Research - (2019) Volume 7, Issue 5
Fetal Transcerebellar Diameter in Estimating Gestational Age in Third Trimester of Pregnancy
Lilyan W Sersam1*, Sura Basil Findakly1 and Najlaa Hanoon Fleeh2
*Correspondence: Lilyan W Sersam, Department of Obstetrics and Gynaecology, College of Medicine, Mustansiriyah University, Baghdad, Iraq, Email:
Abstract
Background: Accurate determination of gestational age (GA) is of critical importance, it can positively affect pregnancy outcomes. In the third trimester, the reliability of any single ultrasound parameter in gestational age determination is poor. Fetal transcerebellar diameter (TCD) can help as an alternative parameter to the most frequently used biometric parameters to date pregnancy.
Aim: To evaluate the accuracy of fetal transcerebellar diameter (TCD) in estimating gestational age at the third trimester of pregnancy using the last menstrual period (LMP) as a reference for the actual period of gestation.
Materials and methods: In this descriptive study, a total of 65 pregnant women with uncomplicated pregnancies between 28 to 40 weeks of gestation with known LMP were enrolled. Ultrasonographic measurement of fetal TCD was done along with routine parameters. TCD was measured by identifying the cerebellum in the posterior fossa and measuring the transcerebellar distance from the outer edge to the outer edge. Gestational age using TCD was calculated and compared with estimated gestational age based on LMP.
Results: Statistical analysis showed a strong and significant correlation between the estimated gestational age by LMP and each of biparietal diameter (BPD), femur length (FL), and TCD; the latter having the highest correlation coefficient (r=0.931, p=0.0001; r=0.931, p=0.0001 r=0.955, p=0.0001; respectively). Even when considering fetal sex whether male or female, the TCD was the best parameter to correlate with gestational age by LMP.
Conclusion: Fetal transcerebellar diameter can be taken as a good indicator of gestational age in late pregnancy.
Keywords
Cerebellum, Fetal, Gestational age, Third trimester, Pregnancy
Introduction
Knowledge of gestational age (GA) is important for the pregnant woman herself, for ensuring the optimal time for arranging antenatal tests and for assessing the progress of pregnancy [1]. Though last menstrual period (LMP) is known to correlate with gestational age, it may act as a false guide. Furthermore, it has been reported in literature that only about one-half of women can accurately recall their LMP. Thus, early verification or correction of dating can solve such misdating. Antenatal ultrasound is the commonly employed method for standard care in monitoring pregnancy. Ultrasound examination in the first trimester (defined by up to and including 13 6/7 weeks of gestation) has been considered as the most accurate method in estimating or in confirming gestational age [2,3]. In the third trimester (defined by 28 0/7 weeks of gestation and beyond), gestational age assessment by ultrasound is considered the least reliable method with an accuracy of only ± 21 to 30 days [2]. No single parameter is reliable for accurate gestational age determination in the third trimester. Still, the transcerebellar diameter (TCD) (which is a measure of the widest anteroposterior diameter of the cerebellum) is a parameter which is not so much affected even in case of severe intrauterine growth retardation, it has got no deformation in its shape being unaffected by extrinsic pressure, thus can be preferably used to date pregnancy more reliably than other ultrasound parameters [1,4]. Its value in millimeters is found to correspond roughly to the period of gestation between 14-40 weeks [5]. In a recent study by Dashottar et al. they reported that for normal pregnancy as well as in pregnancies complicated by intrauterine growth retardation, the mean difference between estimated and actual gestational age was minimum in TCD followed by other established parameters [5]. TCD can be employed as a useful tool to assist in assessing gestational age during the third trimester [6].
The present study aimed to evaluate the accuracy of fetal TCD in estimating gestational age in a sample of Iraqi women in their third trimester of pregnancy.
Materials and Methods
This was a descriptive study conducted at the Obstetrics and Gynaecology department at Al- Yarmouk Teaching Hospital during the period of one year from January 1, 2018, to December 31, 2018. This hospital is the teaching hospital of Mustansiriyah Medical College and is a tertiary referral hospital in Baghdad city.
The protocol was approved by the Local Research Ethics Committee of the Obstetrics and Gynaecology Department at Al-Yarmouk Teaching Hospital. Informed written consent was obtained from all participants in accordance with the Declaration of Helsinki before embarking them in the study. All participants were antenatal care cases attending the hospital for routine ultrasound examination and seeking antenatal care. The study comprised a randomly selected sample of 65 Iraqi pregnant women with uncomplicated singleton pregnancy with cephalic presentation, during their third trimester (at gestations between 28 to 40 weeks) with known last menstrual period. The gestational age was assessed using the LMP that was confirmed by a first-trimester scan (between 8-11 weeks) for measuring the crownrump length (CRL). The LMP-derived gestational age was only considered if it was within 7 days different from ultrasound-derived gestational age. The LMP was used for assigning gestational age. Exclusion criteria were pregnant women unsure of dates of last menstrual period, cases with irregular cycles, those with discordant LMP and ultrasound dating, not willing to participate, those with fetal growth abnormalities, congenital malformations, multiple gestations, intrauterine death (IUD), and medical disorders like diabetes mellitus and hypertensive disease.
Transabdominal ultrasonography was performed to all women using GE Voluson E6 ultrasound device with 3.0–5.0 MHz transabdominal convex transducer. All antenatal scans were performed by a single and experienced sonographer (one of the authors: Fleeh N.). The following fetal biometric parameters were measured transabdominally during each ultrasound examination including biparietal diameter (BPD) and femur length (FL). Additional ultrasound measurement was obtained included the TCD. Fetal transcerebellar diameter was measured in mm using the widest anteroposterior diameter of the cerebellum. The transthalamic view was obtained first; then the probe was slightly rotated below the thalamic plane, towards the fetal neck. The posterior horns of the lateral ventricles disappeared from view and were replaced by the normal dumbbell-shaped cerebellum. The intersection of the cross of the calipers was positioned on the outer edge of each hemisphere to obtain an outer-to-outer measurement at 90 degrees to the midline (Figure 1). Single measurement was used for each pregnancy. The fetal sex was also ascertained at the time of the ultrasound assessment.
Figure 1. Caliper placement to calculate TCD.
Statistical Analysis
Data were analyzed using the available statistical package of SPSS-25 (Statistical Packages for Social Sciences- version 25). Data were presented in simple measures of frequency, percentage, mean, standard deviation, and range. The significance of difference of different means (quantitative data) was tested using Student’s t-test for difference between two independent means or analysis of variance (ANOVA test) for difference among more than two independent means after the data fulfilled the condition for parametric test applications. A level of P value less than or equal 0.05 was considered statistically significant.
For the correlation between two quantitative variables, the Pearson correlation was calculated with its t-test for testing the significance of correlation. The correlation coefficient value (r) was considered either positive (direct correlation) or negative (inverse correlation) with values <0.3 represent no correlation, 0.3-<0.5 indicate weak correlation, 0.5-<0.7 represent moderate strength, >0.7 strong correlation. The coefficient of determination (r2) was also calculated.
Results
The mean maternal age (years) was (27.0 ± 5.7) with a range of (15-39). The mean of parity was (1.0 ± 1.3) with a range of (0-5) and the mean gestational age by LMP in weeks was (34.3 ± 3.4) with a range of (28-40). The majority of pregnant women belonged to age groups 20-24 years (30.8%) and 25-29 years (30.8%). Regarding the parity status, 53.8% were primigravida and 46.2% were multiparas. In the majority of these pregnancies, the gestational age by LMP was between 28 to 30 weeks, accounting for 26.2% of the population. When considering fetal sex, 52.3% were males and 47.7% were females as shown in Table 1.
| Characteristics | No | % | |
|---|---|---|---|
| Age (years) | <20 y | 5 | 7.7 |
| 20-24 | 20 | 30.8 | |
| 25-29 | 20 | 30.8 | |
| 30-34 | 13 | 20 | |
| Parity | 0 | 35 | 53.8 |
| 1 | 8 | 12.3 | |
| 2 | 14 | 21.6 | |
| 3 | 4 | 6.2 | |
| 4 | 3 | 4.6 | |
| 5 | 1 | 1.5 | |
| GA by LMP (weeks) | <31 w | 17 | 26.2 |
| 31-32 ± 6 | 8 | 12.3 | |
| 33-34 ± 6 | 7 | 10.8 | |
| 35-36 ± 6 | 12 | 18.5 | |
| 37-38 ± 6 | 14 | 21.5 | |
| >39 w | 7 | 10.8 | |
| Sex | Male | 34 | 52.3 |
| Female | 31 | 47.7 |
Table 1: Demographic maternal and fetal characteristics.
Table 2 shows fetal biometric parameters (BPD and FL) in addition to TCD measurements according to each gestational age category by LMP by fetal sex. The means for all the three measured parameters significantly increased with the progression of gestation (P < 0.001). When comparing male and female sex for each fetal biometric parameter according to each gestational age category, there were no significant differences in mean measurements (p>0.05).
| GA by LMP (weeks) | BPD (mm) | FL (mm) | TCD (mm) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | Male | Female | Total | |||||||
| No | Mean ± SD | No | Mean ± SD | Mean ± SD (Range) | No | Mean ± SD | No | Mean ± SD | Mean ± SD (Range) | No | Mean ± SD | No | Mean ± SD | Mean ± SD (Range) | |
| GA <31 w | 5 | 75.0 ± 3.1 | 12 | 77.1 ± 3.0 | 76.48 ± 3.08 (70.7-83.0) | 5 | 55.3 ± 2.8 | 12 | 58.1 ± 3.4 | 57.26 ± 3.43 (53.0-65.0) | 5 | 34.7 ± 1.9 | 12 | 36.6 ± 2.2 | 36.02 ± 2.22 (32.2-41.6) |
| 31-32+6 | 5 | 79.2 ± 3.3 | 3 | 79.3 ± 1.4 | 79.23 ± 2.57 (76.0-84.0) | 5 | 58.7 ± 2.2 | 3 | 60.9 ± 1.0 | 59.55 ± 2.05 (56.0-62.0) | 5 | 38.8 ± 0.9 | 3 | 39.2 ± 1.3 | 38.93 ± 0.95 (37.9-40.5) |
| 33-34+6 | 4 | 83.3 ± 3.8 | 3 | 81.8 ± 2.3 | 82.66 ± 3.09 (78.7-87.5) | 4 | 62.2 ± 4.8 | 3 | 62.0 ± 1.1 | 62.11 ± 3.44 (55.2-66.0) | 4 | 41.2 ± 3.2 | 3 | 40.2 ± 1.0 | 40.73 ± 2.38 (38.0-44.0) |
| 35-36+6 | 8 | 89.9 ± 2.1 | 4 | 90.0 ± 1.7 | 89.88 ± 1.89 (86.0-92.0) | 8 | 67.6 ± 1.5 | 4 | 69.3 ± 2.9 | 68.16 ± 2.08 (65.0-73.0) | 8 | 46.5 ± 1.1 | 4 | 47.2 ± 2.9 | 46.75 ± 1.74 (44.0-50.3) |
| 37-38+6 | 7 | 91.8 ± 2.8 | 7 | 89.1 ± 2.8 | 90.44 ± 3.05 (85.0-95.0) | 7 | 71.7 ± 2.1 | 7 | 69.7 ± 2.5 | 70.70 ± 2.45 (64.6-75.0) | 7 | 50.4 ± 3.3 | 7 | 48.6 ± 3.3 | 49.51 ± 3.29 (43.0-54.0) |
| >39 w | 5 | 95.6 ± 1.8 | 2 | 97.0 ± 2.8 | 96.00 ± 2.00 (94.0-99.0) | 5 | 74.8 ± 1.1 | 2 | 76.9 ± 1.3 | 75.39 ± 1.47 (73.0-77.8) | 5 | 54.8 ± 1.3 | 2 | 56.1 ± 0.7 | 55.19 ± 1.26 (53.9-57.0) |
| p value | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | ||||||
* Significant using ANOVA test for difference among different independent means at 0.05 level
Table 2: Fetal biometric parameters (BPD & FL) in addition to trancerebellar diameter (TCD) according to gestational age by LMP by fetal sex.
There was a significant strong correlation between estimated gestational age by LMP and each of biometric parameters in terms of BPD, FL, and TCD; still, the TCD was the mostly correlated parameter when compared to BPD and FL having the highest correlation coefficient (r=0.955, p=0.0001; r=0.931, p=0.0001; r=0.931, p=0.0001 respectively). Even when considering fetal sex whether male or female, the TCD was the most sensitive parameter to correlate with gestational age by LMP. For male sex, the TCD has the highest correlation coefficient when compared to BPD and FL (r=0.965 for TCD vs. r=0.931 and 0.945 for BPD and FL respectively). For female sex, again the TCD has the highest correlation coefficient when compared to BPD and FL (r=0.946 for TCD vs. r= 0.931 and 0.923 for BPD and FL respectively) as shown in Table 3, Figures 2 and 3. When considering the correlation between LMP-based estimated gestational age and TCD (in weeks) for the total group of pregnant women and according to fetal sex differences whether pregnant with male or female fetuses, one can apply the following equations for practical purposes (although there was little sex difference when applied):
| GA by LMP (weeks) | BPD (mm) | FL (mm) | TCD (mm) | |||||||
| Total | Male | Female | Total | Male | Female | Total | Male | Female | ||
| r | 0.931* | 0.931* | 0.931* | 0.931* | 0.945* | 0.923* | 0.955* | 0.965* | 0.946* | |
| P | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | |
| n | 65 | 34 | 31 | 65 | 34 | 31 | 65 | 34 | 31 | |
Table 3: Correlations of different biometric parameters with gestational age by LMP (weeks) by fetal sex.
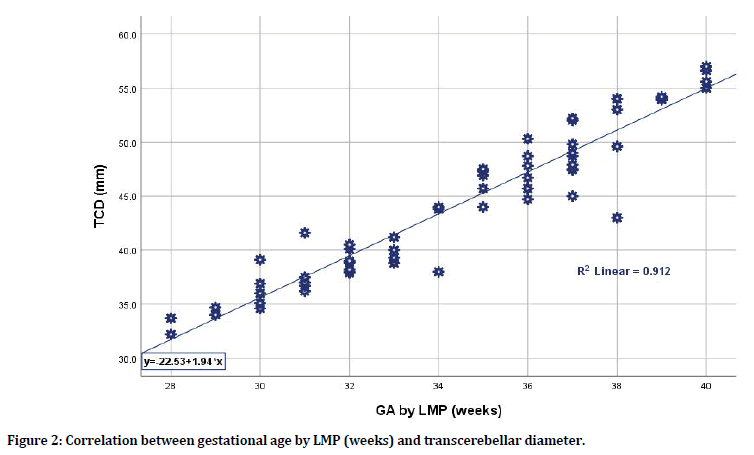
Figure 2. Correlation between gestational age by LMP (weeks) and transcerebellar diameter.
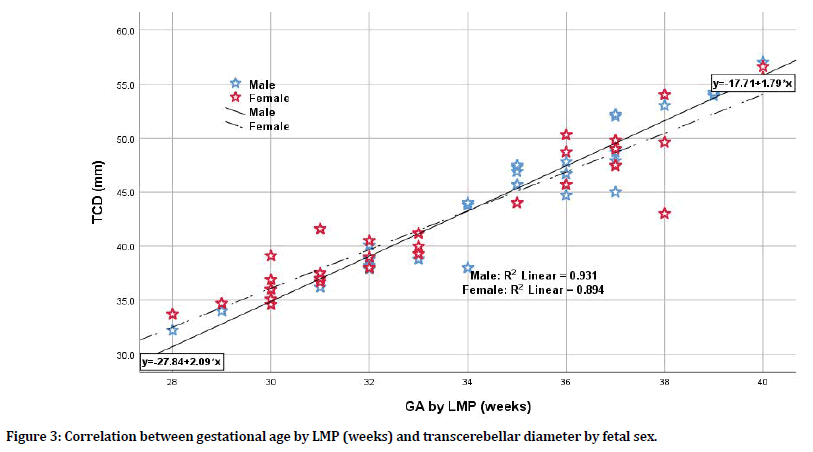
Figure 3. Correlation between gestational age by LMP (weeks) and transcerebellar diameter by fetal sex.
y=-22.53+1.94 × (1) (x=GA by LMP in weeks; y=TCD in mm)
y=-27.84+2.09 × (2) (x=GA by LMP in weeks; y=TCD in mm for male fetuses)
y=-17.71+1.79 × (3) (x=GA by LMP in weeks; y=TCD in mm for female fetuses)
Discussion
Accurate gestational dating is the cornerstone for the management of pregnancy. There are inherent problems in using the menstrual cycle for assessing gestational age. One of these obstacles is that many women do not have regular cycles, and that only 77% have average cycle lengths ranging between 25-31 days adding to this that more than 45 percent of pregnant women are unsure of their LMP due to poor recall, having bleeding in early pregnancy, or recent use of hormonal contraception within two months period of having conception [7]. Added to this some patients in our setup come seeking antenatal care for the first time in the third trimester for either socioeconomic reasons, or being uneducated, and even some women are lactating, which make them unsure of the LMP. This makes the ultrasound assessment of the gestational age a central element and an integral part of obstetric practice.
Using the standard fetal biometric parameters by measuring BPD and FL, have been used to establish ultrasound GA estimation. Still, the precision tolerance for BPD dimensions decreases during the last trimester of pregnancy. Similarly, the accuracy of FL decreases with advancing gestation [8]. The measurement of the TCD is an emerging accurate ultrasound parameter useful for the estimation of gestational age [5,9]. In the current study, and as shown in Table 2, the means for all the three measured biometric parameters (BPD, FL, and TCD) significantly increased with the progression of gestation. This was in consistence with other studies which showed that for different gestational ages, gestational age estimates for fetal biometric parameters showed a progressive trend [4,5,10].
In the present study, and when comparing fetal sex (whether pregnant with male or female fetuses), the mean measurements for each of BPD, FL, and TCD showed insignificant differences at each gestational age category.
Concerning fetal biometrical indices, in fact little researches have been performed focusing on differences in fetal sex. Broere-Brown et al. found that FL for male fetuses was smaller when compared to that of female fetuses. In another study, and similar to the results of the present study, they did not report any effect of fetal sex on FL [11,12]. In one retrospective study by O’Neill et al. and on analysis of a cohort of 1,246 women with unassisted conception in their midgestation, male BPD exceeded female BPD [13], this was inconsistent with our study results which can be explained by small sample size in the present study and that we performed the fetal biometric measurements in the last trimester of pregnancy. Regarding TCD, the present study is one of the first studies showing that TCD mean measurements do not differ between male and female fetuses at each gestational age category as measured according to LMP. This makes TCD as a tool that can be relied upon for estimating fetal age in third trimester of pregnancy.
In this study, between 28-40 weeks of gestation, TCD measurements correlated more with estimated gestational age by LMP when compared to BPD and FL with the highest correlation coefficient ((r=0.955 for TCD vs. r=0.931 and r=0.931 for BPD and FL respectively). Many researchers worked on the correct estimation of gestational age by ultrasound by measuring different fetal biometric parameters at different gestational age categories and correlate it with gestational age as calculated by LMP with findings similar to the present study that TCD was a strong estimator of gestational age. Dashottar et al. [5] conducted their study on two hundred pregnant women with normal pregnancies and intrauterine growth-retarded pregnancies between 15 to 40 weeks of gestations, they found that TCD strongly correlated at 16-20, >20-24 and >24-28 weeks (r>0.7), moderately correlated at >24-28 weeks (r=0.600) and mildly correlated at >32-35 and >36-40 weeks (r=0.376 and 0.387 respectively) and that overall, there was a strong correlation between TCD measurements and gestational age (r=0.984). Adeyekun et al. [14] studied 450 Nigerian Africans women between 14-42 weeks of pregnancy and found that TCD was the best to correlate and significantly with the menstrual age when compared with other routinely used fetal biometric parameters, r=0.984 with p-value=0.000. Bansal et al. [15] studied 650 cases between 14-40 weeks of gestation and found a highly significant correlation between TCD and gestational age in both normal and intrauterine growth-retarded fetuses. Eze et al. in their study also reported that TCD measurement had a direct correlation with GA that is obtained from patient’s LMP in both the second and third trimesters of pregnancy and that TCD has a strong linear relationship with GA [16]. Alalfy et al. [17] studied 60 Egyptian ladies in their second and third trimesters with both uncomplicated and complicated pregnancies, they found that transcerebellar diameter was significantly positively correlated to menstrual gestational age.
In the present study and when focusing on fetal sex, again the TCD was the best parameter to correlate with gestational age by LMP compared to BPD and FL as shown in Table 3, and Figure 3. To the best of our knowledge, this was the first study focusing on fetal sex when considering the correlation between TCD and estimated gestational age by LMP again the TCD was the most sensitive parameter with correlation coefficients for TCD for male and female fetuses of r=0.965 and r=0.946 respectively. This observation seems to make TCD more accurate than other traditional universally accepted parameters in terms of BPD and FL in third trimesters.
In this study and on analysis of data, three formulas were obtained and these can be applied for practical purposes; where x (GA in weeks) can be calculated once y (TCD in mm) is known irrespective of fetal sex and when pregnant with male or female fetuses. Although higher measurements were recorded in general for female fetuses than males but there were no significant differences in mean measurements. Still, separate equations were formulated for male and female fetuses although there is little sex difference when applied. Bansal et al. and Eze et al. in their studies also derived equations for gestational age estimation [15,16].
Furthermore, in this study, the recorded fetal biometric measurements including TCD were performed by the same sonographer to reduce inter-observer variability. Moreover, Eze et al. [16] found that there was no statistically significant difference in the recorded TCD measurements between each sonographer or between two different sonographers, this makes TCD a valuable measure in clinical practice.
In the current study, it is clear that TCD may be used with confidence for GA estimation in late pregnancy and that in a tertiary center with extra expertise and good resolution machine, obtaining TCD as a single biometric measurement has the advantage for reducing scanning time. There are limitations to this study, namely that the sample size is small, at only 65 measurements and that this is from one medical center. Further studies on larger population size are needed to assess the role of TCD in fetal growth and fetal growth retardation.
Conclusion
Fetal transcerebellar diameter can be taken as a good indicator of gestational age in late pregnancy. Its values strongly correlate with estimated gestational age by last menstrual period.
Conflict of Interest
None declared.
References
- Chudleigh T, Smith A, Cumming S. Dating and screening the pregnancy between 10 and 14 weeks. In: Obstetric & Gynaecological Ultrasound: How, Why and When. 4th Edn, Elsevier 2017; 123-157.
- Committee Opinion No 700: Methods for estimating the due date. Obstet Gynecol 2017; 129:150-154.
- Callahan TL, Caughey AB. Pregnancy and prenatal care. In: Blueprints obstetrics & gynecology, 6th Edn. Chapter 1. Lippincott Williams & Wilkins 2013; 1-11.
- Uikey PA, Kedar KV, Khandale SN. Role of trans-cerebellar diameter in estimating gestational age in second and third trimester of pregnancy. Int J Reprod Contracept Obstet Gynecol 2016; 5:3411-3415.
- Dashottar S, Senger KPS, Shukla Y, et al. Transcerebellar diameter: An effective tool in predicting gestational age in normal and IUGR pregnancy. Int J Reprod Contracept Obstet Gynecol 2018;7:4190-4196.
- Orji MO, Adeyekun AA. Ultrasound estimation of fetal gestational age by transcerebellar diameter in healthy pregnant Nigerian women. West Afr J Med 2014; 33:61-67.
- Kalish RB. Sonographic determination of gestational age. Donald school basic textbook of ultrasound in obstetrics & gynecology, Jaypee Brothers Medical Publishers 2016; 59-66.
- Žaliūnas B, Bartkevičienė D, Drąsutienė G, et al. Fetal biometry: Relevance in obstetrical practice. Medicina 2017; 53:357-364.
- Reddy RH, Prashanth K, Ajit M. Significance of foetal transcerebellar diameter in foetal biometry: A pilot study. J Clin Diagn Res 2017; 11:1-4.
- Chavez MR, Ananth CV, Smulian JC, et al. Fetal transcerebellar diameter measurement with particular emphasis in the third trimester: A reliable predictor of gestational age. Am J Obstet Gynecol 2004; 191:979-984.
- Broere-Brown ZA, Baan E, Schalekamp-Timmermans S, et al. Sex-specific differences in fetal and infant growth patterns: A prospective population-based cohort study. Biol Sex Differ 2016; 7:65.
- Schwarzler P, Bland JM, Holden D, et al. Sex-specific antenatal reference growth charts for uncomplicated singleton pregnancies at 15-40 weeks of gestation. Ultrasound Obstet Gynecol 2004; 23:23-29.
- O'Neill KE, Tuuli M, Odibo AO, et al. Sex-related growth differences are present but not enhanced in in vitro fertilization pregnancies. Fertil Steril 2014; 101:407-412.
- Adeyekun AA, Orji MO. Predictive accuracy of trans-cerebellar diameter in comparison with other fetal biometric parameters for gestational age estimation among pregnant Nigerian women. East Afr Med J 2014; 91:138-144.
- Bansal M, Bansal A, Jain S, et al. A study of correlation of transverse cerebellar diameter with gestational age in the normal and growth restricted fetuses in western Uttar Pradesh. Peop J Sci Res 2014; 7:16-21.
- Eze CU, Onwuzu QE, Nwadike IU. Sonographic Reference Values for fetal transverse cerebellar diameter in the second and third trimesters in a Nigerian population. J Diagn Med Sonogr 2017; 33:174–181.
- Alalfy M, Idris O, Gaafar H, et al. The value of fetal trans cerebellar diameter in detecting GA in different fetal growth patterns in Egyptian fetuses. Imaging Med 2017; 9:131-138.
Author Info
Lilyan W Sersam1*, Sura Basil Findakly1 and Najlaa Hanoon Fleeh2
1Department of Obstetrics and Gynaecology, College of Medicine, Mustansiriyah University, Baghdad, Iraq2Department of Radiology, Mustansiriyah University, Baghdad, Iraq
Citation: Lilyan W Sersam, Sura Basil Findakly, Najlaa Hanoon Fleeh, Fetal Transcerebellar Diameter in Estimating Gestational Age in Third Trimester of Pregnancy, J Res Med Dent Sci, 2019, 7(5):60-66.
Received: 09-Sep-2019 Accepted: 26-Sep-2019
