Research - (2019) Volume 7, Issue 2
Evaluating the Effects of Casein Phosphopeptide Amorphous Calcium Phosphate Paste and Nano-hydroxyapatite Solutions on the Re-mineralization of Synthetic Primary Caries in Enamel
Abdolrahim Davari, Alireza Daneshkazemi and Fahime Shafiee*
*Correspondence: Fahime Shafiee, Operative and Aesthetic Dentistry, Social Determinant of Oral Health Research Center, Shahid Sadoughi University of Medical Sciences, Iran, Email:
Abstract
Background and Aim: It has now been well documented that dental caries can be re-mineralized before they become pitted. So this laboratory study examined the effect of CPP-ACP paste and Nano-hydroxyapatite solutions on remineralization of primary enamel caries lesions.
Materials and Methods: 75 pieces of 3 mm × 4 mm were obtained from the buccal crown surface of 75 premolars drawn. Primary enamel lesions were created by putting samples, except for 15 that set aside by random, for 48 hours in demineralization solution. Then these 60 specimens were divided randomly into 4 groups of 15. 10 days pH cycle was completed and in 3 groups after the end of each day of cycle, the concerned test material was applied. Group 1: Healthy (positive control); Group 2: With primary lesions; Group 3: Applying CPP-ACP paste; Group 4: Applying 5% nano-hydroxy apatite solution; Group 5: Applying 10% nano-hydroxy apatite solution on the lesions. The rate of weight of calcium, phosphor and fluoride elements in all samples after the end of work was measured by SEM-EDS. Data analysis was perfumed using one way ANOVA and Post Hoc Bonferroni statistical tests (α=0.05).
Results: There was a significant statistical difference between groups about the amount of calcium, phosphor and fluoride elements (p=.000). The rate of calcium and phosphor in the group having white spot lesions (WSL) significantly was less than the other groups (p=.000). The rate of calcium, phosphor and fluoride elements in the healthy groups wasn’t significantly different from the group under applying CPP-ACP paste and 5 nano-hydroxy apatite solution (p=1.000). But the group under applying 10% nano-hydroxy apatite solution significantly had more calcium and phosphor than the other 4 groups (even healthy or intact group) (p=.000).
Conclusion: All three materials of CPP-ACP, 5% and 10% nano-hydroxy apatite solution are effective on the remineralization of the primary enamel lesions. Also 10% nano-hydroxy apatite solution is more effective than the other groups and results in strength of healthy tooth structure (without lesion).
Keywords
CPP-ACP paste, Nano-hydroxy apatite, Remineralization, Primary enamel lesion
Introduction
Despite extensive efforts made so far to prevent dental caries, dental caries are still considered as the most common chronic disease during childhood and adolescence and they are quite prevalent in some societies. The main cause of such a high prevalence is the increased consumption of glucose-based food and insufficient fluoride exposure [1].
The decay process involves the loss of ivory and enamel minerals by acidic metabolic products derived from bacteria [2].
It has now been well documented that it is possible to remineralize dental caries at the initial stage of formation before cavitation; it is possible to facilitate this process by several factors, including using fluoride in toothpastes and mouthwashes [3,4]. However, the excessive use of fluoride leads to an increased risk of dental fluorosis and poisoning, especially in preschool children [5].
Accordingly, the need for decay re-mineralizing agents is as an effective alternative to fluoride without its harmful effects.
CPP-ACP derived from the protein of cow's milk has shown potent cryogenic effect in studies conducted in laboratory, and on animals and human beings [6].
In-vivo studies have shown that when CPP-ACP is used in sugar-free chewing gum by patients, it re-mineralizes sub-surface enamel decays independent from the frequency and duration of chewing [6-10].
In addition, in clinical trials conducted by Hay et al. [11] on patients with severe oral dry mouth, CPP-ACP used in mouth moisturizing spray (Dentacal, NSI Pty Ltd, Australia], had positive results on decay prevention, and wetting the mouth [12].
Kariya et al. also reported that CPP-ACP has a stronger potential to neutralize acids in comparison with fluoride toothpastes [13].
A systematic review and meta-analysis study has recently examined the effect of CPP-ACP on enamel white spot lesions. This study has shown the promising effects of this substance on decay prevention, induction of wastere- mineralization, treating dentin hypersensitivity and oral dryness [14].
Nano-hydroxyapatite is also one of the most bio-friendly bio-based materials which have been highly regarded in medicine and dentistry in recent years [15]. The morphology and crystalline structure of this nanoparticle is similar to apatite enamel crystals and has been studied as a simulator for the reconstruction of dental enamel in cases where many minerals have failed to do so [16].
The results of Huang et al. study, which investigated the effect of different concentrations of 1%, 5%, 10%, and 15% Nano-hydroxyapatite and sodium fluoride on the remineralization of primary caries lesions, showed that 10% Nano-hydroxyapatite concentration was quite effective for the realization of the re-mineralization process [17].
Therefore, the present experimental study was conducted to compare the effects of CPP-ACP toothpaste and 5% and 10% Nano-hydroxyapatite solution on the re-mineralization of primary caries lesions of enamel.
Zero hypotheses were as follows:
1. There was no difference between CPP-ACP paste and 5% and 10% Nano-hydroxyapatite solution in regard with the degree and extent of re-mineralization.
2. There was no difference between 5% and 10% Nano-hydroxyapatite solution in regard with the degree and extent of re-mineralization.
Materials and Methods
Sample preparation
Seventy five extracted healthy premolar teeth were examined after collecting, by means of a magnifying glass, with magnification power of 20, in order to prevent the presence of cracks or fractures; then, additional tissue was removed and stored in normal saline at room temperature. Normal saline was replaced and refreshed every day. The teeth were sterilized by 0.5% T solution of chloramine prior to the initiation of the project. Then teeth crowns were removed from the root by a brown coarse diamond disc (made in France, BISICO) were assessed in the present study. The surface enamel of the samples was flattened in basal middle part (equal to 10 times the disc movement on them); after leveling with silicon carbide papers with the severity of 400-600-800 grits, a 3 mm × 4 mm piece was collected from the basal area of each tooth by means of a disk; then, it was mounted on self-cure acryl; 15 samples were accidentally dyed in purified water and dismissed.
Preparation of de-and re-mineralization solution
Demineralization and remineralization buffer solutions were prepared by combining chemical substances and purified water. The demineralization solution [16] contained (2/2 mM CaCl2, 2/2 mM KH2PO4, 0.05 M acetic acid, whose pH reached 4/4 with 1 M KOH).
Re-mineralization solution [16] contained (1.5 mM CaCl2, 0.9 mM NaH2PO4, and 0.15 M KCL with pH=7).
Generating white spot lesions (WSL)
60 remaining samples were placed in 10 ml of demineralization solution for 48 hours in order to generate artificial caries [18]. All of these samples were examined for WSL by eye examination in the presence of natural water and by drying with air (APAC): the generation of primary caries was confined by Diagnodent (Diagnostic, Kavo, Germany) (score 13-19) [19]. The samples were, then, randomly assigned to four groups, the classification of which was like the following:
Group 1: Healthy, no process conducted (Positive control).
Group 2: Presence of white spot lesions (WSL) and PH cycle without the use of any other material (Negative control).
Group 3: Presence of WSL and the application of CPPACP paste application after the end of each pH cycle, known as Tooth Mousse (manufactured by GC, Japan).
Group 4: Presence of WSL and the application of 5% nano-hydroxyapatite solution after the end of each pH cycle (Nick Ceram Razi Corporation, Isfahan, Iran) [16,20].
Group 5: Presence of WSL and the application of 10% nano-hydroxyapatite solution after the end of each pH cycle (Nick Ceram Razi Corporation, Isfahan, Iran) [16,20].
Pure Nano-hydroxyapatite powder prepared from Nick- Ceram Razi Company (Milky White) with Ca10(PO4)6(OH)2 formulation was mixed with purified water and 5% and 10% aqueous solution was prepared (the powder is completely dissolved in the water) [21]. Hydroxyapatite particles are less than 150 nm in size, analyzed for TEM, SEM, XRD and in-vivo and in-vitro experiments to evaluate the properties of this product (Figure 1) [22].
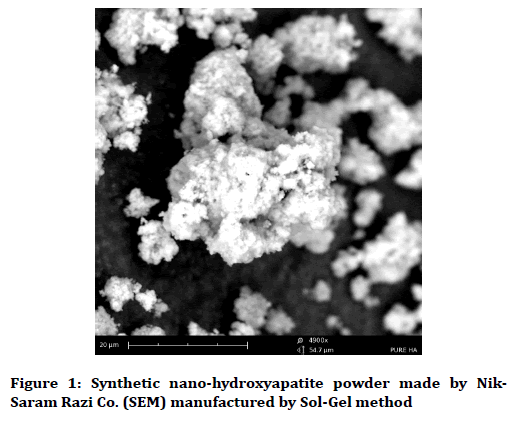
Figure 1. Synthetic nano-hydroxyapatite powder made by Nik-Saram Razi Co. (SEM) manufactured by Sol-Gel method
pH cycle
The samples were, then, placed in a pH cycle for 10 days [17]. Each cycle lasted 8 hours, including 3 hours of demineralization twice a day and 2 hours of remineralization during their intervals [17]. In the negative control group (group 2), three hours of demineralization were performed twice a day with two hours of remineralization during their intervals for 10 days without applying therapeutic agents. After the second demineralization, samples in group 3 were covered with a layer of CPP-ACP paste for 2 minutes removed from the tube at the same moment; then, they were washed with purified water. Samples in groups 4 and 5 were immersed in the solution for 2 minutes (the solution was well disrupted before use to prevent the accumulation and agglomeration of Nano-metric particles); then, each sample was washed with purified water. After completion of each cycle, the samples were placed in purified water until the next day. After the completion of 10-day cycle [17], the samples were checked to measure the amount of the elements.
Measuring the extent of re-mineralization
Calcium, phosphorus and fluoride ions were measured from pieces extracted from the middle of each sample by (SEM-EDS, FESEM, TESCAN Company, France) which is a semi-quantitative analysis. Fluoride ion was measured in order to determine whether or not the two materials tested in the present study had the potential to replace fluoride ion from the tooth structure.
In SEM-EDS method, the entire surface of the studied electron is received, all signals are collected from the surface and reliable data is obtained for each sample surface. The electrons scan the microscope gun scan with a surface depth of 1 μm. When the device voltage reaches a certain level, the electrons collide and reflect the surface of the material. These electrons contain information about the surface of matter, including the type and percentage of its constituent elements, which are calculated by software. The weight and atomic percent of the elements are presented in the table of reporting and graphing of the energy spectrum of the elements.
All data were analyzed by SPSS 17 using one way ANOVA and Post Hoc Bonferroni.
Results
The present study was conducted to evaluate the effect of CPP-ACP paste and two concentrations of and 10% of Nano-hydroxyapatite solution on WSL re-mineralization.
The mean and standard deviation of the groups are presented in Table 1. ANOVA statistical analysis showed that there was a statistically significant difference between the groups in regard with the amount of calcium, phosphorus and fluoride (p=.000) (Table 2).
| Ions | Groups | Number in each group | Mean | Standard deviation | Standard error | Confidence level of 95% for the mean | |
|---|---|---|---|---|---|---|---|
| Minimum | Maximum | ||||||
| Calcium | Healthy | 15 | 30.624 | 3.65056 | 0.94257 | 28.6024 | 32.6456 |
| Primary caries | 15 | 18.7713 | 1.429 | 0.36897 | 17.98 | 19.5627 | |
| cpp-acp paste | 15 | 30.688 | 2.40237 | 0.62029 | 29.3576 | 32.0184 | |
| Nano solution 5% | 15 | 30.0933 | 2.47141 | 0.63811 | 28.7247 | 31.462 | |
| Nano solution 10% | 15 | 39.9367 | 1.10052 | 0.28415 | 39.3272 | 40.5461 | |
| Total | 75 | 30.0227 | 7.15066 | 0.82569 | 28.3774 | 31.6679 | |
| Phosphorus | Healthy | 15 | 25.4107 | 1.28662 | 0.3322 | 24.6982 | 26.1232 |
| Primary caries | 15 | 15.5493 | 2.26247 | 0.58417 | 14.2964 | 16.8022 | |
| CPP-ACP paste | 15 | 25.5973 | 1.09768 | 0.28342 | 24.9895 | 26.2052 | |
| Nano solution 5% | 15 | 24.668 | 1.56093 | 0.40303 | 23.8036 | 25.5324 | |
| Nano solution 10% | 15 | 29.136 | 2.19257 | 0.56612 | 27.9218 | 30.3502 | |
| Total | 75 | 24.0723 | 4.86914 | 0.56224 | 22.952 | 25.1926 | |
| Fluoride | Healthy | 15 | 2.04 | 0.27441 | 0.07085 | 1.888 | 2.192 |
| Primary caries | 15 | 1.5687 | 0.3438 | 0.08877 | 1.3783 | 1.7591 | |
| CPP-ACP paste | 15 | 1.8527 | 0.38644 | 0.09978 | 1.6387 | 2.0667 | |
| Nano solution 5% | 15 | 2.0627 | 0.47599 | 0.1229 | 1.7991 | 2.3263 | |
| Nano solution 10% | 15 | 2.2033 | 0.41412 | 0.10693 | 1.974 | 2.4327 | |
| Total | 75 | 1.9455 | 0.43447 | 0.05017 | 1.8455 | 2.0454 | |
Table 1: Mean and standard deviation of the groups
| Ions | Groups | Sum of squares | df | Mean square | F | p-value |
|---|---|---|---|---|---|---|
| Calcium | Intergroup | 3385.337 | 4 | 846.334 | 148.694 | 0 |
| Intragroup | 398.426 | 70 | 5.692 | |||
| Total | 3783.764 | 74 | ||||
| Phosphorus | Intergroup | 1541.307 | 4 | 385.327 | 126.561 | 0 |
| Intragroup | 213.121 | 70 | 3.045 | |||
| Total | 1754.429 | 74 | ||||
| Fluoride | Intergroup | 3.596 | 4 | 0.899 | 6.068 | 0 |
| Intragroup | 10.372 | 70 | 0.148 | |||
| Total | 13.969 | 74 | ||||
Table 2: Inter- and intra-group comparison with one-way ANOVA
Post Hoc Bonferroni test was used to compare the two groups, the results of which are summarized in Tables 3-5
| Groups | Difference between groups | p-value |
|---|---|---|
| Healthy and caries groups | 11.85267 | 0 |
| Healthy and CPP-ACP groups | -0.064 | 1 |
| Healthy and Nano solution 5% groups | 0.53067 | 1 |
| Healthy and Nano solution 10% groups | -9.31267 | 0 |
| Caries and CPP-ACP groups | -11.9167 | 0 |
| Caries and Nano solution 5% groups | -11.322 | 0 |
| Caries and Nano solution 10% groups | -21.1653 | 0 |
| Nano solution 5% and CPP-ACP groups | 0.59467 | 1 |
| Nano solution 10% and CPP-ACP groups | -9.24867 | 0 |
| Nano solution 5% and Nano solution 10% groups | -9.84333 | 0 |
Table 3: Paired comparison between the groups about the amount of calcium ion with post hoc Bonferroni analysis
| Groups | Difference between groups | p-value |
|---|---|---|
| Healthy and caries groups | 9.86133 | 0 |
| Healthy and CPP-ACP groups | -0.18667 | 1 |
| Healthy and Nano solution 5% groups | 0.74267 | 1 |
| Healthy and Nano solution 10% groups | -0.72536 | 0 |
| Caries and CPP-ACP groups | -10.48 | 0 |
| Caries and Nano solution 5% groups | -9.11867 | 0 |
| Caries and Nano solution 10% groups | -13.5867 | 0 |
| Nano solution 5% and CPP-ACP groups | 0.92933 | 1 |
| Nano solution 10% and CPP-ACP groups | -3.53867 | 0 |
| Nano solution 5% and Nano solution 10% groups | -4.468 | 0 |
Table 4: Paired comparison between the groups about the amount of phosphorus ion with post hoc Bonferroni analysis
| Groups | Difference between groups | p-value |
|---|---|---|
| Healthy and caries groups | 0.47113 | 0.013 |
| Healthy and CPP-ACP groups | 0.18733 | 1 |
| Healthy and Nano solution 5% groups | -0.02267 | 1 |
| Healthy and Nano solution 10% groups | -0.16333 | 1 |
| Caries and CPP-ACP groups | -0.284 | 0.472 |
| Caries and Nano solution 5% groups | -0.494 | 0.008 |
| Caries and Nano solution 10% groups | -0.63467 | 0 |
| Nano solution 5% and CPP-ACP groups | -0.21 | 1 |
| Nano solution 10% and CPP-ACP groups | -0.35067 | 0.15 |
| Nano solution 5% and Nano solution 10% groups | -0.14067 | 1 |
Table 5: Paired comparison between the groups about the amount of fluoride ion with post hoc Bonferroni analysis
The results of this test showed that the amount of calcium and phosphorus was significantly lower in the group with white spot lesions (WSL) in comparison with the other groups (p=.000); additionally, the fluoride content of the WSL group was significantly lower than other groups (p<0.05), except for the group with CPP-ACP paste, which did not show a significant difference in the fluoride content with the WSL group (p=0.472) (Table 5).
Also, the results of this test showed that the levels of calcium, phosphorus and fluoride were not significantly between the healthy groups and the group in which CPP-ACP and 5% Nano-hydroxyapatite solution were used (p=1.000) (Tables 3-5).
However, the group treated with 10% Nanohydroxyapatite solution had significantly higher levels of calcium and phosphorus in comparison with other group (even healthy or intact group) (p=.000), (Table 3 and Table 4); however, the level of fluoride in the group under treatment with 10% solution had no significant difference with healthy groups and the one receiving 5% Nano-hydroxyapatite solution and CPP-ACP (p=1.000, p=1.000 and p=0.150, respectively), although this group had significantly higher content of fluoride in comparison with the group having WSL (p=.000) (Table 5).
The elemental analysis charts of SEM-EDS are shown in Figures 2-6.
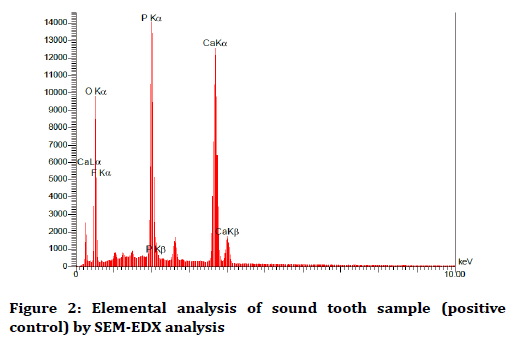
Figure 2. Elemental analysis of sound tooth sample (positive control) by SEM-EDX analysis
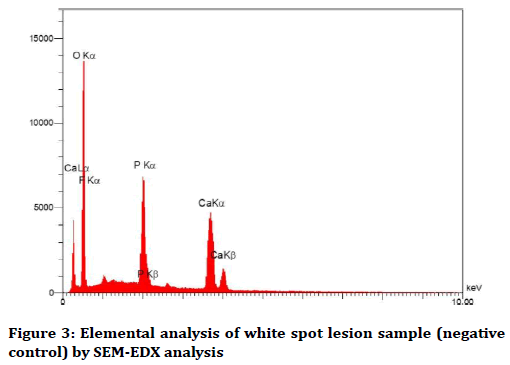
Figure 3. Elemental analysis of white spot lesion sample (negative control) by SEM-EDX analysis
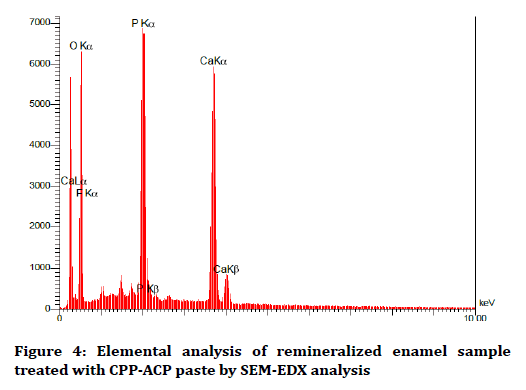
Figure 4. Elemental analysis of remineralized enamel sample treated with CPP-ACP paste by SEM-EDX analysis
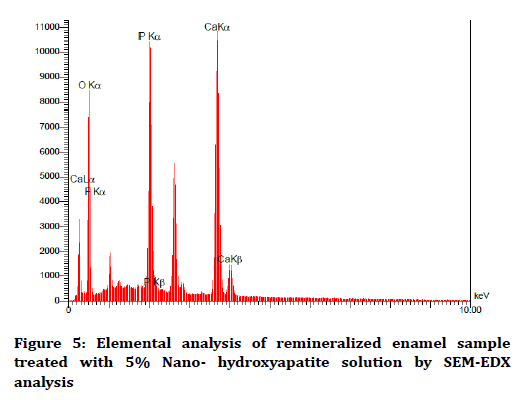
Figure 5. Elemental analysis of remineralized enamel sample treated with 5% Nano- hydroxyapatite solution by SEM-EDX analysis
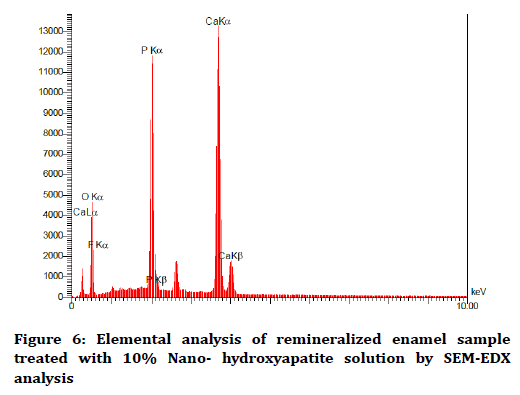
Figure 6. Elemental analysis of remineralized enamel sample treated with 10% Nano- hydroxyapatite solution by SEM-EDX analysis
Discussion
The recent approach to decay management is a noninvasive one which can turn the lesion from active to inactive form. By improving the condition of the patient's mouth and the use of complementary therapeutic agents, it is possible to reverse caries at an earlier stage and stop them from progressing into advanced stages. Substances with re-mineralizing potential have been tested to realize such an objective [17].
The present study compared the effect of CPP-ACP paste and 5% and 10% Nano-hydroxyapatite solutions on WSL re-mineralization. The results of this research showed that all three treatments increased the ions significantly compared to the group with white spot lesions (WSL) and led to re-mineralization; additionally, 10% Nanohydroxyapatite solution increased the amount of calcium and phosphorus significantly in comparison with the other groups, so that the amount of these elements was higher in healthy teeth. However, there was no significant difference in the amount of elements between the CPPACP, 5% Nano-hydroxyapatite solution and healthy teeth groups. So, the null hypotheses were rejected.
By forming the amorphous layer, CPP-ACP locates a large amount of calcium and phosphorus ions on the tooth surface and causes the oversaturation to facilitate the remineralization process. It has also been shown that this substance can be applied to the albumen contained in the pellicle and to inhibit the adhesion of the Streptococcus mutans and Sabrinos to pellicle [21].
Nano-hydroxyapatite is structurally and morphologically similar to dental structure crystals and it has turned out to be highly efficient in the emulsion of enamel waste. This substance is directly implanted by immersion in enamel lesions, which is clearly higher in porosity than healthy teeth, thus resulting in re-mineralization; it also provides a large amount of calcium and phosphate ions, and can result in the growth of existing crystals [23]. Due to high solubility, it can be used in two forms of solution and paste [20].
10% Nano-hydroxyapatite solution resulted in higher remineralization compared to 5% solution in the present study. With increasing concentrations from to 10%, more sediment was carried out on the tooth surface and a higher percentage of porosity was detected by these nanostructured particles [15]. This was quite consistent with the results of Huang et al. study, which investigated the effect of different concentrations of 1%, 5%, 10% and 15% Nano-hydroxyapatite on the re-mineralization of the primary mucous membrane [17]. The results of their study showed that by increasing the concentration up to 10% enhanced the hardness, but there was no difference between 10% and 15% concentrations; so, 10% was selected as the optimum concentration. However, this study is different from the present research, because samples were prepared from cow incisors, the duration for generating (WSL) was 72 hours, as well as different pH cycle and the duration of the cycle was 12 days; on the other hand, the present study was performed on healthy human premolars, samples were immersed in a demineralization solution for 48 hours to generate WSL and the duration of the cycle was 10 days. The tests used in their study were Knoop hardness and SEM images, while the present researchers used the semi-quantitative elemental analysis of SEM-EDS in order to determine the weight of the elements precisely.
It must be stated that the 5% concentration also caused the re-mineralization of the lesion to the healthy tooth and increased the amount of the elements significantly compared to the caries group; it can be concluded that the Iranian nanostructured paste in a concentration of less than 10% also has a significant effect on remineralization of white spot lesions.
CPP-ACP also re-mineralized the lesion up to the healthy tooth surface due to the localization of a large amount of calcium and phosphorus ions and the generation of an oversaturation state against enamel in the present study [21]. CPP-ACP paste was used in form of coating in the present study, because Kumar et al. study showed that CPP-ACP paste has the potential to re-mineralize white spot lesions; additionally, higher re-mineralization rate was observed when CPP-ACP was used in form of topical coating application [24,25].
The advantage of using Nano-HA in comparison with Micro-HA is that the nanoparticles are morphologically similar to dental apatite in size, crystalline structure, solubility and biocompatibility [25]. It has been reported that the re-mineralization efficacy of nanoparticles increases id they are used in combination. As the surfaceto- volume ratio increases, the reaction between the enamel and dentin with the nanoparticles will be more and more affected [26]. Therefore, we also used nanoparticles in our study to increase the penetration of these enhancer materials into porosities in the lesion.
Surface enamel is usually hyper-mineralized and rich in Fluor apatite and is therefore more resistant to demineralization [27]. Hence, using the abrasive discs, the surface of the enamel was removed to eliminate the difference in samples by obtaining a uniform mirror surface [28].
The present researchers used purified water to store samples at intervals between cycles and until the next day. The reason for not using artificial saliva was that it contains various ions, such as PO43-, Cl-, K+, Na+, Mn2+, Ca2+, which, given their re-mineralizing potential, might exaggerate the effect of tested substances [29].
The period for the generation of white spot lesions (WSL) varied from 48 hours to 6 days in different studies [15,17,24,30], a period which was 48 hours in the present study. Since the samples were subjected to a diagnostic device after 48 hours and since white spot lesions were formed, there was not kept in the demineralization solution for a long time.
Different methods, including Vickers and Knoop hardness tests, observation with polarized optical microscopy, SEM images, and microscopy have been used to measure the amount of re-mineralization of samples [15-17,23,24]; the method used in the present study to measure the amount of re-mineralization was SEM-EDS (Dispersive Spectrometry Energy), which provides a specific method for determining the weight percent of the chemical components of the used substances [31].
However, the present experimental study has some limitations. It is recommended to conduct a study in this regard and compare the effect of different percentages of Iranian and foreign Nano-hydroxyapatite materials on enamel re-mineralization.
Conclusion
Considering the limitations of this study, one can conclude that:
1. All three tested substances, i.e. CPP-ACP s, 5 and 10% Nano-hydroxyapatite solutions, are effective in re-mineralizing white spot lesions.
2. 10% Nano-solution is more effective that other tested substances and it helps to strengthen the structure of the tooth (by keeping it without lesion).
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
References
- Walsh T, Worthington HV, Glenny AM, et al. Fluoride toothpastes of different concentrations for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2010.
- Feathestone JD. The continuum of dental caries-evidence for a dynamic process. J Dent Res 2004; 83:C39-42.
- Amaechi BT. Remineralization therapies for initial caries lesions. Curr Oral Health Rep 2015; 2:95-101.
- Singhal RK, Rai B. Remineralization potential of three tooth pastes on enamel caries. Open Access Maced J Med Sci 2017; 5:664.
- Zohoori FV, Duckworth RM, Omid N, et al. Fluoridated toothpaste: Usage and ingestion of fluoride by 4‐to 6‐yr‐old children in England. Eur J Oral Sci 2012; 120:415-21.
- Shen P, Cai F, Nowicki A, et al. Remineralization of enamel subsurface lesions by sugar-free chewing gum containing casein phosphopeptide-amorphous calcium phosphate. J Dent Res 2001; 80:2066-70.
- Walker G, Cai F, Shen P, et al. Increased remineralization of tooth enamel by milk containing added casein phosphopeptide-amorphous calcium phosphate. J Dairy Res 2006; 73:74-8.
- Iijima Y, Cai F, Shen P, et al. Acid resistance of enamel subsurface lesions remineralized by a sugar-free chewing gum containing casein phosphopeptide-amorphous calcium phosphate. Caries Res 2004; 38:551-6.
- Hay KD, Thomson WM. A clinical trial of the anticaries efficacy of casein derivatives complexed with calcium phosphate in patients with salivary gland dysfunction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002; 93:271-5.
- Hay KD, Morton RP. The efficacy of casein phosphopeptideamorphous calcium phosphate complex (DC-CP) as a mouth moistening in patients with severe xerostomia. N Z Dent J 2003; 99:46-8.
- Kariya S, Sato T, Sakaguchi Y, et al. Fluoride effect on acid resistance capacity of CPP-ACP containing material. IADR 2004.
- Kecik D. Effectiveness of Casein phosphopeptide-amorphous calcium phosphate on the prevention of white spot lesions: A systematic review and meta-analysis, Iran J Ortho 2017; 12:e7194.
- Hannig M, Hannig C. Nanomaterials in preventive dentistry. Nature Nanotech 2010; 5:565.
- Vandiver J, Dean D, Patel N, et al. Nanoscale variation in surface charge of synthetic hydroxyapatite detected by chemically and spatially specific highresolution force spectroscopy. Biomaterials 2005; 26:271-83.
- Huang SB, Gao SS, Yu HY. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed Mater 2009; 4:1748-53.
- Bajaj M, Poornima P, Praveen S, et al. Comparison of CPP-ACP, tri-calcium phosphate and hydroxyapatite on remineralization of artificial caries like lesions on primary enamel-An in vitro study. J Clin Pediatr Dent 2016; 40:404-9.
- Swarup JS, Rao A. Enamel surface remineralization: Using synthetic nanohydroxyapatite. Contemp Clin Dent 2012; 3:433.
- Davari A, Daneshkazemi A, Assarzadeh H, et al. Evaluation of Er:YAG laser and fluoride ion effects on the remineralization of enamel white spot lesions. J Mash Dent Sch 2018; 42:210-20.
- Najibfard K, Ramalingam K, Chedjieu I, et al. Remineralization of early caries by a nano-hydroxyapatite dentifrice. J Clin Dent 2011; 22:139.
- nikceram.ir/catalog/NCR%20Medical%20catalogue.pdf
- Roza H, Hamid RH, Farid A, et al. The effect of nano-hydroxyappatite solution on the permanent tooth remineralization following exposure to soft beer (in situ). J Dent Med 2015; 27:233-40.
- Vashisht R, Indira R, Ramachandran S, et al. Role of casein phosphopeptide amorphous calcium phosphate in remineralization of white spot lesions and inhibition of Streptococcus mutans?. J Conserv Dent 2013; 16:342.
- Sharma A, Rao A, Shenoy R, et al. Comparative evaluation of Nano-hydroxyapatite and casein Phosphopeptide-amorphous calcium phosphate on the remineralization potential of early enamel lesions: An in vitro study. J Orofac Sci 2017; 9:28.
- Kumar VL, Itthagarun A, King NM. The effect of casein phosphopeptide‐amorphous calcium phosphate on remineralization of artificial caries‐like lesions: An in vitro study. Aust Dent J 2008; 53:34-40.
- Khoroushi M, Shirban F, Kaveh S, et al. Effect of three nanobiomaterials on microhardness of bleached enamel. Restor Dent Endod 2016; 41:196-201.
- Generosi A, Rau JV, Albertini VR, et al. Crystallization process of carbonate substituted hydroxyapatite nanoparticles in toothpastes upon physiological conditions: An in situ time-resolved X-ray diffraction study. J Mater Sci Mater Med 2010; 21:445-50.
- Kanemura N, Sano H, Tagami J. Tensile bond strength to and SEM evaluation of ground and intact enamel surfaces. J Dent 1999; 27:523-30.
- Deng M, Wen HL, Dong XL, et al. Effects of 45S5 bioglass on surface properties of dental enamel subjected to 35% hydrogen peroxide. Int J Oral Sci 2013; 5:103.
- Efeoglu N, Wood D, Efeoglu C. Microcomputerised tomography evaluation of 10% carbamide peroxide applied to enamel. J Dent 2005; 33:561-7.
- Cardoso CA, Cassiano LP, Costa EN, et al. Effect of xylitol varnishes on remineralization of artificial enamel caries lesions in situ. J Dent 2016; 50:74-8.
- Cakir FY, Korkmaz Y, Firat E, et al. Chemical analysis of enamel and dentin following the application of three different at-home bleaching systems. Oper Dent 2011; 36:529-36.
Author Info
Abdolrahim Davari, Alireza Daneshkazemi and Fahime Shafiee*
Operative and Aesthetic Dentistry, Social Determinant of Oral Health Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, IranCitation: Abdolrahim Davari, Alireza Daneshkazemi, Fahime Shafiee, Evaluating the effects of casein phosphopeptide amorphous calcium phosphate paste and nano-hydroxyapatite solutions on the re-mineralization of synthetic primary caries in enamel, J Res Med Dent Sci, 2019, 7(2): 62-69.
Received: 16-Jan-2019 Accepted: 01-Mar-2019
