Research Article - (2022) Volume 10, Issue 9
Efficacy of Locally Applied K-Carrageenan on the Healing of Thermal Burn in Rat-Experimental Model: Histological Study
Ammar Abood Kanber1* and Enas Fadhil Kadhim2
*Correspondence: Ammar Abood Kanber, Department of Dental Surgery Iraqi Parliament Council, Baghdad, Iraq, Email:
Abstract
Wound is damage or disruption to the normal anatomical structure and function. Carrageenan is sulphated polysaccharide found in Gigartina, Chondrus and Eucheuma species in the red algal family. Having anticancer, ntǦnϔlmmtor and renewal of tissues. Our study aimed to detect the role of kappa carrageenan in the burned skin wound repair. Skin burn were performed in the right and left cheek of 20 male rats (aged 7-8 weeks weighing 300-350 g). Burned skin rats were categorized into two equal groups. Burned areas of right side were treated with a local application of 1 ml of kappa carrageenan solution once daily (treatment group) and the left side receive no treatment (control group). After 5, and 10 days, 5 rats from each group were scrϔced and tissue specimens from the wounded burn areas were obtained for histopathological inspection. Histopathological examination showed acceleration of wound healing in the kappa carrageenan treated rats as compared with control group during the experimental period. Progressions of wound healing were noticed with regard to re-epithelialization, wound closure and nϔltrton of nϔlmmtor cells. In conclusion, local usage of Kappa Carrageenan has curative efϔcenc to hasten wound healing.
Keywords
Facial burn, Kappa carrageenan, Wound healingIntroduction
The biggest organ in the body is skin; it acts as barrier to protect the body from harmful factors in the environment. Skin consists from three layers: hypodermis, dermis, and epidermis. The outer layer is epidermis which is formed by keratinocytes which are set in different layers [1]. Stratum basale, stratum granulosum, stratum spinosum, and stratum cornium. All these four layers act as defensive barrier against multiple pathogens, chemical agents, mechanical agents, and they also responsible for fluid equilibrium. Among keratinocytes there are specialized types of cells responsible for pigmentation which is the melanocytes. Other types of specialized cells also found in this layer which are the langerhans cell which responsible of immunity [2]. Second layer is dermis which is thick and dense, this layer is formed by fibroblasts, these cells work to secret extracellular component such as fibronectin and collagen which is formed the matrix. The ECM is made of glycosaminoglycans, collagen and elastin, and enclosed by fibroblasts, smooth muscle cells, mast cells and endothelial cells. The main protein of extracellular matrix is collagen and the main structural collagen in skin is collagen type I.
Carrageenan is a highly sulphated polysaccharide found in Gigartina, Chondrus and Eucheuma species in the red algal family. It is widely used in the pharmaceutical industries as a gelling agent, stabilizer, binder, thickener and additive. Carrageenan is regarded as safe. It is reported to induce pleurisy and paw edema in experimental rats, which is used to study anti-inflammatory action. Carrageenan induces thrombosis in a tail thrombosis model and is used to study the mechanisms of thrombolysis and antithrombosis in small laboratory animals. Rising evidence proposes the anti-cancer ability. A wound is an injury or disturbance to the normal anatomical structure and function. This injury may be simple break in the epithelial continuity of the skin or it can be deeper, extending into the subcutaneous tissue with damage to other structures such as muscles, tendons, nerves, vessels and bone [3].
Wounds can be categorized according to multiple criteria. Time is an important factor in injury management and wound healing. Thus, wounds can be clinically classified as acute and chronic according to their healing time [4], acute wounds that repair themselves by normal healing pathway [3,5], chronic wounds fail to repair through the normal steps of healing pathway. The phases of wound healing which are hemostasis, inflammation, proliferation and remodelling may be disturbed by different factors, which prolong one or more steps in the phases of wound healing [6], complicated wound is a tissue defect and infection that poses a threat to the wound [7-12].
Burn is an injury that requires special care based on size and depth of the burn. The depth and area of a burn are not static with regard to the burn diagnosis and treatment, and through the first few days after injury, burns can progress in severity. Increases in both body surface area and burn depth. Burn progressions have been implicated by cytokine accumulation, oxidative stress and hypercoagulability, Local and systemic processes involved in the burn sequence of events remain unclear. The process of burn tissue progression is represented by three zones. The zone of coagulation is the tissue that was damaged directly after burn and unable to repair [13], around that is the zone of stasis, consist of hypo perfused tissue which undergo necrosis, the zone of hyperemia is the outer zone and consist of hyper perfused tissue and repairable.
Evaluation of burn severity
To determine the severity of a burn we should know width of the area and the depth of the burn. The depth of the burn may be changed due to infection and edema so that it is necessary to wait 1-2 days after burn to mark the correct burn degree. Variations in the burn depth are affected by the degree of temperature, the type of the causative agent and the vascularity and thickness of the affected skin. The Burn classified into three groups according to the depth.
First degree burn occurs in epidermis (superficial) and this burn is painful with edematous skin. Pain subsides during 12-24 hours and heals after one week with desquamation like sunburns; cold application on erythematous skin can reduce the pain. Fluid supply should be needed if the 2nd degree burns more than 20% of body surface. In third-degree burns the entire skin epidermis, dermis, and hypodermis is infected. More severe burns can affect tendons, muscles and bones. The skin is dry with erythema [14].
Progressions of wound healing were noticed with regard to re-epithelialization, wound closure and infiltration of inflammatory cells.
Materials and Methods
Study design
A total number of 10 Western Albino male rats weighting (300-350) gram and with age of (7-8) months were used in this study in randomized clinical trial design. The experimental procedures were achieved in compliance with the ethical principles of animal experimentation of the University of Baghdad, college of dentistry. Each rat was subjected to thermal burn on the right and left cheek skin. The left side was assigned as the control group and the right side was assigned for the experimental groups. The treatment groups were included the addition of medications on the burned skin to monitor the healing process compared with that of the control group.
Study grouping
- Carrageenan group (n=10): the rats were subjected to thermal burn on the right side of cheek followed by treatment with 1 ml of Carrageenan solution.
- Control group (n=10): the thermal burn was conducted on the left side of the cheek.
Experimental procedure
The experiment was started with check hair shaving using shaving blade and gel (Veet, Bahrain) [14], before shaving the rats should be anesthetized as we mentioned below. After shaving, the rats were anesthetized with inhalation as a general anesthesia. The diethyl ether solution was delivered to the rat by immersion a piece of cotton in 2 ml of the anesthetic solution, and the rat and the immersed cotton were placed in a closed container for 20 s [15]. At this time the rat is ready for the thermal burn procedure for inducing the skin burn. The metal dental instrument (Ash no.6) was heated in boiling water for 5 min and pressed to shaved, disinfected right side of cheek skin surface for 10 sec under light pressure to induce skin burn wound, and this procedure was repeated for the left side [16,17].
Preparation of the treatment medication
The medication used in this study was the Carrageenan. The mentioned medication was prepared by mixing 1 gm of the medication (Carrageenan) and 100 ml of normal saline for 1 min. by hand liquids blender.
Treatment of skin burn: After thermal burn of the skin has been done, the burned skin was treated with the medication mentioned above. This medication was applied immediately after the skin burning procedure for 10 days one time/day. One drop was applied to the burn wound area thoroughly. In the control group no medication was applied after the skin burning procedure.
Specimens’ collection: After 5 days of medication application, 5 rats were sacrificed and the rest of the rats were sacrificed at day 10. The rats were anaesthetized by general anesthesia using the diethyl ether employing the same procedure mentioned in the experiment before the sacrifice procedure. After the sacrifice procedure, the burned wound area was excisioned by scalpel and blade no. 15. The excision border included the wounded area and extended to adjacent intact skin, and the depth of the incision was from skin surface to the underlying bone. The collected samples were placed in 10% formaldehyde immediately after excision.
Histological slides preparation
The histological slides preparation for the incised tissue was conducted as usual protocol to stain using H and E.
Measurement of the epithelial thickness and inflammatory cells count
After histological slides examination and capturing with light microscope, the measurement software (ImageJ V.1.53K, National institute of health, USA) was used to measure the epithelium thickness and inflammatory cells count, and by using the 10x of magnification. The thickness of the epithelium was measured from the inner edge of the basal cell layer until the surface of cornium layer from both sides of the wound area, and then the mean of two reading was measured, by using light microscope.
Visual observation of wound contraction
The wound area of rat animal was measured on days 1-10 post-burning. The size of the skin burn was captured by digital camera with the same conditions every time. The wound area measurements were taken daily from day 1-10 to calculate the percentage of wound contraction, the wound area was analysed by Image J 1.49v software (National Institutes of Health, Bethesda, MD, USA).
The wound contraction rate was measured by using the following formula:
The original wound area was the head area of metal instrument; the particular day wound area was the area of wound measured on that specific day [18].
Statistical analysis: The IBM SPSS V.21 (IBM Corp., Armonk, NY, USA) was used to calculate the research statistics. The descriptive statistics of mean and standard deviation was calculated. The group’s normality was tested using Shapiro-Wilk’s test. The analysis of variance (ANOVA) was employed to test the treatment efficacy and significancy. The post hoc tukey test was used for measurement of groups difference is significant or not. T-test after was used for measurement of groups difference between periods. The significance border was set to be 0.05 and below.
Results
Histological finding (Hematoxylin and eosin) stain
- Control group
- 5 days duration
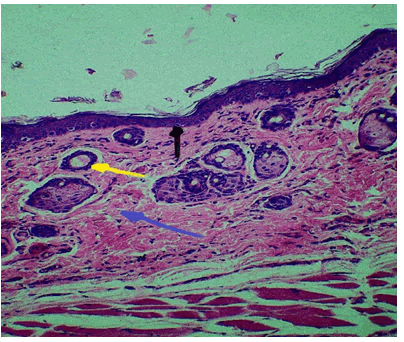
For this duration the histological finding at wound site shows newly formed epithelium with infiltration of inflammatory cells, granulation tissue formation (new collagen fibres and blood vessels) and hair follicle Figure 1.
Figure 1: Control group 5 days duration, collagen fibres, (Blue Arrow), hair follicle (Yellow Arrow), epithelium (black arrow) H and E 10 x.
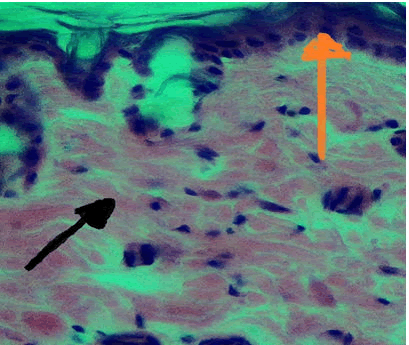
10 days duration: For this duration the histological finding at wound site shows regular arrangement of collagen fibres, hair follicle, new blood vessels and reepithelialization Figure 2.
Figure 2: Control group 10 days duration, collagen fiber (Yellow Arrow), inflammatory cells (Black Arrow) H and E stain 40x.
Skin burn treated with carrageenan
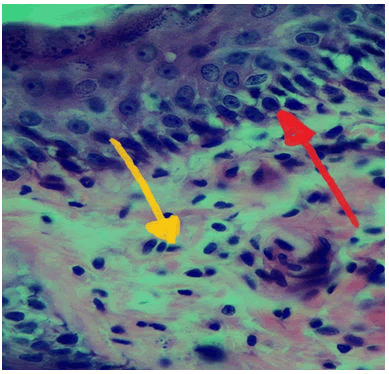
At 5 days duration: Histological view at wound site showed diffuse accumulation of inflammatory cells, granulation tissue formation and migration of epithelial cells to form new epithelium Figure 3.
Figure 3: View of experimental group (carrageenan) at 5 days duration, new epithelium (red arrow), inflammatory cells (yellow arrow) H and E Stain 40x.
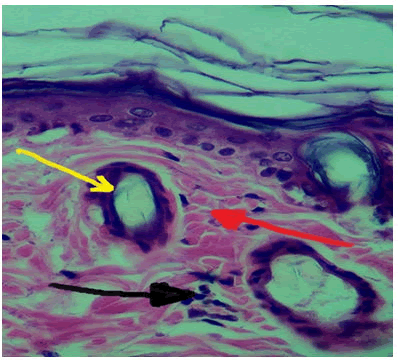
10 days duration: Histological evaluation at wound site displayed few numbers of inflammatory cells and replacement of granulation tissue by fibrous tissue, re epithelialization and keratinocytes differentiate into keratin layer Figure 4.
Figure 4: View of experimental group (Carrageenan) at 10 days duration, inflammatory cells (Black Arrow), collagen fibres (Red Arrow), hair follicle (Yellow Arrow) H and E stain 40x.
Discussion
Histological finding: Wound healing involves several steps of tissue healing including haemostasis, inflammation, proliferation and remodelling with scar tissue formation, in coagulation phase the growth factors released from platelets that activate and attract neutrophils, macrophages, fibroblasts and endothelial cells to the wound region [19].
Epithelial thickness: Epithelialization represents the proliferative phase of wound healing which involve newly formed capillaries and extracellular matrix secretion to fill the defects left after burn. Near the edge of wound, keratinocytes proliferate, around the bulbs of hair follicles stem cells and apocrine glands begin to rearranged into basal cell layer and differentiate to keratinocytes. Then begin to move over the wound edge. Once they enclose the mesenchyme of ECM, new basement membrane begins to form by fusion near the inner wound edge. Later, another row of keratinocytes migrates over the newly laid epithelial cells to fill the defect [20,21]. The epidermis that is close to the wound edge begins thickening within 24 h after injury. Keratinocytes differentiate from stem cells of the stratum basale and begin the migration toward each other over the wound to fill the defect [22,23]. In our study we found that there was gradual increase in epithelial thickness through the days of the experiment in control group that increase was significant with p value 0.000, this finding had agreement with prior study [24] on rabbits’ skin that used Asiaticoside rich hydrogel for wound healing with complete epithelization on day 12 after incision. The local application of Carrageenan persists the wound wet; this helped in re-epithelization, and agrees with [25] who suggest that if the wound kept moist, the rate of epithelialization will be faster.
In summary, this study revealed gradual increase in epithelial thickness in control and carrageenan groups but this increase was highly significantly accelerated from day 5 till the end of the experiment.
These results were in concur with [26] who found similar increase in thickness but she used Krill oil and Osteopontin for wound healing, in contrast with [27] who used interleukin found increase in epithelization only in day 5 and decrease in day 10, this may be due to difference in remodelling process of wound healing or due to the effectiveness of his treatment..
Inflammatory cell count: For Carrageenan group this study shows that there was also a slightly increase in the number of inflammatory cells that entered the wound site as compared with control group in 5-days duration then begin to decrease gradually and become less than the control group in 10-days duration, this finding agrees with study done by [21] who used Carrageenan for burn injury treatment and showed decrease in the number of inflammatory cells on day 7 post injury.
Wound contraction: Wound contraction is a healing process that functions to minimize the size of the tissue injury and to decrease the harmed tissue that needs rehabilitation. This process includes myofibroblasts, which are present with the surrounding the margins of the wound. Newly synthesized collagen fibres are pulled by these myofibroblasts toward the centre of the defect in the damaged tissues to reduce the size of the wound defect. Actin and myosin which are the two main proteins that do this process by interacting with newly formed collagen fibres in the extracellular matrix forming a network base for wound contraction [26]. The advantage of wound healing is reducing the wound margins, which end with wound closure, excessive contraction lead to formation of undesirable contracture and scar formation, leading to functional and cosmetic problems, fibroblasts have mesenchymal origin and can manifest either non-contractile or highly contractile phenotype [24], fibroblasts function to preserve tissue homeostasis by arranging the turnover of extracellular matrix, when tissues are injured, fibroblasts at the periphery of injured region transform into myofibroblasts, that produce abundant ECM proteins [25]. Both fibroblasts and myofibroblasts play a major part in the process of wound healing. The coordinated contraction of myofibroblasts and traction forces of fibroblasts are responsible for wound closure, excessive efficiency of myofibroblast may causes scar formation, leading to loss of function and local immobilization [28]. In our study we found that there was gradual acceleration in wound closure in Achillea group in 5 and 10 days as compared with control group. Our finding agrees with a previous study [29] who found that the krill oil and Osteopontin as an antioxidant and anti-inflammatory substance when used in wound healing. Regarding our study groups we found a significant increase in Achillea group which was studied in this experiment in 5 days duration with p value less than 0.031 while in 10 days duration we found highly significant increase with p value 0.000. Comparing Achillea and control groups using ANOVA test revealed significant difference in day 5 and high significant difference in day 10, thus post hoc test was done to verify the particular difference among the two groups using Tukey test, in 5 days duration there were non-significant difference between the two groups while in 10 days duration there was significant difference between them, because the wound contraction starts nearly one week after primary injury and attains its highest stage after the second week [30].
In our study we found that the Carrageenan group do better result than control in the progression of wound contraction.
Conclusion
The effect of topical application of carrageenan on burned skin in our study is acceleration and improvement of burn wound healing.
References
- Jean K. Anatomy, histology and immunohistochemistry of normal human skin. Eur J Dermatol 2002; 12:390-401.
[Indexed]
- Goodarzi P. Tissue Engineered Skin Substitutes. Adv Exp Med Biol 2018; 1107:143-188.
- Velnar T, T Bailey, V Smrkolj. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res 2009; 37:1528-1542.
- Robson MC, DL Steed, MG Franz. Wound healing: biologic features and approaches to maximize healing trajectories. Curr Probl Surg 2001; 38:72-140.
- Larraneta E, Rebecca EM, Lutton, et al. Micro needle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mat Sci Eng R: Rep 2016; 104:1-32.
- Lazarus GS, Cooper DM, David R et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol 1994; 130:489-493.
- Bischoff M, L Kinzl, A Schmelz. The complicated wound. Unfallchirurg 1999; 102:797-804.
- Prajapati VD, Maheriya PM, Jani GK, et al. Carrageenan: a natural seaweed polysaccharide and its applications. Carbohydr Polym 2014; 105:97-112.
- Lawrence WT. Physiology of the acute wound. Clin Plast Surg 1998; 25:321-340.
- Hart J. 1: Its role in the healing of acute wounds. J Wound Care 2002; 11:205-209.
- Samuels P, AK Tan. Fetal scarless wound healing. J Otolaryngol 1999; 28:296-302.
- Richardson M. Acute wounds: an overview of the physiological healing process. Nurs Times 2004; 100:50-53.
- Tissue Engineered Skin Substitutes
- Kara YA. Burn Etiology and Pathogenesis, in Hot Topics in Burn Injuries. 2018.
- Khassaki A. JN Ahmed. Clinical Assessment of Anti-inflammatory Activity of 940 Nanometer Low Level laser Therapy on Carrageenan Induced Arthritis in Temporomandibular Joint in Wistar Albino Rats. J Pure Applied Microbiol 2019; 13:619-628.
- Barretto SR, de Melo GC, dos Santos JC, et al. Evaluation of anti-nociceptive and anti-inflammatory activity of low-level laser therapy on temporomandibular joint inflammation in rodents. J Photochem Photobiol B 2013; 129:135-142.
- Mohamad N, Mohd Amin MCI, Pandey M, et al. Bacterial cellulose/acrylic acid hydrogel synthesized via electron beam irradiation: Accelerated burn wound healing in an animal model. Carbohydr Polym 2014; 114:312-320.
- Guo HF, Razana M Ali, Roslida A Hamid, et al. A new model for studying deep partial-thickness burns in rats. Int J Burns Trauma 2017; 7:107-114.
- Hunt TK, H Hopf, Z Hussain. Physiology of wound healing. Adv Skin Wound Care 2000; 13:6-11.
- Gantwerker EA, DB Hom. Skin: histology and physiology of wound healing. Facial Plast Surg Clin North Am 2011; 19:441-453.
- Mogensen M, Morsy HA, Thrane L, et al. Morphology and epidermal thickness of normal skin imaged by optical coherence tomography. Dermatology 2008; 217:14-20.
- Al-Zamely ZRA. Histological Evaluation of the effect of local exogenous application of Krill oil/Osteopontin on cutanous facial wound healing in Rats, in Department of oral diagnosis. 2017, University of Baghdad: Iraq–Baghdad 2017; 86.
- Nissen NN, Gamelli RL, Polverini PJ, et al. Differential angiogenic and proliferative activity of surgical and burn wound fluids. J Trauma 2003; 54:1205-1210.
- Li B, Wang Fibroblasts and myofibroblasts in wound healing: force generation and measurement. J Tissue Viability 2011; 20:108-120.
- Tomasek JJ, Gabbiani G, Hinz B, et al. Myofibroblasts and mechano-regulation of connective tissue re-modelling. Nat Rev Mol Cell Biol 2002; 3:349-363.
- Lesperance MM, Francis TL, Norton B. Chapter 1-Postsurgical Soft Tissue Healing. Postsurgical Orthopedic Sports Rehabilitation, R.C. Manske, Editor. 2006, Mosby: Saint Louis. 2006; 3-18.
[Crossref]
- Enoch S, Miller DR, Price PE, et al. Early diagnosis is vital in the management of squamous cell carcinomas associated with chronic non healing ulcers: a case series and review of the literature. Int Wound J 2004; 1:165-175.
- Kozma EM, Miller DR, Price PE, et al. Pathogenesis of Dupuytren's contracture-a review. Chir Narzadow Ruchu Ortop Pol 2002; 67:73-79.
- Bruemmer D, RE Law. Thiazolidinedione regulation of smooth muscle cell proliferation. Am J Med 2003; 115:87-92.
- Ward RS. Chapter 31-Burns. Physical Rehabilitation, M.H. Cameron and L.G. Monroe, Editors. 2007, W.B. Saunders: Saint Louis. 2007, 828-843.
[Crossref]
Author Info
Ammar Abood Kanber1* and Enas Fadhil Kadhim2
1Department of Dental Surgery Iraqi Parliament Council, Baghdad, Iraq2Department of Histology and Embryology, College of Dentistry, University of Baghdad, Iraq
Received: 04-Jul-2022, Manuscript No. JRMDS-22-61109; , Pre QC No. JRMDS-22-61109; Editor assigned: 07-Jul-2022, Pre QC No. JRMDS-22-61109; Reviewed: 21-Jul-2022, QC No. JRMDS-22-61109; Revised: 05-Sep-2022, Manuscript No. JRMDS-22-61109; Published: 12-Sep-2022