Research Article - (2022) Volume 10, Issue 12
Gas Chromatography Mass Spectroscopic Study of Rhyncosia Minima
Satheesh Kumar C1, Prabhu K2*, S Kalaivani3, A Franklin4, MRK Rao5, CS Janaki6 and Shruti Dinakaran6
*Correspondence: Dr. Prabhu K, Department of Anatomy, Sree Balaji Medical College and Hospital, Chennai, Tamil Nadu, India, Email:
Abstract
The present work undertook the gas chromatography mass spectroscopic analysis of one herbal plant Rhycosia minima, which is reported to have medicinal properties such as anthelmintic and antioxidant, anti-nociceptive, anti-diabetic and anti-inflammatory. The plant was collected from the water logged area of Chengalpattu, Tamil Nadu, India and the ethyl acetate extract of the whole plant was obtained. The extract was subjected to gas chromatography mass spectroscopy after due processing. The results indicated some molecules such as 3,4-di-o-methyl-L-arabinopyranose, thiocyanic acid, ethyl ester, 2-isopropenyl-4a,8-dimethyl-1,2,3,4,4a,5,6,7-octahydronaphthalene, 4-(2,4-dimethylcyclohex-3-enyl)but-3-en-2-one, n-hexadecanoic acid, methyl 2-hydroxy-4-methoxybenzoate, tert-butyldimethylsilyl ether, 7-methyl-z-tetradecen-1-ol acetate, gamma tocopherol, cholesterol, pregna-5,8(9),16-triene-3 beta-ol-20-one benzoate, stigmasterol, beta-sitosterol, beta-amyrin, betulin, dl-alpha-tocopherol, hexadecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester, hexadecanoic acid, 1-(hydroxymethyl) etc. Which have medicinal properties supportive of the plants role as an important herbal medicine?.
Keywords
Gas chromatography mass spectroscopy, Herbal, Rhyncosia minima, Gamma tocopherol, Cholesterol, Betaamyrin, Betulin
Introduction
The modern medicine is based mostly on plants. Most of the medicine molecules have been isolated, purified and tested for their medicinal roles. At a later stage these medicine molecules are synthesized chemically to protect the plants form being over exploited. Many more plants are there whose medicinal potentials have not been explored except for ethno botanical use as native medicines. It is high time to record all these unexplored data to develop better, safer and affordable medicines. One such method is to subject the plant material to gas chromatography mass spectroscopic analysis to find the type of biomolecules present in it. Quite a few articles in this regard are available and much more need be done [1-14]. The gas chromatography mass spectroscopic analysis report of analysis of one herbal plant, Rhyncosia minima is presented in this article. The phytochemical composition and biological activities of Rhyncosia minima essential oil was reported by Gundidza, et al. [15]. Rhyncosia has been listed in the plants known to be abort efficient by Kumar, et al. [16]. The anthelmintic and antioxidant role of Rhyncosia minima was reported by Yellasubbaiah, et al. [17]. Jiaaet, et al. has studied the immune function of polysaccharide PRM3 from root extract of Rhycosia minima [18]. Pradeep and Sudhakar have studied the anti-diabetic role of ethanol extracts of a related species, Rhyncosia beddomei [19]. Kumar, et al. has demonstrated the antioxidant, anti-nociceptive and anti-inflammatory activities of this plant [20].
Materials and Methods
Shade dried leaves of Rhyncosia minima were extracted with ethyl acetate and the dried extract was used for gas chromatography mass spectroscopic analysis by standard protocols.
Results
Table 1 and Figure 1 depict the results of gas chromatography mass spectroscopic analysis of Rhyncosia minima. The biomolecules were identified by NIST spectral library from data base national agriculture library, USA and others as shown in Table 1.
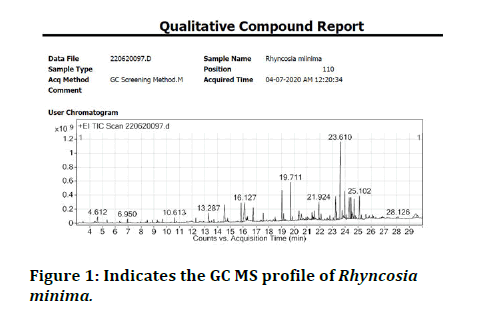
Figure 1: Indicates the GC MS profile of Rhyncosia minima.
| Sl.No | R T | Name | Molecular Formula | Molecular Weight | Peak Area (%) | Possible medical Role |
|---|---|---|---|---|---|---|
| 1 | 4.61 | 3,4-Di-O-methyl-L-arabinopyranose | C7H14O5 | 178.1 | 1.24 | Catechol-O-methyl-transferase inhibitor, methyl donor, antidote, coronary dilator, digestive, diuretic, Diaphoretic |
| 2 | 6.95 | Thiocyanic acid, ethyl ester | C3H5NS | 87 | 0.79 | Acidifier |
| 3 | 9.28 | 2-Isopropenyl-4a, 8-dimethyl-1,2,3,4,4a,5,6,7-Octahydronaphthalene | C15H24 | 204.2 | 0.55 | 5 alpha reductase inhibitor, beta inhibitor |
| 4 | 9.67 | (-)-alpha-panasinsen | C15H24 | 204.2 | 0.55 | Not known |
| 5 | 10.61 | Guaiol | C15H24 | 204.2 | 0.55 | Not known |
| 6 | 12.29 | 4-(2,4-Dimethylcyclohex-3-enyl)but-3-en-2-one | C12H18O | 178.1 | 0.72 | Decrease endothelial platelet adhesion, decrease endothelial leukocyte adhesion, endorphinogenic, endocrine protective, endothelium derived relaxing factor promoter, ergotamine enhancer, enteromotility enhancer |
| 7 | 13.29 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | C20H40O | 296.3 | 1.67 | Provides pligosaccharide |
| 8 | 14.56 | n-hexadecanoic acid | C16H32O2 | 256.2 | 5.35 | Acidifier, arachidonic acid inhibitor, increase aromatic amino acid decarboxylase activity, anaphylactic, antitumor, aryl amine-n-acetyltransferase-inhibitor, decrease norepinephrine production, down regulates nuclear and cytosol androgen reuptake, GABA-nergic, Increase N K cell activity, Inhibit Production of TNF, Myoneuro stimulant |
| 9 | 18.5 | Methyl 4,7,10,13,16-docosapentaenoate | C23H36O2 | 344.3 | 0.65 | Catechol-O-methyl-transferase inhibitor |
| 11 | 19.03 | Butyl 4,7,10,13,16,19-docosahexaenoate | C26H40O2 | 384.3 | 5.1 | Not known |
| 12 | 20.32 | Butyl 9,12,15-octadecatrienoate | C22H38O2 | 334.3 | 0.84 | Not known |
| 13 | 20.55 | tert-hexadecanethiol | C16H34S | 258.2 | 2.45 | Not known |
| 14 | 21.56 | Methyl 2-hydroxy-4-methoxybenzoate, tert-butyldimethylsilyl Ether | C15H24O4Si | 296.1 | 1.24 | Catechol-O-methyl-transferase inhibitor, 17-beta-hydroxysteroid dehydrogenase Inhibitor, aryl hydrocarbon hydroxylase inhibitor, testosterone hydroxylase inducer |
| 15 | 21.92 | 7-Methyl-Z-tetradecen-1-ol acetate | C17H32O2 | 268.2 | 3.13 | Catechol-O-methyl-transferase inhibitor, provide zinc, oligosaccharide, increase zinc bioavailability |
| 16 | 22.78 | gamma-tocopherol | C28H48O2 | 416.4 | 0.98 | Tocopherol synergist, PPAR‑gamma antagonist |
| 17 | 23.05 | Cholesterol | C27H46O | 386.4 | 0.6 | Precursor for steroid synthesis |
| 18 | 23.61 | Pregna-5,8(9),16-triene-3beta-ol-20-one benzoate | C28H32O3 | 416.2 | 21.13 | Oligosaccharide provider, 17-beta-hydroxysteroid dehydrogenase inhibitor, beta-blocker |
| 19 | 23.74 | Camp sterol | C28H48O | 400.4 | 1.39 | Plant steroid use as food additive and has cholesterol lowering role |
| 20 | 23.96 | Stigma sterol | C29H48O | 412.4 | 4.04 | Precursor of steroid hormones, Anti-osteoarthritic, anti-hypercholesterolemia, |
| 21 | 24.3 | beta-sitosterol | C29H50O | 414.4 | 5.45 | Beta blocker |
| 22 | 24.42 | beta-amyrin | C30H50O | 426.4 | 3.35 | Anti-TGF beta, beta blocker |
| 23 | 24.5 | Phytonadione | C31H46O2 | 450.4 | 0.85 | Not known |
| 24 | 24.68 | Betulin | C30H50O2 | 442.4 | 3.43 | It has antiviral, analgesic, anti-inflammatory and antineoplastic activities |
| 26 | 25.1 | dl-alpha-Tocopherol | C29H50O2 | 430.4 | 7.08 | Tocopherol synergist, alpha reductase inhibitor |
| 26 | 28.13 | Hexadecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester | C35H68O5 | 568.5 | 0.99 | Acidifier |
Table 1: Indicates the retentions values, types of possible compound, their molecular formulae, molecular mass, peak area and their medicinal roles of each compound as shown in the GC MS profile of Rhyncosia minima.
Discussion
The GC MS profile of Rhyncosia minima indicated the presence of some important biomolecules such as 3,4-dio- methyl-L-arabinopyranose, thiocyanic acid, ethyl ester, 2-isopropenyl-4a, 8-dimethyl-1, 2, 3, 4, 4a, 5, 6, 7 octahydronaphthalene, 4-(2, 4-dimethylcyclohex-3 enyl)but-3-en-2-one, n-hexadecanoic acid, methyl2- hydroxy-4-methoxybenzoate, tert-butyldimethylsilyl ether, 7-methyl-z-tetradecen-1-ol acetate, gammatocopherol, cholesterol, Pregna-5,8(9),16-triene-3betaol- 20-one benzoate, stigmasterol, beta-sitosterol, betaamyrin, betulin, dl-alpha-tocopherol, hexadecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester, hexadecanoic acid, 1-(hydroxymethyl) etc. which have far reaching medicinal roles as shown in Table 1. These roles of the molecules could support the plant’s which is used as anthelmintic and antioxidant, anti-nociceptive, antidiabetic and anti-inflammatory.
Conclusion
The results and discussion indicate the positive effect of the molecules towards curing diseases for which this plant is used.
References
- Gomathi, Elizabeth AA, Anthony J, et al. The GC MS analysis of one medicinal plant, Premnatomentosa. J Pharma Sci Res 2017; 9:1595-1597.
- Jayakumari S, Prabhu K, Rao MRK, et al. The GC MS analysis of a rare medicinal plant Aloe barbadensis. J Pharm Sci Res 2017; 9:1035-1037.
- Rao MRK, Vijayalakshmi N. Preliminary phytochemical and GC MS analysis of different extracts of Sphaeranthusindicus leaves. Indo Am J Pharma Sci 2018; 5:1511-1520.
[Crossref]
- Rao MRK, Vijayalakshmi N, Lakshmi Sundaram R. Preliminary phytochemical and GC MS analysis of different extracts of Psophocarpus tetragonolobus leaves. Indo Am J Pharma Sci 2018; 5:1649-1656.
[Crossref]
- Rao MRK, Anisha G. Preliminary phytochemical and GC MS study of one medicinal plant Carissa spinarum. Indo Am J Pharama Res 2018; 8:414-421.
- Rao MRK, Balasubramanuam M. TLC, GC MS and antibacterial study of methanol extracts of Tribulusterres tristhorns and Morinaga oleifera flowers. Indo Am J Pharm Sci 2018; 5:3300-3308.
[Crossref]
- Vijayalakshmi N, Rao MRK. The antioxidant studies of two medicinal plants, Sphaeranthusindicus and Psophocarpustetragonolobus. Asian J pharm Clinical Res 2019; 12:321-327.
[Crossref]
- Yuvaraj R, Rao MRK, Prabhu K, et al. The GC MS study of one medicinal plant, Stachyterphetaindica. Drug Invention Today 2019; 12:1665-1669.
- Kumar MH, Prabhu K, Rao MRK, et al. The GC MS study of one medicinal plant, Dodoneaangutifolia. Drug Invention Today 2019; 12:1661-1664.
- Rao MRK, Anisha G, Prabhu K, et al. Preliminary phytochemical and GC MS study of one medicinal plant Carissa carandas. Drug Invention Today 2019; 12:1629-1634.
- Rao MRK, Vijayalakshmi N, Prabhu K, et al. The gas chromatography mass spectrometry study of Moringa oleifera seeds. Drug Invention Today 2019; 12:2172-2175
- Kumar MH, Prabhu K, Rao MRK, et al. The GC MS study of one medicinal plant, Aristolochia Indica. Drug Invention Today 2020; 12:2919-2923.
- Vijayalakshmi N, Rao MRK. ‘Preliminary phytochemical and antioxidant studies of leaf extracts of one medicinal plant, Vitexnegundo”. Res J Pharm Technol 2020; 13:2167-2173.
[Crossref]
- Gundidza M, Gweru N, Magwa ML, et al. Phytochemical composition and biological activities of essential oil of Rhynchosia minima (L) (DC) (Fabaceae). African J Biotechnol 2009; 8:721-724.
[Crossref]
- Ranjith Kumar Y, Elumalai A, Chinna Eswaraiah M. An updated review on herbal Abortifacients. J Pharm Biol 2013; 3:40-41.
- Yellasubbaiah N, Nagasudha B, Suresh Kuamr SV, et al. Evaluation of anti-oxidant and anthelminthic activity of Rhynchosia minima (linn) DC. J. Glob. Trends Pharm Sci 2015; 6:2579-2588
- GJiaa X, Lianga YB, Chao Zhang, et al. Polysaccharide PRM3 from Rhynchosia minima root enhances immune function through TRL4 pathway. Biochim Biophys Acta Gen Subj 2018; 1862:1751-1759.
- Pradeep TP, Sudhakar Y. Antidiabetic activity of ethanolic extracts of Rhyncosia beddomei and Glycosmispentaphylla in Alloxan induced diabetic rats. Int J Pharm Sci Rev Res 2014; 26:328-333.
- Kumar V, Kumar SR, Sudhakar P, et al. Antioxidant, anti-nociceptive and anti-inflammatory activities of Rhyncosia minima (L) DC. Res J Phar tech 2020; 13:1853-1858.
[Crossref]
- Duke, James A. Phytochemcial and ehno botanical databases. U.S. Department of agriculture, agricultural research service. Ag Data Commons, U.S, 2021.
Author Info
Satheesh Kumar C1, Prabhu K2*, S Kalaivani3, A Franklin4, MRK Rao5, CS Janaki6 and Shruti Dinakaran6
1Department of Anatomy, Bharath Institute of Higher Education and Research, Melmaruvathur Adhiparasakthi Institute of Medical Sciences and Research, Melmaruvathur, Chennai, Tamil Nadu, India2Department of Anatomy, Sree Balaji Medical College and Hospital, Chennai, Tamil Nadu, India
3Department of Anatomy, Vel’s Medical College and Hospital, Chennai, Tamil Nadu, India
4Department of Microbiology, Anna Medical College, University of Technology, Port Louis, Mauritius
5Department of Anatomy, Amritha University, Thiruporur, Tamil Nadu, India
6Department of Anatomy, Ayurvedic Medical Practioneer, Kottakkal Arya Vaidya Sala, Kerala, India
Citation: Satheesh Kumar C, Prabhu K, S Kalaivani, A Franklin, MRK Rao, CS Janaki, Shruti Dinakaran, Gas Chromatography Mass Spectroscopic Study of Rhyncosia Minima, J Res Med Dent Sci, 2022, 10 (12): 128-131
Received: 02-Sep-2022, Manuscript No. JRMDS-22-77099; , Pre QC No. JRMDS-22-77099(PQ); Editor assigned: 05-Sep-2022, Pre QC No. JRMDS-22-77099(PQ); Reviewed: 19-Sep-2022, QC No. JRMDS-22-77099; Revised: 21-Nov-2022, Manuscript No. JRMDS-22-77099(R); Published: 02-Dec-2022
