Research - (2022) Volume 10, Issue 7
Evaluating the Effects of Administration of the Ethanolic Leaf Extracts of Jatropha curcas and Ascorbic Acid on the Male Reproductive Functions in Alloxan-Induced Diabetic Rats
Akin Olanipekun1, John Ogedengbe2, Olorunshola Kolawole2 and Senol Dane1*
*Correspondence: Senol Dane, Department of Physiology, Faculty of Basic Medical Sciences, College of Health Sciences, Nile University of Nigeria, Nigeria, Email:
Abstract
Introduction: The focus of this study is to compare the androgenic, antioxidant, and antidiabetic properties of ethanolic leaf extract of Jatrophas curcas when co-administered with ascorbic acid to when administered separately in alloxan-induced diabetic rats. Method: 48 adult male Wistar rats were divided into 8 groups. Group I: Normal control rats, Group II, Group III, Group IV were normal rats fed with Jatropha curcas (500mg/kg/d), Ascorbic acid (250mg/kg/d), and Jatropha curcas+ascorbic acid respectively. Diabetic rats were untreated in Group V, while diabetic rats in Groups VI, VII, and VIII were given Jatropha curcas (500mg/kg/day), ascorbic acid alone (250mg/kg/d), and Jatropha curcas+ascorbic acid, respectively. They were treated for 28 days, and the body weights and fasting blood glucose were checked weekly. The level of testicular antioxidant enzymes, sperm quality, and reproductive hormone profile were determined. Results: There was a significant reduction in serum glucose levels, increased levels of testicular antioxidant enzymes, increased serum levels of testosterone, and improved sperm quality in groups separately treated with Jatropha curcas and ascorbic acid compared to the diabetic untreated group. There was significant reduction in sperm quality of groups treated with co-administration of Jatropha curcas and ascorbic acid compared to when used separately. Conclusion: The findings demonstrate that ethanolic leaf extracts of Jatropha curcas could trigger profertility properties in male diabetic rats, due to their potent hypoglycemic, antioxidant, and androgenic effects. However, co-administration of Jatropha and ascorbic acid could be deleterious to male reproductive functions.
Keywords
Diabetes mellitus, Male infertility, Jatropha curcas, Ascorbic acid
Introduction
Diabetes mellitus is a group of metabolic diseases marked by persistent hyperglycemia caused by deficiencies in insulin secretion, insulin action, or both [1]. Carbohydrate, lipid, and protein metabolism are all disrupted in DM [2]. Diabetes is currently one of the most rapidly growing public health issues. 547 million adults worldwide had diabetes in 2021 and has caused 6.7 million annual deaths worldwide in 2021, with longterm human, social, and financial consequences [1,3].
Diabetes mellitus has been demonstrated to have deleterious impacts on reproductive functions (male and female), resulting in higher rates of male infertility [4,5]. As the prevalence of diabetes rises, so will the prevalence of infertility among males of reproductive age [6]. When we look at fertility statistics in modern nations, we can see that the rising prevalence of diabetes is linked to lower birth rates and lower fertility [7]. Several clinical and experimental studies have discovered different levels of male reproductive deterioration in diabetics, including disruption of the hypothalamic-pituitary-gonadal axis, testicular and epididymal oxidative stress, reduction in the number of spermatogonia, reduction in the number of Leydig and Sertoli cells, abnormal testicular energy metabolism, and altered sperm parameters (decreased sperm concentration, motility, abnormal morphology) [8-10].
Because of the expensive cost and negative side effects of anti-diabetic medicines, researchers are looking for medicinal plants that have effective hypoglycaemic, antioxidant, and male reproductive capabilities. Medicinal plants offer anti-hyperglycemic and antioxidant properties and are important in the development of novel medications because they contain bioactive substances (phytochemicals) with a diverse set of biological activities [11].
Jatropha curcas (purging nut or physic nut) is a droughttolerant shrub or tree from the Euphorbiaceae family that is grown in Africa, India, Southeast Asia, Central and South America [12]. It is known as Lapalapa in Yoruba, Bi ni da zugu in Hausa, Gyedan in Tiv, and Ochigbede in Idoma, and is widely grown throughout Nigeria. It can withstand high levels of aridity and thrive in harsh environments such as drought, limited nutrient availability, and salinity, allowing it to be grown in deserts [13]. According to recent research, the Jatropha curcas plant contains hypoglycemic and antioxidant characteristics, and has the ability to reverse diabetesrelated tissue oxidative damage [14,15]. However, there is scarcity of information about this plant's androgenic properties.
Ascorbic acid is an endogenous antioxidant that shields lipids from oxidative damage caused by non-lipid peroxyl radicals produced in the aqueous phase [16]. Because there are several linkages between diabetes mellitus, oxidative stress, and male factor infertility, its favorable effect on male fertility would be expected [17].
Therefore, the focus of this study is to compare the androgenic, antioxidant and antidiabetic properties of the Jatrophas curcas ethanolic leaf extract when coadministered with ascorbic acid to when administered separately in alloxan-induced diabetic rats.
Materials and Methods
Jatropha curcas ethanolic leaf extracts preparation
The freshly collected leaves of Jatropha curcas gotten from a local farm in Abuja was identified and authenticated at the herbarium unit NIPRD, Abuja with (VSN= NIPRD/H/ 6736). The ethanolic leaf extract of Jatropha curcas (ELEJC) was done in-accordance as described by [18]. The leaves were air-dried for four days before being ground up in an industrial blender. 50 g of crude fiber was extracted with ethanol by soaking in 1500 ml of 98% ethanol for 72 hours. The filter paper used to filter the sample was Whatman. A rotary evaporator (IKA RV 10, Staufen, Germany) was used to concentrate the extract. The extract was kept in the fridge until it was time to conduct the experiment.
Phytochemical Analysis of ELEJC
Tannin, alkaloids, flavonoids, saponins, and triterpenoids were discovered in a 50 percent ethanolic leaf extract of Jatropha curcas during preliminary phytochemical screening [19].
Oral acute toxicity test
The LD50 of ELEJC was determined using mice according to [20]. Fifteen fasted male albino mice (25-30 g, 10 weeks old) were divided into five groups A, B, C, D, and E, each with three animals. Group A animals had only distilled water, while groups B, C, D, and E had 5, 50, 300, and 2000 mg/kg body weight of ELEJC in distilled water via orogastric tubes, respectively. To detect changes in autonomic or behavioral responses, the animals were monitored at 2, 6, 24, and 48 hours following ELEJC administration. Mortality was observed for 24 hrs.
Result: The ELEJC was shown to be safe to use in animals and exhibited no mortality at 2000 mg/kg body weight. Dosages of up to 500 mg/kg BW were assumed to be safe and used for the present study.
Ascorbic acid (Vitamin C)
Daily preparation of Ascorbic acid (Nice Chemicals Edappally, India) was done by diluting the appropriate quantity (250mg/kg/day for each rat) in warm water [17] and was administered orally. It was kept in dark containers to avoid exposure to light.
Experimental animals
For the study, healthy adult male albino rats of the Wistar strain weighing between 170 and 200g were procured from the Animal house of National Institute of Pharmaceutical Research and Development (NIPRD) Abuja. The rats were housed in their cages with a 12- hour light-dark cycle and were kept at room temperature (27 2°C) and humidity (55 percent). They were fed with rat chows and water ad-libitum. They had 2 weeks of acclimatization before the experiment. The animal studies were carried out in accordance with the policies specified in the US National Institute of Health's "Guide for the Care and Use of Laboratory Animals" [21]. The experimental protocol was approved by the local Ethics Committee (UAECAU/2021/002).
Experimental induction of diabetes
After two weeks of acclimating to their cages, all animals were fasted overnight and injected intraperitoneal with 150 mg/kg of 5 percent alloxan monohydrate freshly dissolved in normal saline using 2ml disposable needles and syringes (19). They had unrestricted access to food and water subsequently. Determination of Fasting blood glucose (FBG) was done 48 hours after alloxan administration, using capillary samples obtained from tail snips and only animals with FBG above 12mmol/L were utilized in the investigation (15). ON CALL PLUS GLUCOMETER (ACON Laboratories San Diego, USA), a glucose test meter, was used to measure FBG, and the results were compared to the relevant standard values. FBG was done weekly.
Experimental design
A total of 48 male wistar rats were used in the experiment. The rats were split into eight groups, each with six (6) rats. After induction of diabetes, they were given Jatropha curcas ethanolic leaf extract (ELEJC) and ascorbic acid (AA) once daily orally for 28 days.
Group I (NC): Normal control animals were given rat chow and water liberally
Group II (JCE): Jatropha curcas only(500mg/kg/day)+rat chow and water
Group III (AA): Ascorbic acid (250mg/kg/day)+rat chow and water.
Group IV(JAA): Co-administration of Jatropha curcas (500mg/kg/day) & Ascorbic acid(250mg/kg/day) (DJA)+rat chow and water
Group V (DC): Untreated diabetic rats+rat chow and water.
Group VI (DJCE): Diabetic animals treated with Jatropha curcas only (500mg/kg/day).
Group VII (DAA): Diabetic animals treated with Ascorbic acid (250mg/kg/day).
Group VIII (DJAA): Diabetic animals treated with coadministration of Jatropha curcas (500mg/kg/day) & Ascorbic acid (250mg/kg/day)
Sample collection
The rats were weighed, anesthetized with diethyl ether for 5 minutes and decapitated after 28days of ELEJC and ascorbic acid treatment. Na+ EDTA bottle was used for sample collection and centrifuged at 3000 rpm for 5 minutes. Each bottle's plasma is transferred into a new plain specimen vial and maintained at -200C until analysis time. Reproductive organs (testes and epididymis) were promptly removed, rinsed in ice-cold physiologic saline solution (0.9 percent w/v), blotted, and weighed using a digital balance (RADWAG, Poland) with a sensitivity of 0,0001 gr. The caudal epididymal contents were used for sperm analysis and the tissue homogenates were prepared from the testis.
Reproductive hormone assays
Using the enzyme-linked immunosorbent assay (ELISA) kits (CALBIOTECH Inc. CA, USA), blood samples were tested for testosterone, LH, and FSH levels, according to the techniques reported by [22].
Preparation of testes homogenate and determination of testicular antioxidant markers
The homogenization of testes tissue was in 0.9 percent sodium chloride (ice cold) at a ratio of 1:5. To obtain the final supernatant, the initial supernatant was centrifuged at 3500rpm for 20 minutes at 40°C. The final supernatant was then utilized to evaluate testicular antioxidant indicators such as:
Catalase (CAT), which was tested using the method described by [23].
Superoxide dismutase activity was measured spectrophotometrically using the method of [24]. Glutathione peroxidase activity were determined spectrophotometrically in-accordance with [25] method.
Malonaldehyde (MDA), lipid peroxidation's breakdown product and thiobarbituric acid reactive substances (TBAR), were determined as described by [26].
Semen analysis/determination of sperm quality Determination of sperm concentration: The rats’ epididymal content were retrieved by cutting the epididymis' tail, tingling it with 2 mL of normal saline, and then teasing each rat's cauda epididymis. To avoid any additional tissue contamination, the suspension was stirred through a steel mesh. Sperm cell concentration was examined microscopically with the aid of hem cytometer according to the method described by [27].
Determination of sperm motility: Motility of spermatozoa was determined according to the methods of [28].
Determination of sperm morphology: Eosin–nigrosin was used to stain the slide to measure the percentage of morphologically abnormal spermatozoa. On each slide, 400 sperm cells (2000 cells per group) were studied, and the total abnormality (which include head and tail) rates of spermatozoa were expressed as a percentage.
Statistical analysis
The findings were presented as mean+standard error of mean (SEM). One-way ANOVA was used to determine the significance of differences between groups, followed by post hoc (LSD) analysis for significant value using the Statistical Package for Social Science (SPSS) version 23.0. if P<0.05, the differences were deemed statistically significant.
Results
Figure 1 shows the effect of ethanolic leaf extract of Jatropha curcas and ascorbic acid on the body weights of rats. There is significant reduction in the body weight of diabetic control groups compared to other groups. There is significant reduction in the body weight of rats in groups that received Jatropha curcas only after one week of treatment, however, regain their weight back subsequently.
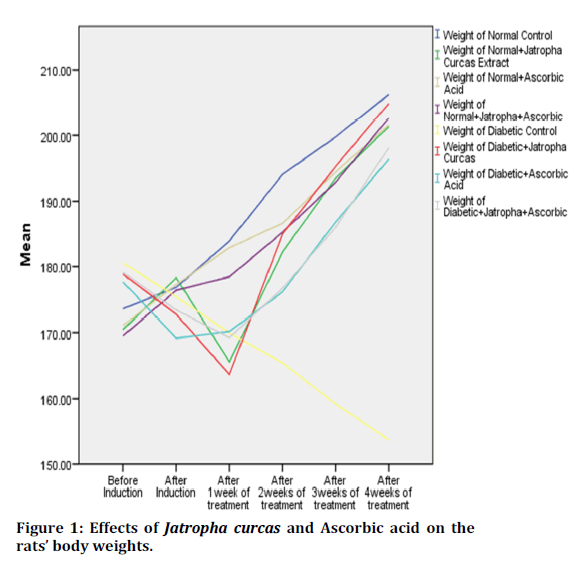
Figure 1. Surgical training questionnaire
Figure 2 shows the effect of ethanolic leaf extract of Jatropha curcas and ascorbic acid on weekly fasting blood glucose levels. There is significant increase in FBG after alloxan induction as seen in DC, DJCE, DAA, and DJAA compared to other groups. There is significant decrease in FBG of DJCE, DAA and DJAA in comparison to DC.
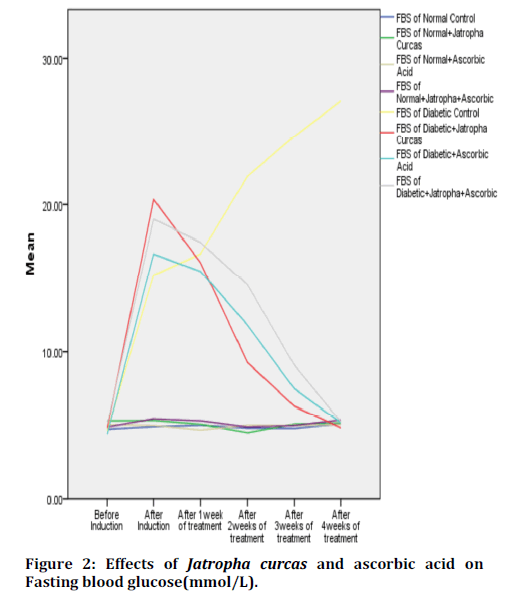
Figure 2. Surgical training questionnaire
Table 1 shows the effect of ethanolic leaf extract of Jatropha curcas and ascorbic acid on glutathione peroxidase, superoxide dismutase, catalase and malonaldehyde. The antioxidant enzymes (CAT, SOD & GSH) increased significantly in all treated groups compared to DC. There is significant increase in MDA level of DC, JAA and DJAA compared to NC. There is significant reduction in MDA level of DJCE and DAA compared to DC.
| Group/Parameters | Malonaldehyde (ng/mL) | Catalase (Ku/L) | Superoxide Dismutase (%IN) | Glutathione Peroxidase(ng/mL) |
|---|---|---|---|---|
| Normal Control (NC) | 837.91 ± 39.93 | 63.81 ± 1.48b | 157.49 ± 8.17b | 16.79 ± 0.39b |
| Normal+Jatropha curcas (JCE) | 893.04 ± 70.25 | 58.38 ± 1.90b | 154.64 ± 10.85b | 14.01 ± 0.46b |
| Normal+Ascorbic Acid (AA) | 857.73 ± 60.61 | 60.86 ± 2.01b | 156.66 ± 6.38b | 14.38 ± 0.87b |
| Normal+Jatropha curcas+Ascorbic Acid (JAA) | 1174.44 ± 44.76a | 50.07 ± 1.89b | 124.42 ± 4.80b | 14.83 ± 1.15b |
| Diabetic Control (DC) | 1620.51 ± 85.82a | 30.68 ± 3.77 | 48.11 ± 6.82 | 9.03 ± 0.66 |
| Diabetic+Jatropha Curcas (DJCE) | 699.45 ± 31.04bc | 53.71 ± 0.81b | 140.10 ± 4.80b | 15.34 ± 0.35b |
| Diabetic+Ascorbic Acid (DAA) | 985.90 ± 37.62bc | 58.68 ± 1.19b | 158.33 ± 3.90b | 16.06 ± 0.76b |
| Diabetic+Jatropha Curcas+Ascorbic Acid (DJAA) | 1469.28 ± 61.10a | 57.55 ± 0.74b | 164.99 ± 4.10b | 17.38 ± 0.65b |
| a=P<0.001 when compared to NC | ||||
| b=P<0.001 when compared to DC | ||||
| c=P<0.001 when compared to DJAA | ||||
Table 1: Effects of Jatropha curcas and ascorbic acid on testicular antioxidant enzymes and malonaldehyde.
Table 2 shows the effect of ethanolic leaf extract of Jatropha curcas and ascorbic acid on serum reproductive hormones (testosterone, FSH and LH). The testosterone deceased significantly in DC when compared to other groups. The testosterone decreased significantly in JCE, AA, DJCE & DJAA when compared to NC. The level LH and FSH of DC increase significantly when compared to other groups. The LH of JAA increased significantly when compared to JCE & AA. There is significant decrease in LH of DJAA compared to DJCE & DAA.
| Groups/ Parameters | Testosterone (ngh/mL) | FSH (mIU/mL) | LH (mIU/mL) |
|---|---|---|---|
| Normal Control (NC) | 38.21 ± 1.47a | 4.21 ± 0.08 | 8.86 ± 0.37d |
| Normal+Jatropha curcas (JCE) | 23.74 ± 1.06ab | 4.47 ± 0.19 | 8.81 ± 0.52d |
| Normal+Ascorbic Acid (AA) | 24.08 ± 1.60ab | 4.05 ± 0.07 | 8.11 ± 0.31d |
| Normal+Jatropha curcas+Ascorbic Acid (JAA) | 29.46 ± 1.00ab | 4.31 ± 0.20 | 15.93 ± 1.05 |
| Diabetic Control (DC) | 14.76 ± 0.58b | 11.20 ± 0.68 | 20.14 ± 1.18 |
| Diabetic+Jatropha curcas (DJCE) | 27.57 ± 0.81ab | 4.47 ± 0.12a | 13.08 ± 0.73ac |
| Diabetic+Ascorbic Acid (DAA) | 26.06 ± 1.27ab | 4.95 ± 0.85a | 14.25 ± 0.85ac |
| Diabetic+Jatropha curcas+Ascorbic Acid (DJAA) | 27.22 ± 1.84ab | 4.00 ± 0.32a | 4.06 ± 0.53a |
| a=P<0.001 in comparison to diabetic group | |||
| b=P<0.001 in comparison to control group | |||
| c=P<0.001 in comparison to Diabetic+Jatropha Curcas+Ascorbic acid group | |||
| d=P<0.001 in comparison to Normal+Jatropha Curcas+Ascorbic acid group | |||
Table 2: Effects of Jatropha curcas and ascorbic acid on the serum testosterone, FSH and LH.
Table 3 shows the effect of ethanolic leaf extract of Jatropha curcas and ascorbic acid on sperm concentration, sperm motility, sperm morphology and sperm PH. The sperm concentration, sperm motility and normal sperm morphology of DC, JAA and DJAA decreased significantly when compared to other groups. The sperm concentration of ascorbic acid treated groups is significantly reduced compared to Jatropha curcas treated groups. There is significant increase in abnormal sperm morphology of DC, JAA and DJAA compared to other groups.
| Group/ Parameters | Sperm Concentration (106/mL) | Sperm Motility (%) | Normal Morphology (%) | Abnormal Morphology (%) | Sperm PH |
|---|---|---|---|---|---|
| Normal Control (NC) | 212.60 ± 9.99a | 82.92 ± 1.20a | 93.02 ± 0.82a | 6.98 ± 0.82 | 6.60 ± 0.24 |
| Normal+Jatropha curcas (JCE) | 201.40 ± 4.03ace | 78.72 ± 1.38ac | 90.60 ± 1.35ac | 9.40 ± 1.35 | 6.60 ± 0.24 |
| Normal+Ascorbic Acid (AA) | 180.40 ± 3.17ac | 81.64 ± 1.64ac | 91.26 ± 1.65ac | 8.74 ± 1.65 | 6.80 ± 0.20 |
| Normal+Jatropha curcas +Ascorbic Acid (JAA) | 32.00 ± 3.51 | 59.18 ± 1.91 | 67.00 ± 2.14 | 33.00 ± 2.14f | 6.80 ± 0.12 |
| Diabetic Control (DC) | 56.20 ± 3.15 | 49.58 ± 2.35 | 61.14 ± 1.33 | 38.86 ± 1.33f | 6.50 ± 0.22 |
| Diabetic+Jatropha curcas (DJCE) | 224.20 ± 3.84abd | 77.06 ± 1.50ab | 89.90 ± 1.54ab | 10.10 ± 1.54 | 6.40 ± 0.24 |
| Diabetic+Ascorbic Acid (DAA) | 174.20 ± 4.93ab | 75.66 ± 1.52ab | 92.64 ± 1.65ab | 7.36 ± 1.65 | 6.70 ± 0.20 |
| Diabetic+Jatropha curcas+Ascorbic Acid (DJAA) | 30.40 ± 3.93 | 53.80 ± 1.47 | 58.44 ± 1.62 | 41.56 ± 1.62f | 7.0 ± 0.00 |
| a=P<0.001 in comparison to diabetic group | |||||
| b=P<0.001 in comparison to diabetic+Jatropha+Ascorbic group | |||||
| c=P<0.001 in comparison to Normal+Jatropha+Ascorbic group | |||||
| d=P<0.001 in comparison to diabetic+ascorbic acid | |||||
| e=P<0.001 in comparison to normal+ascorbic group | |||||
| f=P<0.001 in comparison to normal group | |||||
Table 3: Effects of Jatropha curcas and ascorbic acid on sperm quality (concentration, morphology, and motility).
Discussion
The body weight of untreated diabetic rats was significantly lower than that of the normal and treated groups. This is consistent with research that found considerable weight loss in diabetic rats that were not treated [29]. Weight reduction is one of the clinical features of diabetes mellitus and is due to poor glucose uptake because of insulin deficiency or resistant to insulin action. It was also observed that there was significant weight reduction in groups treated with ELEJC only after one week of treatment; however the weight increased subsequently throughout the course of experiment.
The anti-hyperglycemic impact of the ELEJC utilized in this study was significant, as blood glucose levels were corrected within three weeks of treatment. This is consistent with those obtained by [14,15,29].Medicinal plants with hypoglycemic and antidiabetic properties typically contain significant levels of alkaloids and flavonoids [30]. The presence of high amounts of alkaloids and flavonoids (2.26mg and 3.83mg, respectively) in the ethanolic leaf extract of Jatropha curcas may explain its hypoglycemic and antidiabetic effects [31]. When ELEJC and ascorbic acid were given together, there is no significant difference in the hypoglycemic impact compared to when they are given separately. Jatropha curcas had a faster hypoglycemic impact than ascorbic acid. [32] reported that ascorbic acid is protective and prevents diabetic end organ damage because of its antioxidant properties.
One of the main causes of diabetes-induced injury has been proposed by [33] as the generation of lipid peroxides (MDA) by free radical derivatives, which leads to tissue damage and the inability of antioxidant defense systems to prevent the development of uncontrolled free radicals. In this present study, the testicular antioxidant enzymes (GPx, SOD, and CAT) decreased significantly in DC compared to groups treated with Jatropha curcas and ascorbic acid. Decreased production of antioxidants-glutathione peroxidase (GSH–Px), catalase (CAT), superoxide dismutase (SOD) may contribute to increased ROS levels in diabetes.
Ascorbic acid and ELEJC has free radical scavenging abilities and could decrease the free radical-induced peroxidation of cellular structures. After 28 days of therapy with both Jatropha curcas and ascorbic acid, the testicular antioxidant enzymes (GPx, SOD, and CAT) and MDA activities have significantly improved. This is consistent with previous research that found that ELEJC reduced MDA levels and increased testicular level of GPx, CAT, SOD in diabetic rats, which could be linked to an increase in antioxidant enzyme activities in treated rats, resulting in the lipid peroxidation inactivation [14].
There is a connection between diabetes mellitus and alteration of the hypothalamic–pituitary–gonadal (HPG) axis, changing LH, FSH and testosterone concentrations in males [8-10]. In the present study, there is significant reduction in the serum testosterone level of DC compared to other groups. This may be due decreased Leydig cell function or suppression of the hypothalamic-pituitarygonadal axis. There is significant increase in the serum LH and FSH levels of DC compared to other groups. This may be a case of hyper gonadotropic hypogonadism due to lack of feedback inhibition to gonadotrophins as a result of decreased testosterone secretion. The findings are consistent with [34], who reported that hyper gonadotropic hypogonadism is the more common form of hypogonadism in men with Type II diabetes mellitus.
The testosterone level decreased significantly in groups treated with ELEJC only and ascorbic acid only; and their co-administration compared to control group. This is the same as [35], who reported that ELEJC decreased the levels of serum testosterone in comparison with control group. Decreased levels of LH and the damage of Leydig cells might account for reduced testosterone production [35].
The testosterone level of diabetic groups treated with Jatropha curcas and ascorbic acid increased significantly as compared to diabetic untreated group. This may be due to ELEJC and ascorbic acid free radical scavenging abilities and were able to reverse the initial damage (Leydig cell malfunction) caused by diabetes mellitus. However, there is also no significant difference in the testosterone level of groups treated with coadministration of ELEJC and ascorbic acid compared to those treated with ELEJC only and ascorbic acid only.
Diabetes Mellitus has been demonstrated to negatively alter spermatogenesis and sperm–related parameters [7-10]. The present study also shown similar findings, in which there is significant reduction in the sperm concentration, sperm motility and an increase in abnormal sperm morphology of diabetic untreated groups when compared to groups treated with ELEJC only and ascorbic acid only. The findings showed that ELEJC has androgenic property. This may be due to its antioxidant properties and was able to reverse the testicular oxidative stress, thereby promoting spermatogenesis and other sperm parameters. This is different from what was observed by Airaodion et al., 2020 who reported antifertility propensity of Jatropha curcas in male rats.
The ascorbic acid also shown profertility properties in this study. It prevents oxidative stress of sperm by inhibiting H2O2, superoxide & hydroxyl radicals; improves sperm motility and boosts sperm concentration [36]. However, the ELEJC has more androgenic property than ascorbic acid as the present study revealed that there was significant increase in the sperm concentration of rats treated with ELEJC only compared to rats treated with ascorbic acid only.
The present study also revealed that co-administration of ELEJC and ascorbic may be anti-androgenic, as the rats in this category had significant decreased in sperm concentration, reduced sperm motility and an increased in abnormal sperm morphology compared to rats treated with ELEJC only and ascorbic acid only. There may be an antagonistic interaction between ELEJC and ascorbic acid on spermatogenesis, leading to reduced sperm quality in rats treated with their co-administration.
Conclusion
Dietary supplementation of ethanolic leaf extract of Jatropha curcas and ascorbic acid for 28days after alloxan treatment resulted in reduced serum glucose levels, increased level of testicular antioxidant enzymes, increased serum level of testosterone, low FSH and LH, and improved sperm concentration, motility, and normal morphology. The androgenic effect is more in Jatropha curcas than in ascorbic acid. However, there is significant reduction in sperm quality of groups treated with coadministration of Jatropha curcas and ascorbic acid.
Recommendation
Dietary supplementation with Jatropha curcas leaf could be a simple, low-cost, and potential pharmaceutical agent that may prevent diabetic complication of reproductive dysfunction in men.
Jatropha curcas and ascorbic acid should not be taken together due to their anti-fertility effects.
Further research at the molecular level is needed to determine the exact mechanism of action of the Jatropha curcas plant in experimental animals.
As this study was done on experimental animal (rats), the outcome may not be the same in human. There is a need to carry-out further studies on the effect of Jatropha curcas plant in human subjects.
References
- https://www.who.int/publications-detail-redirect/9789241565257
- Innes JA. Davidson’s essentials of medicine. 2nd Ed. Churchill Livingstone Elsevier 2016.
- http://www.diabetesatlas.org/resources/2021-atlas.html
- Du Plessis SS, Cabler S, McAlister DA, et al. The effect of obesity on sperm disorders and male infertility. Nat Rev Urol 2010; 7:153-161.
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010; 33:S62-S69.
- http://www.diabetesatlas.org/resources/2017-atlas.html.
- Omolaoye TS, du Plessis SS. Diabetes mellitus and male infertility. Asian Pac J Reprod 2018; 7:6-14.
- Alves MG, Oliveira PF. Diabetes mellitus and male reproductive function: Where we stand? Int J Diabetol Vasc Dis Res 2013; 1:1-2.
- Agbaje IM, Rogers DA, McVicar CM, et al. Insulin dependant diabetes mellitus: Implications for male reproductive function. Hum Reprod 2007; 22:1871-1877.
- Nna VU, Abubakar AB, Mohammed M. Diabetes mellitus induced male reproductive impairment: The role of natural product. J Appl Pharmacol Sci 2017; 7:233-242.
- Chiekezie PC, Ojiako AO, Nwafor KC. Overview of antidiabetic medicinal plants. The Nigerian research experience. J Diabetes Metab 2015; 6:546.
- Abobatta W. Jatropha curcas: An overview. J Adv Agric 2019; 10.
- Kamal S, Manmohan S, Birendra S. A review on chemical and medicobiological applications of Jatropha curcas. Int Res J Pharm 2011; 2:61-66.
- Asuk AA, Dasofunjo K, Okafor AI, et al. Antidiabetic and antioxidative effects of Jatropha curcas extracts in streptozotocin-induced diabetic rats. Br J Med Med Res 2015; 5:341-349.
- Mishra SB, Vijayakumar M, Ojha SK, et al. Antidiabetic effect of Jatropha curcas leaves extract in normal and alloxan-induced diabetic rats. Int J Ph Sci 2010; 2:482-487.
- Aguirre-Arias MV, Velarde V, Moreno RD. Effects of ascorbic acid on spermatogenesis and sperm parameters in diabetic rats. Cell Tissue Res 2017; 370:305-317.
- Benabbou A, Khaled MB, Alchalabi AS. Evaluation of the efficiency of combined and separated antioxidant supplementation of vitamin C and E on semen parameters in streptozotocin-induced diabetic male wistar rats. South Asian J Exp Biol 2017; 7:166-172.
- Ugwu OPC, Nzubechukwu E, Ogbanshi ME, et al. The effect of ethanol leaf extract of jatropha curcas on chloroform induced hepatotoxicity in albino rats. Global J Biotech Biochem 2015; 10:11-15.
- Kokate CK. Practical Pharmacognosy, New Delhi, India, Vallabh Prakashan 1994; 107-110.
- https://www.oecd-ilibrary.org/environment/test-no-423-acute-oral-toxicity-acute-toxic-class-method_9789264071001-en
- Institute of Laboratory Animal Resources (US). Committee on care, use of laboratory animals. guide for the care and use of laboratory animals. US Department of Health and Human Services, Public Health Service, National Institutes of Health 1986.
- Mohammadi J, Motlagh FT, Mohammadi N. The effect of hydroalcoholic extract of watercress on parameters of reproductive and sex hormones on the diabetic rats. J Pharm Sci Res 2017; 9:1334–1338.
- Aebi H. Catalase in vitro. Methods Enzymol 1984; 105:121-126.
- Martin P, Dailey M, Sugarman E. Negative and positive assays of superoxide dismutase based on heamatoxylin autoxidation. Arch Biochem Biophys 1987; 255:329-336.
- Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol 1984; 105:114-121.
- Buege JA, Aust SD. Microsomal lipid peroxidation. Method Enzymol 1978; 52: 302-310.
- Okey SM, Olorunshola KV. Comparative effects of halofantrine hydrochloride and artesunate on the testis of guinea pigs. Curr Res J Biol Sci 2012; 4:247-249.
- Tijee DY, Ontoeng S. The viscosity of human semen and the percentage of motile spermatozoa. Fertile Steril 1968; 19:562-565.
- Aina VO, Ibrahim MB, Abdulsalami MS, et al. Anti-diabetic activities of the leaf and bark extracts of Jatropha curcas on alloxan-induced diabetic albino rats. J Nat Sci Res 2016; 6:91-95.
- Oladele SM, Ayo JO, Adaudi AO. Medicinal and physiological properties of flavonoid cermiriva derivatives and anthraquines of plant origin. West Afri J Pharmaocol Drug Res 1995; 2:134-144.
- Nwamarah JU, Otitaju O, Otitaju GTO. Chemical composition and antidiabetic properties of Jatropha curcas leaves extract on alloxan induced diabetic wister rats. Afri Biotechnol 2015; 14:1056-1066.
- David C, Girgis CM, Gunton JE. Effects of vitamin C and vitamin D in diabetes mellitus. Nutr Diet 2015;17:21-28.
- Amresh G, Rao CV, Singh PN. Antioxidant activity of issampelospareira on benzo (a) pyrene induced mucosal injury in mice. Nutr Res 2007; 27:625-632.
- Musa E, El-Bashir JM, Sani-Bello F, et al. Hypergonadotropic hypogonadism in Nigerian men with type 2 diabetes mellitus. Clin Diabetol 2021; 10:129-137.
- Airaodion AI, Ayanleke IA, Agunbiade AP, et al. Antifertility propensity of Jatropha curcas linn. leaves on male wistar rat. Int J Res Rep Gynaecol 2020; 3:21-29.
- Ashamu EA, Salawu EO, Oyewo OO. Efficacy of vitamin C and ethanolic extract of Sesamum indicum in promoting fertility in male Wistar rats. J Hum Reprod Sci 2010; 3:11-14.
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Author Info
Akin Olanipekun1, John Ogedengbe2, Olorunshola Kolawole2 and Senol Dane1*
1Department of Physiology, Faculty of Basic Medical Sciences, College of Health Sciences, Nile University of Nigeria, Abuja, Nigeria2Department of Human Physiology, Faculty of Basic Medical Sciences, College of Health Sciences, University of Abuja, Abuja, Nigeria
Received: 02-Jul-2022, Manuscript No. JRMDS-22-68311; , Pre QC No. JRMDS-22-68311 (PQ); Editor assigned: 04-Jul-2022, Pre QC No. JRMDS-22-68311 (PQ); Reviewed: 19-Jul-2022, QC No. JRMDS-22-68311; Revised: 22-Jul-2022, Manuscript No. JRMDS-22-68311 (R); Published: 29-Jul-2022
