jan-2023, Manuscript No. JRMDS-22-87198(R); Published: 16-Jan-2023
