Research - (2020) Volume 8, Issue 3
Evaluating Cytotoxic Effects of Resin Based CAD/CAM Blocks
Numan Ayd├?┬▒n*, Serpil Karaoglanoglu and Elif Aybala Oktay
*Correspondence: Numan Ayd├āŌĆ×├é┬▒n, Department of Restorative Dental Treatment, Gulhane Faculty of Dentistry, University of Health Sciences, Ankara, Turkey, Email:
Abstract
Background: The effects of CAD/CAM blocks containing resin on oral cells, which are widely used in the restoration of teeth recently, are unknown. The aim of this study is in vitro examination of cytotoxic effect of resin and ZLS (zirconia-reinforced lithium silicate) including CAD/CAM blocks on human gingival keratinocyte (HGK) cells.
Material and Methods: In our study; samples were prepared (1.5 × 7 × 12 mm) under water cooling, using CAD/CAM blocks. Both surfaces of the prepared samples were polished using a diamond polishing kit (Clearfil Twist Dia, Kururay). Samples, whose surface (3 cm2/ml) were calculated according to the International Standards Organization (ISO 10993-12: 2012), were left for 1, 3- and 7-days incubation in DMEM. Cell viability of extracts of 1:1 ratio of filtered CAD/CAM blocks were examined by MTT test. Cell viability results were evaluated using one-way analysis of variance (ANOVA) test (p<0.05).
Results: There was no statistically significant difference between the cell viability values of the extracts of CAD/CAM blocks after 1 and 3 days and the control group (p>0.05). Statistically significant differences were observed in the cell viability values of the extracts of composite resin and ZLS CAD/CAM blocks at the end of 7 days compared to the control group (p<0.05).
Conclusion: Although the extracts of CAD/CAM blocks after 7 days caused a decrease in the viability of HGK cells, they showed cell viability above the cell viability rate (>70%) stated in ISO standards.
Keywords
CAD/CAM block, Cytotoxicity, Gingival keratinocyte
Introduction
Today, different restorative materials are preferred to meet the expectations of individuals who demand whiter and more esthetic teeth. The development of computer aided design and computer aided manufacturing (CAD/ CAM) technology has facilitated the production of esthetic restorations [1]. CAD/CAM blocks may contain feldspatic glass ceramics, leucitereinforced glass ceramics, lithium disilicate glass ceramics aluminum-oxide and composite resin [2].
Recently developed resin and ZLS containing CAD/CAM blocks are among the popular materials to produce tooth color restorations [3]. As an alternative to ceramic blocks, polymer infiltrated ceramic network (PICN) material and resin ceramic blocks have been developed. Della Bona et al. [4] reported that the properties of PICN materials are between porcelain and highly filled composite resin. Composite resinbased CAD/CAM blocks are ceramics integrated into a polymer network polymerizing at higher temperatures and pressures [5]. These blocks are reported to have better or comparable fracture toughness and higher abrasion potential than commonly used composite resin materials [6]. ZLS, lithium metasilicate and lithium disilicate offer a pair of microstructures consisting of very fine crystals and glassy matrix with highly dispersed zirconium oxide [7-10]. This material can be used without the need for a crystallization process unless more durability is required. Although the optical and mechanical properties of resin-based composite CAD/CAM blocks are low compared to ceramics CAD/CAM blocks, being close to Young's modulus dentine are the biggest advantages with easy bonding and repair [11].
Although the mechanical and physical properties of restorative materials meet clinicians' expectations, restorative materials used in the oral environment should be biocompatible for both hard and soft oral tissues. These restorative materials should not contain toxic substances that can cause harmful local effects or a systemic reaction. Cytotoxic substances can cause shortand long-term harmful tissue reactions ranging from postoperative sensitivity to irreversible pulp damage [12].
Restorative materials containing resin can release monomers in their structures depending on the physical and chemical effects in the mouth environment [13]. It is stated that bisphenol-A glycidylmethacrylate (Bis-GMA), triethylene glycol dimethacrylate (TEGDMA) and urethane dimethacrylate (UDMA), which are one of the basic monomers in the organic matrix of these materials, cause cytotoxic and mutagenic effects on cells [14].
The aim of this study is in vitro examination of the cytotoxic effect of resin based and ZLS containing CAD/CAM blocks on HGK cells. Our null hypothesis is that resin-based and ZLS containing CAD/CAM blocks will not decrease the viability of HGK cells.
Materials and Methods
Preparation of CAD/CAM materials
In the study, four resin CAD/CAM blocks (Cerasmart; GC Europe, Brilliant Crios; Coltene, Vita Enamic; Vita Zahnfabrik and Grandio Blocs, VOCO GmbH) and ZLS block (Celtra Duo, Dentsply Sirona) were used (Table 1). The materials used in the study were obtained by slicing from CAD/ CAM blocks with a low speed (150 rpm) water cooled diamond disc precision cutting machine in a size of 1.5x7x12 mm. Both surfaces of the prepared samples were polished using a diamond polishing system (Clearfil Twist Dia, Kururay) under water cooling. Then, all samples were cleaned with ultrasonic cleaner (Pro-Sonic 600; Sultan Healthcare, NJ, USA) for 10 seconds with deionized water and then dried with air pressure. Samples (n:4) whose surface area is calculated according to ISO 10993-12:2012 [15] standards (3 cm2/ml) incubated in 3 ml serumfree Dulbecco’s Modified Eagle Medium (DMEM) and control group in serum-free medium, for 1, 3 and 7 days at 37°C, with 5% CO2 oven. The extracts of CAD/CAM samples that were kept in serum-free DMEM medium, which were filtered after 1, 3 and 7 days, were used for the experiments in cytotoxicity at a ratio of 1:1.
| Material type | Materials | Composition by weight | Manufacturer | Lot No | |
|---|---|---|---|---|---|
| Filler | Polymer | ||||
| Resin-based (Hybrid ceramic) | Vita Enamic | 86% Fine structure Feldspat ceramic | Methacrylate Polymer, UDMA, TEGDMA | VITA, Zahnfabrik, Germany | 81060 |
| Cerasmart | 71% silica and barium glass nanoparticles | Bis-MEPP, UDMA, DMA | GC Europe, Japan | 1612151 | |
| Resin-based (Composite) | Grandio Blocs | 86% nanohybrid fillers | 14% UDMA+DMA | VOCO GmbH, Germany | 1904625 |
| Brilliant Crios | 70% of glass and amorphous silica | Cross-linked methacrylates (Bis-GMA, Bis-EMA, TEGDMA) | Coltene, Switzerland | I89523 | |
| Zirconia-reinforced lithium silicate (ZLS) | Celtra Dou | SiO2, ZrO2, Lithium-silicate | - | Dentsply Sirona, Germany | 5365411011 |
Table 1: Materials tested and manufacturer’s information.
Cell culture
The first passage was performed after the cell line (HGKs) used in the study was dissolved under appropriate conditions and required experimental conditions, after 90% confluence. The number of cells with the desired density was calculated in the 96-well cell production plates. Then, for the experiment, it was homogenized with 10% FBS (fetal bovine serum) and 1% antibiotic medium and its suspension was prepared as 1×105 cells/ml [16]. This cell suspension was divided into 96-well cell production plates at 100 μl/well and incubated for 24 hours in a 5% CO2 incubator. At the end of this period, the culture medium was aspirated and removed from the culture medium in 96-well cell production plates, and the extracts in which fillers were kept were placed in the wells as 100 μl/well, and then incubated in a 5% CO2 incubator for 24 hours and then MTT ([3-(4,5-dimethylthiazol-2- yl)-2,5-diphentyltetrazolium bromide] test was performed.
Cytotoxicity test
The MTT solution (Sigma, USA) was homogenized by mixing with serum-free and antibiotic-free oral keratinocyte medium and the final concentration was prepared as 5 mg/ml. 24 hours later, 10 μl/ well of MTT solution was placed in each well of incubated 96-well cell production plates and they were incubated for 4 hours at 37oC in a dark environment. Then, it was read in the optical reader (BIO-TEK μQuant, BIO-TEK Instruments, Inc, USA) at 550 nm and the output values were compared with the control wells. Experiments were repeated at least three times.
Statistical analysis
Statistical investigations were made in SPSS 22.00 (Statistical Package for Social Sciences, IBM Inc., USA) package program. Cell viability results obtained from MTT test were evaluated using one-way analysis of variance (ANOVA) and Tukey post hoc test (p<0.05).
Results
Extracts of CAD/CAM blocks at the end of 1 day showed 100% cell viability on HGK cells. Extracts of CAD/CAM blocks at the end of 3 and 7 days caused a decrease in HGK cell viability values. However, the decrease in cell viability at 3 day was not statistically significant. In addition, the PICN CAD/CAM block (Vita Enamic) showed 100% cell viability on HGK cells in all-time extracts (1:1).
In our study, only cell viability values of the extracts of composite resin (Brilliant Crios) and ZLS (Celtra Dou) CAD/CAM blocks after 7 days showed statistically significant differences compared to the control group (p<0.05). Of the materials, PINC CAD/CAM block (Vita Enamic) produced the highest (100%) cell vitality on cells, while composite enhanced (Brilliant Crios; 72.1%) and ZLS (Celtra Dou; 73.2%) showed minimal cell viability. (Figure 1, Table 2).
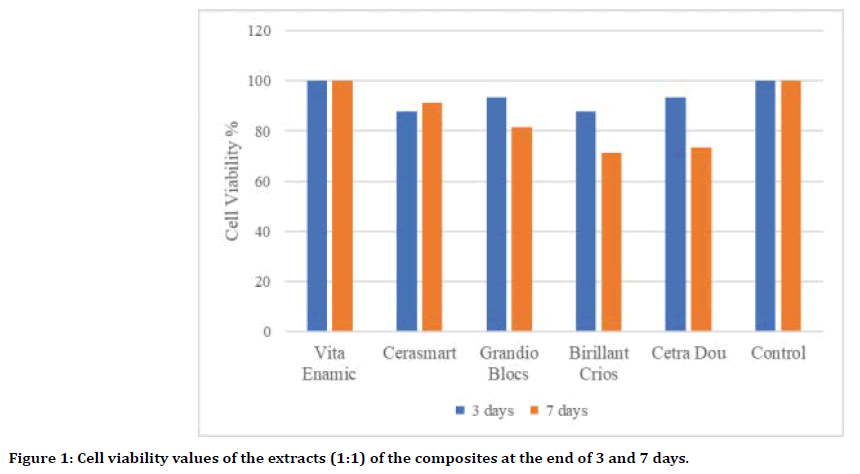
Figure 1. Cell viability values of the extracts (1:1) of the composites at the end of 3 and 7 days.
| CAD/CAM bloks | Cell absorbance value 3 days | Cell Viability value 3 days (%) | Cell absorbance value 7 days | Cell Viability value 7 days (%) |
|---|---|---|---|---|
| Vita Enamic | 0.41 ± 0.07a | 100 | 0.50 ± 0.11a | 102 |
| Cerasmart | 0.36 ± 0.03a | 87.8 | 0.45 ± 0.03ab | 91.2 |
| Grandio Blocs | 0.38 ± 0.03a | 93.6 | 0.40 ± 0.06ab | 81.6 |
| Birillant Crios | 0.36 ± 0.02a | 87.8 | 0.35 ± 0.09b | 71.4 |
| Cetra Dou | 0.38 ± 0.04a | 93.6 | 0.36 ± 0.04b | 73.5 |
| Control | 0.41 ± 0.04a | 100 | 0.49 ± 0.07a | 100 |
| P | 0.25 | 0.006 |
Table 2: Cell viability values of the 1:1 extracts of the composites at the end of 3 and 7 days.
Discussion
Recently, manufacturers have developed new CAD/CAM formulations that combine the advantageous properties of ceramics such as durability and color stability, and improved bending and low abrasiveness of composite resins [17]. In this context, CAD/CAM blocks with resin content are presented to the use of clinicians with the advantages of easy preparation, polishing and reparability. Dental materials are generally physically and chemically examined while their biological effects are ignored. All CAD/CAM restorations are in long-term contact with oral soft tissues, especially keratinized epithelium. These materials should be thoroughly examined for their biocompatibility before clinical applications. Although various test methods are used in research evaluating the biocompatibility of restorative materials used in dentistry, animal experiments and cell culture tests are widely preferred [18]. Cell culture tests are used more because they are better standardized and reproducible and compared to animal experiments; they are easy to apply, less time consuming and economical tests [19].
To assess the cytotoxicity of dental materials, ISO 10993-12:2012 proposed several cell culture test models [15]. These are test methods of direct contact (direct method), indirect contact with a barrier (indirect method) and the method in which extracts from biomaterials are added to the cells (extract method). Lim et al. [20] in their study comparing these in vitro test models used to evaluate the cytotoxicity of composite resins, suggested the extract test due to higher sensitivity if a single test model will be used. In our study, we as well used the extract test method on HGK cells. As a result of our study, in which we analyzed the toxicity of different CAD/ CAM blocks on HGK cells with the extract test method; our null hypothesis was rejected as the extracts (1:1) of CAD/CAM blocks at the end of 7 days caused a decrease in cell viability values.
Monomers similar to traditional composite resins are used in the structure of resincontaining CAD/CAM blocks [21,22]. In their study on monomer elution of CAD/CAM blocks, Mourouzis et al. stated that TEGDMA and UDMA and Bis-EMA monomers elute but Bis-GMA monomer elution was not observed [23]. In many studies, it has been stated that Bis-GMA, UDMA and TEGDMA monomers are cytotoxic [24- 26]. Grenade et al. [27] in fact, PICNs achieved similar results to lithium disilicate glassceramic in terms of HGF behavior (attachment, proliferation and spreading), despite the presence of dimethacrylate resin infiltrating the glass-ceramic network. Yet regarding PICNs, take Tassin et al. [11] did not show any direct cytotoxic effect, contrary to conventional lightcured composites, nor any effect in terms of proliferation, extracellular matrix synthesis, morphology or inflammatory response. Those results were explained by the high degree of conversion of PICNs. In our study, PINC block (Vita Enamic) from resin-containing blocks did not show toxic effects, while compositereinforced block (Brilliant Crios) showed the least cell viability.
It has been reported in the literature that the resin-containing blocks' not having a toxic effect on cells, which may be due to controlled polymerization under the optimum pressure and temperature, and the firm binding of the UDMA monomer to the ceramic network [27]. In addition, it is thought that the reason for PICN CAD/CAM block's not having a decrease in the cell viability is due to not having Bis-GMA monomer [28] in its structure, which is determined as toxic and depends on the high degree of conversion. In their study, Pabst et al. [29] examined the effects of CAD/CAM all-ceramic (e.max CAD LT, e.max CAD HT, Empress CAD, Mark II) materials on cell viability on human gingival fibroblasts; The cell viability of all materials did not show a significant decrease in all time periods (3, 6, 9 and 12 days). Of the materials, only a significant decrease in cell viability was noted for the 7th day of the Empress CAD group test period. In their study, Rafaelli et al. [30] evaluated the cytotoxicity of zirconia and feldspatic CAD/CAM discs on L929 mouse fibroblast cells by MTT method in vitro and stated that zirconia exhibits more biocompatibility.
Rizo-Gorrita et al. [31] analyzed human gingival fibroblast (HGF) between zirconium (Y-TZP) and new zirconia-reinforced lithium silicate ceramics (ZLS) in their study and the results suggest that HGF cultured on Y-TZP have a greater cell proliferation, coverage, and spreading than those cultured on ZLS, which translates in a greater affinity for the surface of Y-TZP. They stated that Y-TZP resulting in better cellular response was associated with less surface roughness.
In our study, the ZLS CAD/CAM block did not produce a significant cell reduction in cell viability for 1 and 3 days but showed less cell viability in 7 days compared to the control group. The fact that the ZLS-containing material causes a decrease in cell viability at the end of 7 days is considered to be due to the absence of a second crysterization process. Within the limitations of this in vitro study is to select only a single cell line and test model in the evaluation of the cytotoxic effects of CAD/CAM blocks. The use of different test models using other cells associated with mouth tissue as well as differences in cell lines may cause variability in cytotoxic responses. In subsequent studies, it may be considered to work with more cell types and test models to confirm cytotoxic properties of these CAD/CAM blocks.
Conclusion
In our study where we examined the toxic effects of resin containing CAD/CAM blocks on HGK cells.
Extracts of CAD/CAM blocks at the end of 3 and 7 days (1:1 ratio) caused a decrease in the viability values of HGK cells. (except PINC CAD/CAM block).
While the block (Vita Enamic) prepared by PINC method showed the highest cell viability from the materials, the composite reinforced block (Brilliant Crios) and ZLS content (Celtra Dou) CAD/CAM block showed the least cell viability.
All CAD/CAM blocks that we used in the study showed cell viability above the rate of acceptable cell viability (70%) specified by ISO, at the end of 7 days.
CAD/CAM blocks other than the block prepared by PINC method decrease the viability values of HGK cells as time progresses.
References
- Coldea A, Swain MV, Thiel N. Mechanical properties of polymer-infiltrated ceramic network materials. Dent Mater 2013; 29:419-426.
- Lauvahutanon S, Takahashi H, Shiozawa M, et al. Mechanical properties of composite resin blocks for CAD/CAM. Dent Mater J 2014; 33:705-170.
- Awada A, Nathanson D. Mechanical properties of resin-ceramic CAD/CAM restorative materials. J Prosthet Dent 2015; 114:587-593.
- Della Bona A, Corazza PH, Zhang Y. Characterization of a polymerinfiltrated ceramic-network material. Dent Mater 2014; 30:564-569.
- Chen C, Trindade FZ, de Jager N, et al. The fracture resistance of a CAD/CAM Resin Nano Ceramic (RNC) and a CAD ceramic at different thicknesses. Dent Mater 2014; 30:954-962.
- Acar O, Yilmaz B, Altintas SH, et al. Color stainability of CAD/CAM and nanocomposite resin materials. J Prosthet Dent 2016; 115:71-75.
- Silva LHD, Lima E, Miranda RBP, et al. Dental ceramics: A review of new materials and processing methods. Braz Oral Res 2017; 31:131-146
- Hallmann L, Ulmer P, Kern M. Effect of microstructure on the mechanical properties of lithium disilicate glass-ceramics. J Mech Behav Biomed Mater 2018; 82:355-370
- Traini T, Sinjari B, Pascetta R, et al. The zirconia-reinforced lithium silicate ceramic: Lights and shadows of a new material. Dent Mater J 2016; 35:748-755.
- Elsaka SE, Elnaghy AM. Mechanical properties of zirconia reinforced lithium silicate glass-ceramic. Dent Mater 2016; 32:908-914.
- Tassin M, Bonte E, Loison-Robert LS, et al. Effects of high-temperature pressurepolymerized resin-infiltrated ceramic networks on oral stemcells. PLoS One 2016; 11:1-16
- Geurtsen W. Biocompatibility of resin-modified filling materials. Crit Rev Oral Biol Med 2000; 11:333-355.
- Reichl FX, Seiss M, Kleinsasser N, et al. Distribution and excretion of Bis GMA in guinea pigs. J Dent Res 2008; 87:378-380.
- Geurtsen W, Lehmann F, Spahl W, et al. Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblast cultures. J Biomed Mater Res 1998; 41:474-480.
- ISO 10993-12:2012. Biological evaluation of medical devices. Part 12: Sample preparation and reference materials, Geneva, 2012
- ISO 10993-5:2009. Biological evaluation of medical devices. Part 5: Tests for in vitro cytotoxicity. Genava 2009
- Coldea A, Swain MV, Thiel N. Mechanical properties of polymer-infiltratedceramic- network materials. Dent Mater 2013; 29:419-426.
- Murray PE, García Godoy C, García Godoy F. How is the biocompatibilty of dental biomaterials evaluated? Med Oral Patol Oral Cir Bucal 2007; 12:258-266.
- Aydın N, Karaoğlanoğlu S, Oktay EA, et al. Evaluating of cytotoxic effects of highly esthetic dental composites. Braz Dent Sci 2020; 23:1-8.
- Lim SM, Yap AUJ, Loo CSL, et al. Comparison of cytotoxicity test models for evaluating resin-based composites. Human Exp Toxicol 2016; 34:339-348.
- Ferracane JL. Elution of leachable components from composites. J Oral Rehabil 1994; 21:441-452.
- Altintas SH, Usumez A. Evaluation of monomer leaching from a dual cured resin cement. J Biomed Mater Res Part B: Appl Biomater 2008; 86B:523-529.
- Mourouzis P, Andreasidou E, Samanidou V, et al. Short-term and long-term release of monomers from newly developed resin-modified ceramics and composite resin CAD-CAM blocks. J Prosthet Dent 2020; 123:339-348.
- Yoshii E. Cytotoxic effects of acrylates and methacrylates: relationships of monomer structures and cytotoxicity. J Biomed Mater Res 1997; 37:517-524.
- Harorli OT, Bayindir YZ, Altunkaynak Z, et al. Cytotoxic effects of TEGDMA on THP-1 cells in vitro. Med Oral Patol Oral Cir Bucal 2009; 14:489-493.
- Moharamzadeh K, Noort RV, Brook IM, et al. Cytotoxicity of resin monomers on human gingival fibroblasts and HaCaT keratinocytes. Dent Mater 2007; 23:40-44.
- Grenade C, De Pauw-Gillet MC, Pirard C, et al. Biocompatibility of polymer-infiltrated-ceramicnetwork (PICN) materials with human gingival keratinocytes (HGKs). Dent Mater 2017; 33:333-343.
- Krifka S, Spagnuolo G, Schmalz G, et al. A review of adaptive mechanisms in cell responses towards oxidativestress caused by dental resin monomers. Biomaterials 2013; 34:4555-4563.
- Pabst AM, Walter C, Grassmann L, et al. Influence of CAD/CAM all-ceramic materials on cell viability, migration ability and adenylate kinase release of human gingival fibroblasts and oral keratinocytes. Clin Oral Investig 2014; 18:1111-1118.
- Raffaelli L, Rossi Iommetti P, Piccioni E, et al. Growth, viability, adhesion potential, and fibronectin expression in fibroblasts cultured on zirconia or feldspatic ceramics in vitro. J Biomed Mater Res A 2008; 86:959-968.
- Rizo-Gorrita M, Luna-Oliva I, Serrera-Figallo MÁ, et al. Comparison of cytomorphometry and early cell response of human gingival fibroblast (HGFs) between zirconium and new zirconia-reinforced lithium silicate ceramics (ZLS). Int J Mol Sci 2018; 19:1-16.
Author Info
Numan Ayd├?┬▒n*, Serpil Karaoglanoglu and Elif Aybala Oktay
Department of Restorative Dental Treatment, Gulhane Faculty of Dentistry, University of Health Sciences, Ankara, TurkeyCitation: Numan Aydın, Serpil Karaoglanoglu, Elif Aybala Oktay, Evaluating Cytotoxic Effects of Resin Based CAD/CAM Blocks, J Res Med Dent Sci, 2020, 8 (3):131-136.
Received: 06-Mar-2020 Accepted: 14-May-2020
