Research Article - (2023) Volume 11, Issue 6
Evaluation of the Anti-inflammatory of Leucaena leucocephala extracts in Experimental Rats.
Azal Satar Al-Baaj*, Thukaa Z Abdul-Jalil and Ammar A Fadhil
*Correspondence: Dr. Azal Satar Al-Baaj, Department of Pharmacognosy and Medicinal Plants, College of Pharmacy, University of Baghdad, Baghdad, Iraq, Email:
Abstract
A lot of previous studies are concerned with the evaluation of the anti-inflammatory activity of medicinal plants because it considered cheap and are believed to possess minimal side effects. Leucaena leucocephala didn’t evaluate globally for its anti-inflammatory effect yet though some of it’s already separated and identified secondary metabolites were studied and proved to exert many pharmacological activities besides their effect on lowering the pro-inflammatory cytokines like TNF-α and IL-6. So, there was an interest to evaluate the biological effect of Leucaena leucocephala as a novel anti-inflammatory agent was the first motivation to start an in vivo study using a rat population. The N-butanol and ethyl acetate extracts were chosen to undergo this study since they contain a lot of the polyphenolic compounds (flavonoids, stilbenes and phenolic acids) which is natural bioactive compounds. Thirty albino rats weighing (150-200) grams of both sexes were used and divided into five groups, each group containing 6 rats: Group I: Negative control, group II: Positive control, group III: Treatment with n-butanol extract, group IV: Treatment with EA extract, group V: Treatment with diclofenac sodium. The oral route of administration using gastric gavage was dependent and for consecutive seven days of supplementation. The induction of inflammation was done by insertion of cotton pellets subcutaneously into the ventral region that is previously shaved and sterilized, with one pellet on each side. So, Leucaena leucocephala proved its novel anti-inflammatory effect by a significant decrease in the inflammation in albino rat models by reducing the percentage of exudate and granuloma and decreasing the serum concentrations of TNF-α and IL-6.
Keywords
Leucaena leucocephala, Anti-inflammatory, Polyphenolic, Flavonoids, Albino rats
Introduction
Aim: This study focused on extracting secondary metabolites that are present in the leaves of Iraqi Leucaena leucocephala and evaluating their antiinflammatory effect in vitro on albino rats.
The inflammation process is considered the immune system’s response to exogenous and endogenous damaging stimuli, such as toxic components, infections, damaged cells and irradiation. It works by eliminating damaging stimuli and allowing the body to initiate the healing process. As a result, inflammation is a necessary defense mechanism for health [1].
Inflammation can be acute or chronic and untreated acute inflammation can lead to the development of several chronic inflammatory diseases, including diabetes, heart disease, cancer, stroke and obesity, which are among the world's leading causes of mortality [2].
Redness, swelling, heat, pain and loss of tissue function are inflammatory indications and symptoms in the tissues, which are caused by local immunological, vascular and inflammatory cell responses to infection or injury. The major microcirculatory events that occur during the inflammation include vascular permeability changes, leukocyte recruitment and accumulation and inflammatory mediator release [3-7].
Anti-inflammatory drugs are available in a wide range; they differ in the percent of their effect, side effects and contraindications with other drugs and diseases. The most effective anti-inflammatory drugs are statins, Non- Steroidal Anti-Inflammatory Drugs (NSAIDs) and corticosteroids, which treat inflammation through different mechanisms [8-11].
There is still a need for discovering and developing safe and effective therapeutic alternatives. Many studies have been done on several plants to determine their antiinflammatory activity. Leucaena leucoceohala contains high content of flavonoids which are poly-phenolic compounds that have many pharmacological effects such as analgesic activity, antibacterial activity, antidiarrheal activity, anthelmintic activity, larvicidal activity, antidiabetic activity, anti-oxidative, antiinflammatory, anti-mutagenic and anti-carcinogenic properties. Also, they have a potent inhibitory effect on several enzymes, such as Xanthine Oxidase (XO), Cyclo-Oxygenase (COX), lipoxygenase and phosphoinositide 3-kinase [12-15].
Flavonoids that presence in Leucaena leucoceohala include apigenin, chrysin, luteolin, resveratrol, catechin, epigallocatechin, quercetin, quercitrin, kaempferol, myricetin kaempferol, rutin, tiliroside glc (6’’-O-p-coumaroyl) and Fisetin etc. that have antiinflammatory activity, they will inhibit the inflammation by a reduction in the levels of several cytokines including tumor necrotic factor-α, interleukin-6 and Interleukin-1β (IL-1β), Interleukin-10 (IL-10) and suppression of COX1 and COX-2, prostaglandins, reactive oxygen species, prostaglandins, histamine, leukotriene and platelets activated factors [16-18].
N-butanol and ethyl acetate fractions of Leucaena leucocephala were proved to be rich with flavonoids and stilbenes which are both poly-phenolic compounds that exert their anti-inflammatory activity by reducing the pro-inflammatory cytokines like TNF-α and IL-6, this reduction was due to inhibiting the expression of IL-6 and TNF-α via suppressing nuclear factor kappa light chain enhancer of activated B cell (NF)-κB signalling which is a protein complex that plays a rule in DNA transcription, cytokine production and cell continuance. NF-kB translocate into the nucleus and up regulates genes associated with inflammation (IL-6 and TNF-α)[19,20].
Also, they increase the Siritin-1 (SIRT-1) expression which is an enzyme located in the cell nucleus and has a cellular regulatory job, decreases the NF-κB activation and decreases the messenger ribonucleic acid mRNA expression for IL6, TNF-α in the visceral adipose tissue [21]. In the same way, polyphenols inhibit the expression of COX-2 enzymes which are responsible for the metabolism of arachidonic acid to prostaglandin that is activated during inflammation and participates in swelling, pain and redness (Figure1) [22].
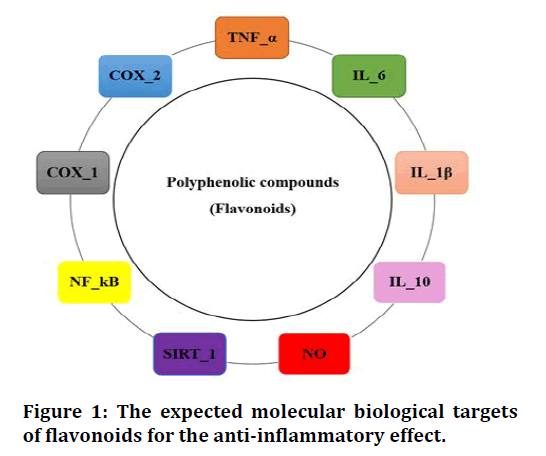
Figure 1: The expected molecular biological targets of flavonoids for the anti-inflammatory effect.
Materials and Methods
Evaluation of the anti-inflammatory effect of Leucaena leucocephala
Plant material collection and preparation: The plant was collected from one of the farms located in Al- Diwaniyah city in the middle of Iraq in September 2021. The leaves were isolated, cleaned, dried for a week under the shade to be dried completely and ground to be ready for extraction.
Animal preparations: Albino rats (both sexes, male and female) were used in this study, brought from the animal house of the college of pharmacy/university of Baghdad/ Iraq. They acclimatized under a light (12 hours): Dark (12 hours) cycle for 1 week before starting experiments and at 25°C ± 2°C. The experimental work was made in accordance with the rules of laboratory animal care and the ethical rules for the use of experimental animals in research [24].
In this study thirty albino rats weighing (150-200) grams of both sexes were used and divided into five groups, each group containing 6 rats. The oral route of administration using gastric gavage was dependent and for consecutive seven days of supplementation.
• Group I: Negative control (no inflammation, no
treatment).
• Group II: Positive control that treated with vehicle,
distilled water. (Induction of inflammation, no
treatment).
• Group III: Induction of inflammation, treatment with
n-butanol extracted fraction of Leucaena leucocephala
(250 mg/kg orally).
• Group IV: Induction of inflammation, treatment with
EA extracted fraction of Leucaena leucocephala (250
mg/kg orally).
• Group V: Induction of inflammation, treatment with
diclofenac sodium (10 mg/kg orally) [25].
Cotton pellet induced granuloma: The induction of inflammation in albino rats was done by using cotton pellets weighing (10 ± 1) mg for induced granuloma formation. The cotton pellets were sterilized in the autoclave at a temperature equal to 120°C and 15 Ib pressure for 15 minutes. The rats were anesthetized under diethyl ether anesthesia, two cotton pellets were placed subcutaneously into the ventral region that is previously shaved and sterilized, one pellet on each side [26].
Animal scarifying and anti-inflammatory parameters collection: On the eighth day, the animals were sacrificed by cervical dislocation after being anesthetized by using diethyl ether, the animal’s blood (from their hearts) was collected in test tubes separated from each other and set aside for 15 minutes at room temperature then centrifuged for 15 minutes under 1500 round/min. The separated serum was collected (1-1.5 ml) and kept in labeled Eppendorf that were incubated in a fridge at -21°C for ELISA quantitative analysis of TNFα and IL-6 as pro-inflammatory cytokines [27,28].
Then the pellets were carefully removed from the animals together with the granuloma tissues and made free from extraneous tissues. The wet pellets were weighed by a sensitive electrical balance for the determination of wet weight and then dried at 60°C until a constant weight was obtained when all the exudates dried, after that the dried pellets were weighed again [29,30].
The exudate amount was calculated by subtracting the constant dry weight of the pellet from the immediate wet weight of the pellet, while the granulation tissue formation (dry weight of granuloma in mg.) was calculated after subtracting the weight of the cotton pellet (initial weight=11 mg) from the constant dry weight of pellet and taken as a measure of granuloma tissue formation. The percent inhibitions of exudate and inhibition of granuloma tissue formation were calculated according to the following equations:
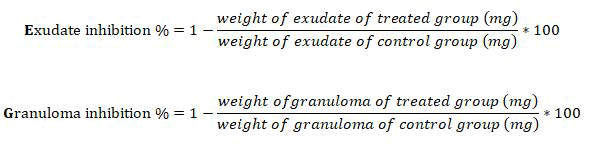
ELISA analysis: Human reader HS ELISA apparatus was used for the detection of Tumor Necrotic Factor-Alpha (TNF-α) and Interleukin-6 (IL-6) levels by using rat TNF- α and IL-6 ELISA kits from Shanghai YL Biotech Co., Ltd, China and Jiaxing Korean Biotech Co., Ltd.
Statistical analysis: All experiment data are expressed as mean ± SEM. Statistical analysis was carried out by using ANOVA followed by the tukey test. The values are significant at (p<0.05) (Figure 2).
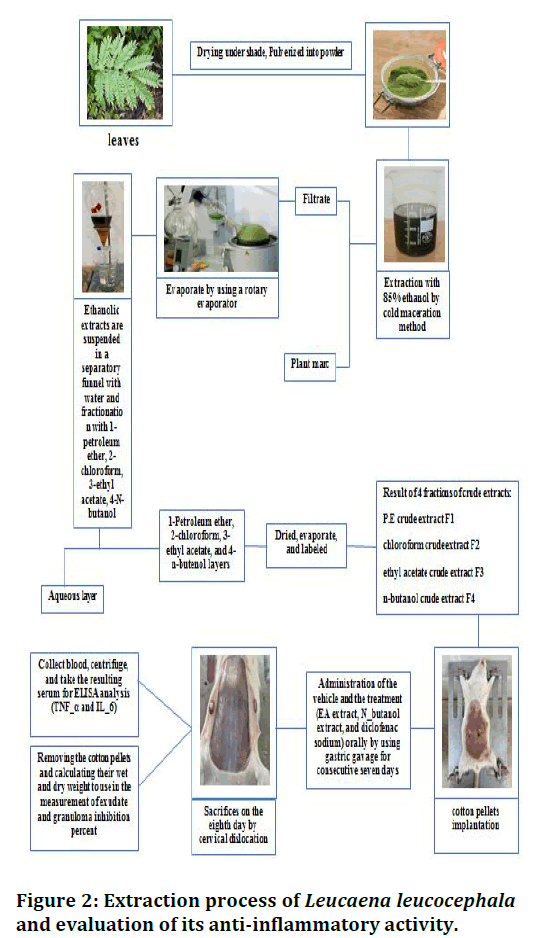
Figure 2: Extraction process of Leucaena leucocephala and evaluation of its anti-inflammatory activity.
Results and Discussion
A lot of previous studies are concerned with the evaluation of the anti-inflammatory activity of medicinal plants because it considered cheap and are believed to possess minimal side effects [31].
Leucaena leucocephala didn’t evaluate globally for its anti-inflammatory effect yet though some of its already separated and identified secondary metabolites were studied and proved to exert many pharmacological activities like resveratrol, rutin, quercitrin, quercetin etc. besides their effect on lowering the pro-inflammatory cytokines like TNFα and IL-6 [32-37].
So, there was an interest to evaluate the biological effect of Leucaena leucocephala as a novel anti-inflammatory agent was the first motivation to start an in vivo study using a rat population.
The ethyl acetate and n-butanol extracted fractions were chosen to undergo this study since they contain a lot of the poly-phenolic compounds (flavonoids, stilbenes and phenolic acids) found in the plant which is naturally bioactive compounds.
Effect of N-butanol and ethyl acetate fractions on the percent of exudate inhibition and percent of granuloma inhibition
The mean values of wet weight and dry weight of cotton pellets, percent of exudate inhibition and percent of granuloma inhibition were calculated, the calculations show an interesting result in which the greatest values of inhibition were recorded for the nbutanol and ethyl acetate fraction of Leucaena leucocephala advanced over the NSAID drug diclofenac sodium that used as a reference for its anti-inflammatory effect (Table 1). Group III, group IV and group V show a significant difference in their anti-inflammatory effect regarding the control group (II) (Figures 3 and 4).
| Groups | N | Mean exudate weight in mg Mean ± SD | Percent of exudate inhibition % | Mean granuloma weight in mg Mean ± SEM | Percent of granuloma inhibition % |
|---|---|---|---|---|---|
| Group II (positive control) | 6 | 119.5 ± 25.04 | - | 24 ± 10.93 | - |
| Group III (treated with n-butanol extract) | 6 | 85.66 ± 9.54 | 28.31 | 18.16 ± 9.60 | 24.3 |
| Group IV (treated with EA extract) | 6 | 88.66 ± 20.50 | 25.8 | 19.83 ± 11.65 | 17.36 |
| Group V (treated with diclofenac sodium) | 6 | 95.5 ± 15.97 | 20.08 | 20.5 ± 14.09 | 14.58 |
Table 1: Show the effect of Leucaena leucocephala extracts and diclofenac sodium on the exudate weight, granuloma weight, percent of exudate inhibition and percent of granuloma inhibition.
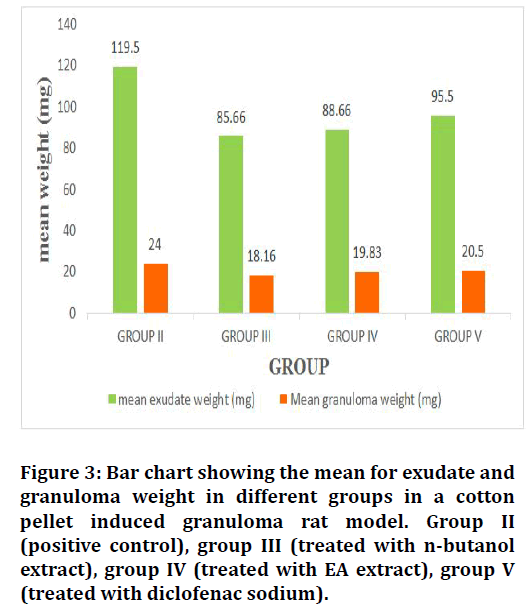
Figure 3: Bar chart showing the mean for exudate and granuloma weight in different groups in a cotton pellet induced granuloma rat model. Group II (positive control), group III (treated with n-butanol extract), group IV (treated with EA extract), group V (treated with diclofenac sodium).
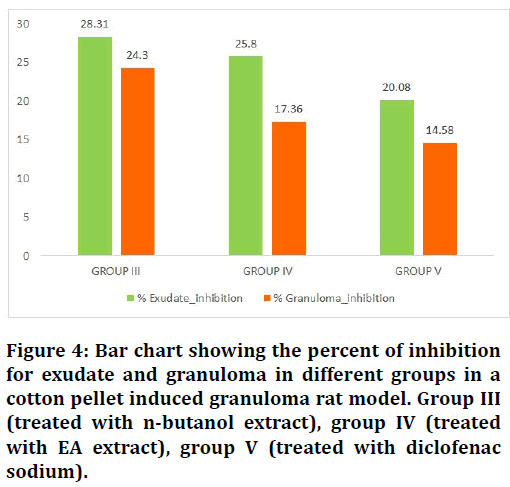
Figure 4: Bar chart showing the percent of inhibition for exudate and granuloma in different groups in a cotton pellet induced granuloma rat model. Group III (treated with n-butanol extract), group IV (treated with EA extract), group V (treated with diclofenac sodium).
Effect of N-butanol and ethyl acetate fractions on Tumor Necrotic Factor (TNF-α) and Interleukin-6 (IL-6) levels in rat groups
By evaluating the data obtained from the inflammatory markers TNF-α and IL-6, it showed that the N-butanol and ethyl acetate fractions of Leucaena leucocephala plant that administration to rats (group III, IV) resulted in a significant decrease in the serum level of TNF-α and IL-6 compared to the positive control group (non-treated group, II) (Table 2). On the other hand, TNF-α mean values ± SD and IL-6 mean values ± SD of groups III and IV in comparison with the diclofenac treated group (group V) didn’t show a large significant difference in their levels, this led to the fact that the plant extracts have comparable anti-inflammatory activity to that of NSAID drug diclofenac sodium (Figures 5 and 6).
| Group | N | Mean TNF-α level ± SD | Mean IL-6 level ± SD |
|---|---|---|---|
| Group I (negative control) | 6 | 167.59 ± 50.5 | 8.06 ± 0.78 |
| Group II (positive control) | 6 | 777.1 ± 167.06 | 21.37 ± 6.50 |
| Group III (treated with n-butanol extract) | 6 | 114.57 ± 9.39 | 6.94 ± 0.55 |
| Group IV (treated with EA extract) | 6 | 136.8 ± 37.3 | 7.48 ± 0.53 |
| Group V (treated with diclofenac sodium) | 6 | 148.185 ± 7.88 | 8.26 ± 1.91 |
Table 2: The effect of Leucaena leucocephala extracts and diclofenac sodium on TNF-α and IL-6 levels in rat groups.
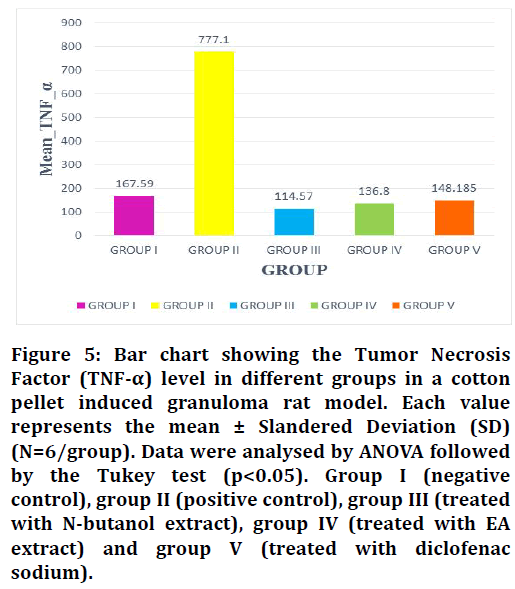
Figure 5: Bar chart showing the Tumor Necrosis Factor (TNF-α) level in different groups in a cotton pellet induced granuloma rat model. Each value represents the mean ± Slandered Deviation (SD) (N=6/group). Data were analysed by ANOVA followed by the Tukey test (p<0.05). Group I (negative control), group II (positive control), group III (treated with N-butanol extract), group IV (treated with EA extract) and group V (treated with diclofenac sodium).
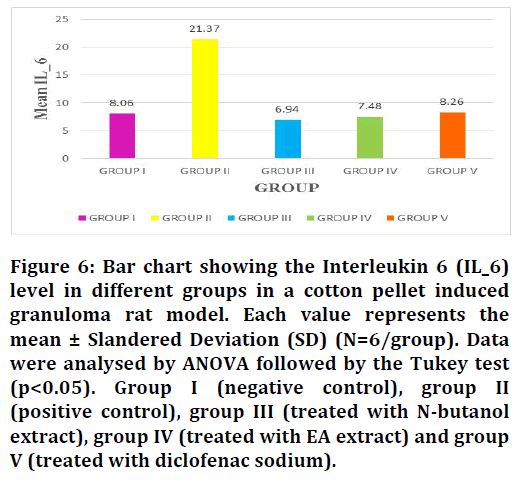
Figure 6: Bar chart showing the Interleukin 6 (IL_6) level in different groups in a cotton pellet induced granuloma rat model. Each value represents the mean ± Slandered Deviation (SD) (N=6/group). Data were analysed by ANOVA followed by the Tukey test (p<0.05). Group I (negative control), group II (positive control), group III (treated with N-butanol extract), group IV (treated with EA extract) and group V (treated with diclofenac sodium).
So, Leucaena leucocephala proved its novel antiinflammatory effect by a significant decrease in the inflammation in albino rat models by reducing the percentage of exudate and granuloma. Furthermore, it is significantly decreasing the serum concentrations of TNF-α and IL-6.
Conclusion
N-butanol and ethyl acetate fractions were very valuable due to their richness with many secondary metabolic substances such as flavonoids, stilbene and phenolic acids (rutin, resveratrol, quercetin, isorhamnetin, luteolin, caffeic acid and coumaric acid) that were identified and characterized to be found in plant n-butanol and ethyl acetate fractions by using TLC and HPLC chromatographic analysis.
A novel anti-inflammatory activity was proven by using the rich n-butanol and ethyl acetate fractions in a rat model in vivo study, the plant fractions were very potent and of strong activity in comparison to the positive control and NSAID diclofenac sodium.
References
- Chen L, Deng H, Cui H, et al. Inflammatory responses and inflammation associated diseases in organs. Oncotarget 2018; 9:7204-7218.
[Crossref] [Google Scholar] [PubMed]
- Ginwala R, Bhavsar R, Chigbu DGI, et al. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 2019; 8:35.
[Crossref] [Google Scholar] [PubMed]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010; 140:805-820.
[Crossref] [Google Scholar] [PubMed]
- Netea MG, Balkwill F, Chonchol M, et al. A guiding map for inflammation. Nat Immunol 2017; 18:826-831.
[Crossref] [Google Scholar] [PubMed]
- Ma KC, Schenck EJ, Pabon MA, et al. The role of danger signals in the pathogenesis and perpetuation of critical illness. Am J Respir Crit Care Med 2018; 197:300-309.
[Crossref] [Google Scholar] [PubMed]
- Wang RX, Zhou M, Ma HL et al. The role of chronic inflammation in various diseases and anti-inflammatory therapies containing natural products. Chem Med Chem 2021; 16:1576-1592.
[Crossref] [Google Scholar] [PubMed]
- Afonina IS, Zhong Z, Karin M, et al. Limiting inflammation the negative regulation of NF-B and the NLRP3 inflammasome. Nat Immunol 2017; 18:861-869.
[Crossref] [Google Scholar] [PubMed]
- Gandhi J, Khera L, Gaur N, et al. Role of modulator of inflammation cyclooxygenase-2 in gamma herpes virus mediated tumorigenesis. Front Microbiol 2017; 8:1â??12.
[Crossref] [Google Scholar] [PubMed]
- Bleich SN, Sherrod C, Chiang A, et al. Systematic review of programs treating high need and high cost people with multiple chronic diseases or disabilities in the United States, 2008-2014. Prev Chronic Dis 2015; 12:4-7.
[Crossref] [Google Scholar] [PubMed]
- Nguyen NH, Khera R, Machado OL, et al. Annual burden and costs of hospitalization for high need, high cost patients with chronic gastrointestinal and liver diseases. Clin Gastroenterol Hepatol 2018; 16:1284-1292.
[Crossref] [Google Scholar] [PubMed]
- Li P, Zheng Y, Chen X. Drugs for autoimmune inflammatory diseases: From small molecule compounds to anti-TNF biologics. Front Pharmacol 2017; 8:1â??12.
[Crossref] [Google Scholar] [PubMed]
- Chatchanayuenyong R, Sujayanont P, Vuttivirojana A. Effects of Leucaena leucocephala (Lam.) de wit leaves extracts in culture of human umbilical vein cells. Pharmacogn J 2018; 10:148-153.
- Zhou Q, Xu H, Yu W, et al. Anti-inflammatory effect of an apigenin maillard reaction product in macrophages and macrophage endothelial co-cultures. Oxid Med Cell Longev 2019; 2019.
[Crossref] [Google Scholar] [PubMed]
- Santiago LA, Mayor ABR. Lupeol: An antioxidant triterpene in Ficus pseudopalma Blanco (Moraceae). Asian Pac J Trop Biomed 2014; 4:109â??118.
[Crossref] [Google Scholar] [PubMed]
- Syamsudin, Sumarny R, Simanjuntak P. Antidiabetic activity of active fractions of Leucaena leucocephala (lmk) dewit seeds in experiment model. Eur J Sci Res 2010; 43:384-391. [Crossref]
[Google Scholar] [PubMed]
- Zayed MZ, Sallam SMA, Shetta ND. Review article on Leucaena leucocephala as one of the miracle timber trees. Int J Pharm Pharm Sci 2018; 10:1-7. [Crossref] [Google Scholar] [PubMed]
- Septina E, Yetti RD, Rivai H. Overview of traditional use, phytochemical and pharmacological activities of Chinese Petai (Leucaena leucocephala). Int J Pharm Sci Med 2020; 5:1â??10.
[Crossref] [Google Scholar] [PubMed]
- Gomes A, Fernandes E, Lima J, et al. Molecular mechanisms of anti-inflammatory activity mediated by flavonoids. Curr Med Chem 2008; 15:1586â??1605.
[Crossref] [Google Scholar] [PubMed]
- Padmaja TK, Naidu PB, Hanuma Kumar GE, et al. Anti-obesity activity of Bauhinia purpurea extract: Effect on hormones and lipid profile in high calorie diet induced obese rats. Adv Biosci Biotechnol 2014; 5:861-873.
- Lian JJ, Cheng BF, Gao YX, et al. Protective effect of kaempferol, a flavonoid widely present in varieties of edible plants, on IL-1β-induced inflammatory response via inhibiting MAPK, Akt and NF-κB signalling in SW982 cells. J Funct Foods 2016; 27:214-222.
- Jimenez-Gomez Y, Mattison JA, Pearson KJ, et al. Resveratrol improves adipose insulin signalling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high fat, high sugar diet. Cell Metab 2013; 18:533-545.
[Crossref] [Google Scholar] [PubMed]
- Martin AR, Villegas I, Sanchez-Hidalgo M, et al. The effects of resveratrol, a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. Br J Pharmacol 2006; 147:873-885.
[Crossref] [Google Scholar] [PubMed]
- Kareem AA, Aziz TA, Ahmed ZA, et al. Anti-Inflammatory activity of Gingko biloba extract in cotton pellet induced granuloma in rats: A comparative study with Prednisolone and Dexamethasone. Iraqi J Pharm Sci 2022; 31:184-193.
- Ismail NR. Phytochemical investigation, isolation of new medicinal compounds and evaluation of anti-inflammatory activity of Parthenocissus quinquefolia cultivated in Iraq Nour Rasim Esmail. 2005.
- Khalaf HA, Ahmed HJ, Ataimish AA, et al. Evaluation of the anti-inflammatory and anti-oxidant activity of crab components and crab shell in experimental rats in comparison with dexamethasone and diclofenac sodium. Int J Drug Deliv Technol 2021; 11:1360-1366.
- Rohit Kumar, Yogendra Kumar Gupta SS. Anti-inflammatory and anti-granuloma activity of Berberis aristata DC in experimental models of inflammation. Indian J Pharmacol 2016; 48:155â??161.
[Crossref] [Google Scholar] [PubMed]
- Yin J, Huang Y, Gao G, et al. Changes and significance of inflammatory cytokines in a rat model of cervical spondylosis. Exp Ther Med 2018; 15:400-406.
[Crossref] [Google Scholar] [PubMed]
- Al-Hejjaj WK, Numan IT, Al-Saâ??ad RZ, et al. Anti-inflammatory activity of telmisartan in rat models of experimentally induced chronic inflammation: Comparative study with dexamethasone. Saudi Pharm J 2011; 19:29-34.
[Crossref] [Google Scholar] [PubMed]
- Al-saad Z, Hussain SA. Republic of Iraq college of pharmacy dose response relationship of the anti-inflammatory activity of doxycycline and pentoxifylline in rats models of induced chronic inflammation by supervised by. 2009.
- Dharmasiri MG, Jayakody JR, Galhena G, et al. Anti-inflammatory and analgesic activities of mature fresh leaves ofVitex negundo. J Ethnopharmacol 2003; 87:199-206.
[Crossref] [Google Scholar] [PubMed]
- Koushki M, Amiri-Dashatan N, Ahmadi N, et al. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci Nutr 2018; 6:2473â??2490.
[Crossref] [Google Scholar] [PubMed]
- Harikumar KB, Aggarwal BB. Resveratrol: A multi targeted agent for age associated chronic diseases. Cell Cycle 2008; 7:1020-1035.
[Crossref] [Google Scholar] [PubMed]
- Garcia-Martinez BI, Ruiz-Ramos M, Pedraza-Chaverri J, et al. Hypoglycemic effect of resveratrol: A systematic review and meta-analysis. Antioxidants 2021; 10:1-25.
[Crossref] [Google Scholar] [PubMed]
- Li Y, Yao J, Han C, et al. Quercetin, inflammation and immunity. Nutrients 2016; 8:1â??14.
[Crossref] [Google Scholar] [PubMed]
- Sato S, Mukai Y. Modulation of chronic inflammation by quercetin: The beneficial effects on obesity. J Inflamm Res 2020; 13:421-431.
[Crossref] [Google Scholar] [PubMed]
- Wang H, Wang L, Guo S, et al. Rutin loaded stimuli responsive hydrogel for anti-inflammation. ACS Appl Mater Interfaces 2022; 14:26327-26337.
[Crossref] [Google Scholar] [PubMed]
- Choi SS, Park HR, Lee KA. A comparative study of rutin and rutin glycoside: Antioxidant activity, anti-inflammatory effect, effect on platelet aggregation and blood coagulation. Antioxidants 2021; 10:1696
[Crossref] [Google Scholar] [PubMed]
Author Info
Azal Satar Al-Baaj*, Thukaa Z Abdul-Jalil and Ammar A Fadhil
Department of Pharmacognosy and Medicinal Plants, College of Pharmacy, University of Baghdad, Baghdad, IraqCitation: Azal Satar Al-Baaj, Thukaa Z Abdul-Jalil, Ammar A Fadhil, Evaluation of the Anti-Inflammatory of Leucaena leucocephala Extracts in Experimental Rats, J Res Med Dent Sci, 2023, 11 (06): 025-031
Received: 30-Apr-2022, Manuscript No. JRMDS-22-69997; , Pre QC No. JRMDS-22-69997 (PQ); Editor assigned: 03-May-2022, Pre QC No. JRMDS-22-69997 (PQ); Reviewed: 18-May-2022, QC No. JRMDS-22-69997; Revised: 01-Jun-2023, Manuscript No. JRMDS-22-69997 (R); Published: 09-Jun-2023
