Research - (2021) Volume 9, Issue 9
Green Synthesis of Zinc Oxide Nanoparticles using Phyllanthus Niruri and Stevia Leaf Extracts and Their Antibacterial Potential
Shreyam Barthwal1, Subhasree R2*, Thiyaneswaran N3 and S Rajeshkumar4
*Correspondence: Subhasree R, Department of Prosthodontics, Saveetha Dental College and Hospital, Saveetha Institute of Medical and Technical Science, Saveetha University Tamilnadu, India, Email:
Abstract
The growing demand for dental implants has propelled the development of the implant industry thereby leading to more advanced research in this area. The plant extract mediated nanoparticles in dental materials has many advantages over conventional materials that are synthesized by physico-chemical methods. Emergence of natural products and their derivatives has caused a paradigm shift in the synthesis of dental materials and are highly preferred over conventional drugs with the notion of reducing adverse effects. Zinc oxide nanoparticles are biocompatible, safe, and nontoxic metal oxide that possesses promising antimicrobial potential against a broad range of bacteria and fungi. Therefore, the aim of the present study was to evaluate the antimicrobial potential of ZnO nanoparticles prepared from the leaf extracts of Phyllanthus Niruri and Stevia that can be incorporated into dental implants. Zinc oxide (ZnO) nanoparticles were synthesized using leaf extracts of two medicinal plants- Phyllanthus Niruri and Stevia and Zinc sulphate as a precursor. The structural and visual characterization of nanoparticles were performed by ultraviolet-visible spectrophotometry (UV-Vis) and transmission electron microscopy (TEM). Change in color of the reaction mixture from turbid yellow to light brown indicated the formation of ZnO nanoparticles. UV peaks at 320 nm and 324 nm, confirmed the synthesis of ZnO nanoparticles. The antibacterial potential of ZnO NPs was examined by paper disc diffusion method against various clinical strains of bacteria based on the zone of inhibition.
Keywords
Dental implants, nanoparticles, zinc oxide, Phyllanthus Niruri, Stevia, antibacterial
Introduction
The advent of ‘dental implant" in dentistry has revolutionized the approach to patient care and has become increasingly important in rehabilitation [1]. The growing demand for dental implants has propelled the development of implant industry and led to its further research and development. Not only has this introduced new or modified implant designs, materials, and surface treatments, but also advances in hard and soft tissues. In addition to this, technological improvements in regenerative surgical approaches, devices, and materials have contributed to the application and success of dental implant therapy, and will continue to do so [2].
It is imperative to understand natural healing events after the implant wound is created. Unlike teeth, which develop synchronously with the surrounding periodontal tissue, dental implants are surgically placed directly into native or regenerated bone, limiting the number of cell types that migrate, attach, and differentiate on the surface during healing [3].
Postoperative infections are considered a rare complication with a prevalence ranging from 1.6% to 11.5% [4,5]. They usually appear in the first month after the dental implant is inserted [6]. Furthermore, changes in oral flora caused by short-term postoperative topical and systemic antibacterial therapy can also encourage the invasion of opportunistic pathogens. Nevertheless, as with any biomaterial-cantered infection, such complications are non-responsive to antibiotics and often persist until the implant is removed [7,8]. Consequently, postoperative infections have been suggested to be a risk factor for osseointegration which increases the risk of early failure of dental implant by almost 80-fold [9-11].
In the current era, herbal medicines have caused a shift in paradigm and are preferred over conventional drugs with the notion of causing lesser adverse effects. According to the WHO Herbal Medicines Strategies and Guidelines proposed in 2002, any plant is considered to have therapeutic potential if careful research has shown that part of the plant has beneficial effects in treating a disease [12]. One such herb is Phyllanthus Niruri (keezhanelli in Tamil) that has been widely studied for its antihepatotoxic, anti hepatitic-B and antidiabetic potential [13]. Sunitha et al. conducted a study wherein the antimicrobial effect of the leaves of P. niruri and Solanum nigrum on caries causing bacteria was evaluated. They reported to have found a significant antibacterial activity against cariogenic organisms [14].
Therefore, the aim of the present study was to evaluate the antibacterial activity after coating suture material with an antiseptic agent P. niruri .
Materials and Methods
Preparation of plant extract
Fresh healthy leaves of Phyllanthus Niruri and Stevia were collected from Chennai, TN and were thoroughly washed with tap water and distilled water to remove dirt and contamination. The leaves were then air dried at room temperature and was ground separately to fine powder using an electric grinder. To prepare leaf extract, 1 g each of P. niruri and Stevia leaf powder were mixed with 100 ml of distilled water and then boiled at 70°C for 10 min. The leaf extract was cooled down and filtered using Whatman filter paper No.1 and was stored at 4 °C.
Synthesis of nanoparticles
A solution of 30 mm zinc sulphide (ZnS) was prepared in 70 mL Distilled water. ZnO nanoparticles were synthesized by mixing 30 ml of plant extract with 70 ml of ZnS solution under continuous stirring and were incubated at 37O C for 48 h. The mixture was allowed to precipitate and settle down at RT for …h and was finally centrifuged at 10,000 rpm for 5 min. The resulting precipitate was washed twice with distilled water and then dried in hot air oven at 80 °C. The powder of ZnO NPs was scraped and further characterized using different techniques.
Characterization of zinc oxide NPs
UV-Visible Spectrophotometry
ZnO nanoparticles were suspended in distilled water. The absorption spectrum of synthesized NPs was measured using UV–visible spectrophotometer (Esico, India) in wavelength range between 200–800 nm. Distilled water was used as a reference.
Transmission electron microscopy
Transmission electron microscopy (TEM) was performed to characterize size and shape of ZnO nanoparticles using a high resolution transmission electron microscope (HRTEM) Model: FEI-TECNAI G2 -20 at an accelerating voltage of 200 kV. The specimens for TEM measurement were prepared by depositing a drop of nanoparticles on a 400 mesh copper grid coated by an amorphous carbon film and evaporating the solvent in air at room temperature. The sutures were soaked in P. niruri for a period of 24 hours; dried and characterization was done with the help of Transmission electron microscopy.
Assessment of antioxidant activity of Zinc Oxide nanoparticles
The antioxidant potential of ZnO nanoparticles was assessed using the DPPH assay [15-f17] based upon spectrophotometric detection of reduction of DPPH• free radical into DPPH-H. The purple colored DPPH. With absorption maximum at 517 nm accepts hydrogen from an antioxidant compound and is reduced to a yellow coloured DPPH-H. The reduction in color is directly proportional to the number of hydrogen atoms absorbed from the antioxidant. Hence, the reduction in absorbance can be directly correlated with the antioxidant activity of the nanoparticles. Different volumes of nanoparticles were added to 1 mL of 0.1mM DPPH solution in methanol. The mixture was incubated for 30 min at room temperature in the dark. Absorbance of nanoparticles and DPPH mixture was measured at 517 nm using ELISA reader Ascorbic acid (vitamin C) mixed with DPPH solution was used as reference standard. Methanol plus nanoparticles solution was used to set up blank. DPPH free radical scavenging activity was calculated using the following formula:
Percent Inhibition={(Absorbance of control-Absobance of Sample)/(Absorbance of control)}*100
Assessment of antimicrobial activity
Bacterial strains
Antimicrobial activity was tested against Candida albicans, Streptococcus mutans, E. faecalis and S. aureus.
Evaluation of antimicrobial activity using agar disc diffusion assay
Antimicrobial activity of nanoparticles was assessed using disk diffusion method. Different concentrations of nanoparticles (specify range of NP concentrations used) were prepared. Bacterial strains were cultured in Luria Bertani broth at 37 °C under continuous shaking overnight. Bacterial suspension was prepared and 0.1 mL of suspension was spread uniformly on nutrient agar plates. Sterile discs (10 mm in diameter, HiMedia Laboratories Pvt. Ltd., India) soaked in different concentrations of the nanoparticles, were placed aseptically onto the surface of the inoculated agar plates. The plates were incubated at room temperature for 1 h to diffuse the nanoparticles into the medium. Plates were then incubated at 37 °C overnight (14-16 h). Post incubation, zones of inhibition was measured as an indicator of activity. Each experiment was replicated at least three times.
Results and Discussion
Visual and optical analysis of nanoparticles formation
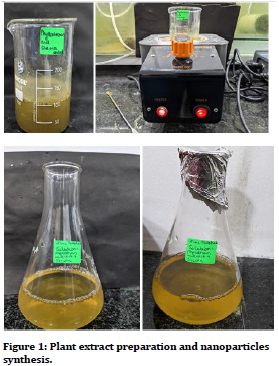
Mixing zinc sulphide with leaf extracts of P. niruri and Stevia led to physio-chemical reactions in the solution. The colour of the reaction mixture changed slightly from yellow to light brown. Plant extracts are enriched in flavonoids and phenolic compounds that strongly facilitate precipitation of Zn ions into Zn nanoparticles. Figure 1 clearly depicts change in color of the reaction mixtures due to formation of ZnO nanoparticles. These results were consistent with the previous reports of color changes in plant-based synthesis of nanoparticles.
Figure 1. Plant extract preparation and nanoparticles synthesis.
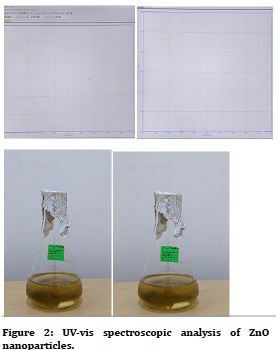
ZnO nanoparticles were further characterized by UV spectrophotometry. Figure 2 shows the UV peaks recorded by the spectrophotometer. The maximum absorption peak for ZnO NPs synthesized via P. niruri and Stevia was recorded at wavelength range nm corresponding to ZnS nanoparticles. These findings are in agreement with the standard ZnS absorption spectra.
Figure 2. UV-vis spectroscopic analysis of ZnO nanoparticles.
Antimicrobial effect of Zn nanoparticles
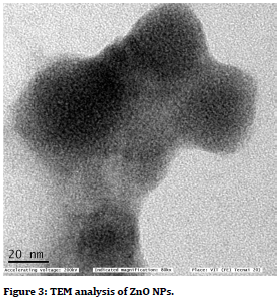
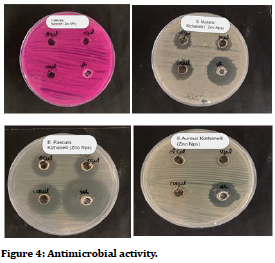
It was observed that as the concentration increases, there is an increase in the zone of inhibition. The present study reflected that silk sutures coated with ZnS nanoparticles, had an effective antimicrobial reaction against Lactobaacilli, S mutans and C. Albicans which are most commonly found in oral microbiota. The plants and many biological materials are playing major role in the zinc oxide nanoparticles synthesis [15-23] (Figures 3 and Figure 4).
Figure 3. TEM analysis of ZnO NPs.
Figure 4. Antimicrobial activity.
Conclusion
Along with zinc, nanoparticles can be effective agents to be used with silk suture. Future scope of the study will be to carry out animal trials followed by clinical trials which will pave way for it to be incorporated into everyday oral surgical procedures.
References
- Albrektsson T, Zarb GA. The brånemark osseointegrated implant. Implant Dent 1992; 1:93
- Villar CC, Huynh-Ba G, Mills MP, et al. Wound healing around dental implants: Wound healing around dental implants. Endod Top 2011; 25:44–62.
- Sculean A, Gruber R, Bosshardt DD. Soft tissue wound healing around teeth and dental implants. J Clin Periodont 2014; 41:S6-22.
- Camps-Font O, Figueiredo R, Valmaseda-Castellón E, et al. Postoperative infections after dental implant placement: Prevalence, clinical features, and treatment. Implant Dent 2015; 24:713-9.
- Resident P. Survival and success rates of dental implants placed using osteotome sinus floor elevation without added bone grafting: A retrospective study with a follow-up of up to 10 years. Periodont 2016; 36:s89-97.
- Campsâ?Font O, Martínâ?Fatás P, Cléâ?Ovejero A, et al. Postoperative infections after dental implant placement: Variables associated with increased risk of failure. J Periodont 2018; 89:1165-73.
- Gristina AG, Hobgood CD, Webb LX, et al. Adhesive colonization of biomaterials and antibiotic resistance. Biomaterials 1987; 8:423-6.
- Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science 1987; 237:1588-95.
- Roosâ?Jansåker AM, Lindahl C, Renvert H, et al. Nineâ?to fourteenâ?year followâ?up of implant treatment. Part I: Implant loss and associations to various factors. J Clin Periodont 2006; 33:283-289.
- Esposito M, Hirsch JM, Lekholm U, et al. Biological factors contributing to failures of osseointegrated oral implants,(I). Success criteria and epidemiology. Eur J Oral Sci 1998; 106:527-51.
- Figueiredo R, Camps-Font O, Valmaseda-Castellón E, et al. Risk factors for postoperative infections after dental implant placement: A case-control study. J Oral Maxillofac Surg 2015; 73:2312-8.
- World Health Organization. WHO Traditional Medicine Strategy 2002-2005. 2002.
- Bagalkotkar G, Sagineedu SR, Saad MS, et al. Phytochemicals from Phyllanthus niruri Linn. and their pharmacological properties: A review. J Pharm Pharmacol 2010; 1559–70.
- Sunitha J. Antimicrobial effect of Leaves of Phyllanthus niruri and Solanum nigrum on caries causing bacteria: An In vitro study. J Clin Diagnost Res 2017.
- Malarkodi C, Rajeshkumar S. Invitro bactericidal activity of bio-synthesized semiconductor nanoparticles against UTI causing pathogens. Inorganic Nano-Metal Chem 2017; 47:9.
- Happy A, Soumya M, Kumar SV, et al. Phyto-assisted synthesis of zinc oxide nanoparticles using Cassia alata and its antibacterial activity against Escherichia coli. Biochem Biophysics Reports 2019; 17:208-11.
- Rajeshkumar S, Agarwal H, Venkat Kumar S, et al. Brassica oleracea mediated synthesis of zinc oxide nanoparticles and its antibacterial activity against pathogenic bacteria. Asian J Chem 2018; 30:2711-5.
- Rajeshkumar S. Synthesis of Zinc oxide nanoparticles using algal formulation (Padina tetrastromatica and Turbinaria conoides) and their antibacterial activity against fish pathogens. Res J Biotechnol 2018; 13:15-9.
- Sujatha J, Asokan S, Rajeshkumar S. Antidermatophytic activity of green synthesised zinc oxide nanoparticles using cassia alata leaves. J Microbiol Biotechnol Food Sci 2021; 2021:348-52.
- Rajeshkumar S, Kumar SV, Ramaiah A, et al. Biosynthesis of zinc oxide nanoparticles using Mangifera indica leaves and evaluation of their antioxidant and cytotoxic properties in lung cancer (A549) cells. Enzyme Microbial Technol 2018; 117:91-5.
- Rajeshkumar S, Agarwal H, Kumar SV, et al. One-pot synthesis of zinc oxide nanoparticles using orange peel extract and its potential anti-bacterial activity. Int J Pharm Res 2018; 10:574-8.
- Santhoshkumar J, Kumar SV, Rajeshkumar S. Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resource-Efficient Technol 2017; 3:459-65.
- Srinisha M, Rajeshkumar S, Lakshmi T, et al. Antibacterial activity of zinc oxide nanoparticles synthesized using amla fruit against oral pathogens. Drug Invention Today 2019; 11.
Author Info
Shreyam Barthwal1, Subhasree R2*, Thiyaneswaran N3 and S Rajeshkumar4
1Department of Implantology, Saveetha Dental College and Hospital, Saveetha Institute of Medical and Technical Science, Saveetha University Tamilnadu, Chennai, India2Department of Prosthodontics, Saveetha Dental College and Hospital, Saveetha Institute of Medical and Technical Science, Saveetha University Tamilnadu, Chennai, India
3Department of Prosthodontics and Implantology, Saveetha Dental College and Hospital, Saveetha Institute of Medical and Technical Science, Saveetha University Tamilnadu, Chennai, India
4Department of Pharmacology, Saveetha Dental College and Hospital, Saveetha Institute of Medical and Technical Science, Saveetha University Tamilnadu, Chennai, India
Citation: Shreyam Barthwal, Subhasree R, Thiyaneswaran N, S Rajeshkumar,Green Synthesis of Zinc Oxide Nanoparticles using Phyllanthus Niruri and Stevia Leaf Extracts and Their Antibacterial Potential, J Res Med Dent Sci, 2021, 9(9): 40-43
Received: 29-Jun-2021 Accepted: 06-Sep-2021