Research - (2021) Volume 9, Issue 7
Efficacy of Diode Laser (980 Nm) and Non-Surgical Therapy on Management of PeriodontitisâA Randomized Clinical Trial
Amel I Faragalla1*, Alhadi M Awooda2, Ahmed K Bolad3 and Ibrahim A Ghandour4
*Correspondence: Amel I Faragalla, Department of Periodontic, College of Dentistry, Al-Neelain University, Saudi Arabia, Email:
Abstract
Introduction: Laser technology, in conjugation to non-surgical therapy, could improve the clinical periodontal parameters and reduce the total bacterial count. This study aimed to evaluate the clinical and microbiological effects of Diode laser (980 nm) when applied to scaling and root planing in the management of periodontitis. Subjects and Methods: Randomized controlled prospective clinical trial, designed with split-mouth technique and conducted to 28 men’s and women’s patients, diagnosed with Generalized Periodontitis Stage II/III Grade B. Ethical approval received from the Federal Health Ministry, Khartoum. All participants received oral hygiene instructions, bacterial sampling, and measurement of clinical periodontal parameters. Full-mouth scaling, polishing, and root planing were applied. One sextant received in addition, single dose of Diode laser (980 nm). Probing Pocket Depth, Gingival Index, Plaque Index, and Sulcus Bleeding Index were measured three times during the nine months of the study. Comparison between different parametric data produced by independent t-test with the level of statistical significance set at P <0.05. Results: The analysis of the clinical parameters in the laser group showed a significant reduction in pocket depth from 4.73 ± 0.96 to 3.69 ± 1.10 (P=0.02), plaque index from 1.3427 ± 0.25 to 1.06 ± 0.30 (P=0.021), and sulcus bleeding index from 2.20 ± 0.56 to 1.30 ± 0.06 (P=0.002). General improvement of all clinical parameters- (GI, PLI, PPD, and SBI) reported for both groups (p<0.001). Conclusion: Diode laser (980 Nm), when applied in conjugation to SRP, effectively reduces gingival inflammation and periodontal pockets (4-6 mm) depth than SRP alone in managing periodontitis.
Keywords
Diode laser, Non-surgical periodontal therapy, Periodontal scaling, Periodontal pockets, Root planing.
Introduction
Periodontitis is a bacterial plaque-related inflammatory disease that contributes to the loss of tooth supportingtissue that raises tooth morbidity. In 2018, the European Federation of Periodontology (EFP) published a new global classification system for periodontal health, diseases, and conditions [1]. Treatment of periodontitis is based on eliminating bacterial deposits that adhere to the tooth surface through scaling, root planing (SRP), and plaque control [2]. In certain situations, antimicrobial therapy is needed in conjugation with conventional treatment to reduce the bacterial load, where SRP is challenging to perform. Diode laser (DL) is a semiconductor laser mixed with other elements such as gallium, arsenide, aluminium, and indium to transform electrical energy into light energy. Diode laser is soluble in hemoglobin and other pigments and considered excellent in soft tissue surgical management [3]. The diode laser can be safely used for surgical intervention. The diode laser is superior in soft-tissue control to other forms of lasers. These include cutting, coagulating, and soft tissue curettage; however, it does not affect the hard dental tissues [4,5]. Due to its practicality, low cost [6], biocompatibility [7], and ability to minimize bacterial count, the diode laser has been widely used in dentistry [8]. In certain circumstances, the traditional periodontal therapy fails, these include difficulties in achieving meticulous scaling procedures, microorganisms’ pathogenicity resistance [9], or the presence of a systemic condition that compromises the host response to treatment [10]. For effective periodontal therapy, the addition of antimicrobial agents is therefore necessary. The laser diode has been suggested as an alternative or adjunctive phototherapy to traditional SRP [5].
Lasers are generally effective in reducing anaerobic microorganisms that inhabit periodontal pockets and participate in the destruction of periodontal tissues. No previous research was performed in Sudan using the laser in conjugation to non-surgical therapy to manage periodontitis. Therefore, the objectives of the current study directed towards evaluation of the efficacy of conjugation of diode laser (980 nm) to SRP in the management of periodontitis in terms of clinical parameters such as probing pocket depth (PPD), Plaque Index (PLI), Gingival Index (GI), and Sulcus Bleeding Index (SBI) and assessment of anaerobic pathogen following laser application through quantitative analysis.
Methods
A randomized controlled prospective clinical trial was conducted in 13 male and 15 female patients with an age range of 35-55 years. Patients diagnosed with Generalized Periodontitis Stage III Grade B [1], and who had at least two affected sextants with a minimum pocket depth of (4-6 mm) were included. Patients recruited from the University of Al-Neelain, College of Dentistry, and Khartoum Dental Hospital. Patients were treated and followed-up for nine months (August 2018-May 2019). Ethical approval received from the Federal Health Ministry, Khartoum, Sudan No. (4-7-19). The selected patients were tested for debilitating diseases such as diabetes mellitus, hypertension, and cancer. Candidates neither receive periodontal therapy during the last six months nor take antibiotics during the previous three months. The exclusion criteria included smokers, alcoholics, orthodontic appliance wearers, patients with rampant dental caries, pregnant ladies or women under oral contraceptives, and patients with a cardiac pacemaker.
Study protocol
To analyze the clinical efficiency of diode laser (980 nm) application along with SRP, 56 sites (28 patients) were selected for the study as our sampling unit were sites. Split mouth study design was applied. Therefore, one sextant of the mouth was taken as a control group (received SRP alone) and the other contralateral sextant as a test group (received SRP + diode laser (980 nm). Following the research protocol explanation, all candidates signed a written consent form and provided verbal agreement for participation. Oral hygiene instructions were given. A double-blind, randomized controlled trial was conducted to test the efficacy of the addition of diode laser (980 nm) to SRP in the management of periodontitis patients. Double blinding was ensured by blinding the periodontal examiner and statistician about the treatment assigned to both groups. Participants who had at least two affected sextants with a minimum pocket depth of 4-6 mm were included. Each site was randomly allocated to either SRP or SRP + Diode laser (980 nm) groups by block randomization (block size: 4). The sequence of allocation was generated using a computer. The assigned sites were enclosed in sealed, opaque envelopes that were numbered sequentially. A split-mouth design introduced in two randomly allocated contralateral sextants. Plaque samples obtained before treatment using endodontic paper points (size-40) which inserted up to the base of the selected pockets (test and control) for 15 seconds. Plaque biofilm samples from each pocket were collected and transported in a sealed tube containing cooked meat agar as a suitable transport medium for anaerobic bacteria for the quantitative analysis of colony-forming units (CFU). The primary measured outcome was recorded by Williams graduated periodontal probe, as improvements to the Plaque Index (PLI), Gingival Index (GI), Probing Pockets Depth (PPD), and Sulcus Bleeding Index (SBI). Full mouth scaling, polishing, and root planing were performed using manual scalers, Gracey curettes, and ultrasonic scalers (Woodpeckers).)
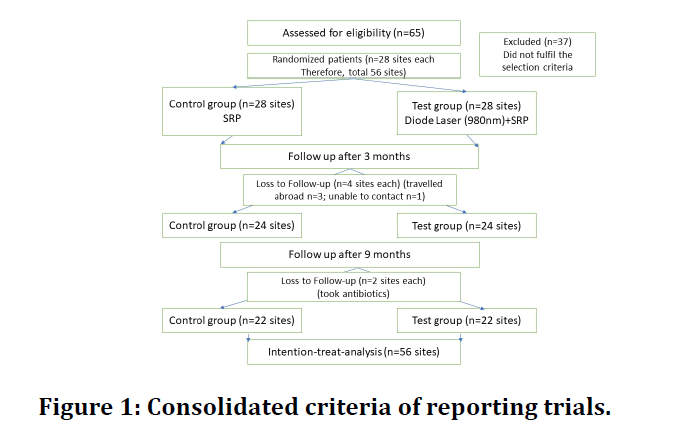
One patient needed local anaesthesia. The deepest pockets in group I, test group (n=28 sites), received SRP plus Diode laser (980 nm). The contralateral sextant, group II control group (n=28 sites), received SRP alone. Patients were informed about brushing twice daily to ensure adequate plaque control. The clinical parameters (PLI, GI, PPF, and BOP) were recorded during the follow up (three and nine months). The Diode laser was not applied in the second and third visits. An experienced dentist who was blinded to the study procedure collected the clinical data at the baseline and during the follow-up visits. At the same time, the author carried out SRP and radiation of the diode laser. The author assessed the results according to the flowchart CONSORT (Consolidated Criteria of Reporting Trials) Figure 1.
Figure 1: Consolidated criteria of reporting trials.
Microbiological assays
Fifty-six plaque samples were collected from 56 sites incubated in the anaerobic agar media for 48 hours at 37 degrees Celsius. Quantitative analysis of anaerobic bacteria performed by the MacConkey agar test that used to isolate gram-negative enteric bacteria and to differentiate lactose fermenting from lactose nonfermenting gram-negative bacteria. The standard microbiological procedure relied on counting the quantity and detection of gram +ve and gram-ve bacteria before and after the periodontal treatment. The microbiological testing was repeated after three months.
Application of diode-laser
Following periodontal probing and non - surgical treatment (SRP), soft tissue diode laser (Quick lase), (The United Kingdom–QW laser 6D-12D), (class IV laser system) was employed in the deepest pockets in the test group. The laser fibre calibration against the periodontal probe was marked (one mm) shorter than the actual pocket depth. Clinicians, patients, and assistants used special eyeglasses for protection during the lasing procedure. A single laser session was operated in a continuous mode of up and down movement inside the periodontal pockets for 15 seconds. The laser beam guided through a flexible fibre-optic cable (400 μm diameter) terminated with the stainless-steel handpiece. The diode laser was irradiated with wavelength (980 Nm), a frequency of 50 Hz, and one watt’s power. The tip of the glass fibre was revaluated after each application to check the need for re-cleaning. Finally, the pocket was sprayed with distilled water and compressed with sterile gauze. The clinical outcomes were measured three and nine months following the treatment.
Clinical parameters
The measured periodontal parameters included plaque Index [11], gingival index [12] probing pocket depth (PPD) in mm, and sulcus bleeding index (SBI) [13]. The reproducibility of the intra-examiner calibration was assessed by evaluating five non-study patients. Each patient had at least two affected contralateral sites and probing depths of (4-6 mm) on at least one part of each tooth. The investigator examined the patients on two different occasions, 48 h apart, and the calibration was approved because the measurement outcomes were similar in more than 90 percent of the cases at baseline and 48 h.
The efficacy of the treatment recorded at a baseline level, after three and nine months in terms of changes in clinical parameters and microbial changes. The effects of the two interventions were analyzed within and between the groups with different intervals and over time. The level of significance was recorded.
Calculation of Sample Size:
The sample size was based on the formula: [14-16].
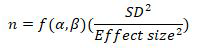
Where are the function of power and significance level.
Based on the results of our pilot study, we calculated 0.5 mm as effect size and a SD of 0.7 mm. If such a difference exists, at the 5% significant level with 90% power, the required sample size for this paired design would be:
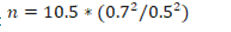
Therefore, 21 patients were required for a total of 42 sites. In order to compensate for loss during follow-up, 28 patients were enrolled in the current study and 56 sites were evaluated.
Data analysis
Data were entered into a spread sheet and proofed for data-entry errors before the statistical analyses. Analyses performed by a statistician who was masked, without the knowledge of the assigned treatment groups. A subjectlevel analysis was performed statistically for each of the parameters using SPSS software for Windows, Version 16.0. (SPSS Inc., Chicago, IL, USA). Median for the clinical variables was calculated for each treatment. Test of normality conducted to the clinical parameters at baseline. As the data distribution of three clinical parameters in the present study did not obey the Gaussian law by Kolmogorov-Smirnov test/Shapiro Wilk test (p<0.05), nonparametric methods were used for analysis. The significant difference between the test and the control groups for categorical data was assessed using the Chi-square test; in contrast the Mann-Whitney U-test was employed to the continuous variable. Likewise, the Wilcoxon’s Signed Rank Test was used for finding significant changes from baseline to various intervals within the test and control groups. Comparison of clinical parameters between groups was analyzed using the Wilcoxon test.
Results
A total of 28 patients included in the current study. Three patients were lost to follow during the three-month follow-up as they travelled abroad, and one did not respond to our telephonic appointment scheduling calls. Similarly, during the nine months follow up two extra patients reported having taken antibiotics. Hence, they were discontinued from the study.
Intention to treat analysis was done for the 28 patients, and missing data were handled by the last observation carried forward (LOCF) method.16 Healing of periodontal disease occurred in all candidates without any adverse reaction or complications such as pain, bleeding, burning sensation, or discomfort. The frequency distribution of biographic, demographic data and sample size were exemplified.
The information related to the clinical parameters (PLI, GI, PPD, and BOP) at the baseline showed no statistical difference between these parameters (Table 1). Exemplified the median values for plaque index at baseline (0.30) for controls and (0.743) for the test group. For the control group, the values of plaque index after three months were (1.12), P.value 0.038, and nine months showed (1.05) P.value 0.011, demonstrating a statistically significant difference. Simultaneously, the Laser group showed a highly statistically significant difference in plaque index values at nine months following the treatment (P.value 0.003) (Table 2). The gingival index median values at baseline were (1.30) for controls and (1.35) for test groups. The GI values following three months of the non-surgical therapy were (1.25), (P.value 0.042), and nine months showed (1.20) (P.value 0.044), demonstrating a statistically significant reduction. No significant reduction in (GI) values of three months compared to the nine months' findings. In comparison, the Laser group showed a statistically significant decrease in gingival index values at three and nine months following the treatment (P-value 0.049) and (P.value 0.009), respectively.
Table 1: Differences in plaque index at baseline, three months, and nine months of treatment.
| Parameter | Baseline | After 3 months | After 9 months | p-values | ||
|---|---|---|---|---|---|---|
| Median (Min-Max; IQR) | Median (Min-Max; IQR) | Median (Min-Max; IQR) | 0-3 months | 0-9 months | 3-9 months | |
| Plaque Index | ||||||
| Control | 0.30 (0.50-1.95; 0.35) | 1.12 (0.79-1.95; 0.2) | 1.05 (0.65-1.92; 0.23) | 0.038* | 0.011* | 0.361 ns |
| Test | 1.30 (0.50-1.95; 0.32) | 1.1(0.79-1.95; 0.25) | 1.05 (0.65-1.92; 0.23) | 0.008* | 0.003** | 0.564 ns |
| p-values | 0.743ns | 0.767 ns | 1.000 ns | |||
PPD-probing pocket depth; SBI-sulcular bleeding index; IQR-inter quartile range; Min-Max-Minimum-Maximum; ns-not significant; *p<0.05 **p<0.005
Table 2: Differences in gingival index at baseline, 3 months, and 9 months of treatment.
| Parameter | Baseline | After 3 months | After 9 months | p-values | ||
|---|---|---|---|---|---|---|
| Median (Min-Max; IQR) | Median (Min-Max; IQR) | Median (Min-Max; IQR) | 0-3 months | 0-9 months | 3-9 months | |
| G. Index | ||||||
| Control | 1.30 (1.04-1.75; 0.23) | 1.25(1.0-1.58; 0.16) | 1.2(1.0-1.62; 0.25) | 0.042* | 0.044* | 0.299 ns |
| Test | 1.35 (1.04-1.75; 0.30) | 1.29(1.0-1.58; 0.14) | 1.2 (1-1.62;0.25) | 0.049* | 0.009* | 0.109 ns |
| p-values | 0.697 ns | 0.471 ns | 1.000 ns | |||
PPD-probing pocket depth; SBI-sulcular bleeding index; IQR-inter quartile range; Min-Max-Minimum-Maximum; ns-not significant; *p<0.05 **p<0.005
Table 3 showed the median values for probing pocket depth at baseline, three-, and nine months following management of periodontitis. Regarding the control group, the medium values for both three months (4.0) and nine months (4.0) follow-up showed a statistically significant reduction in the probing pocket depth (pvalue 0.005 and 0.004), respectively. However, a comparison of the results between the three- and nine months values was not statistically significant. On the other hand, the test group revealed a highly statistically significant reduction in pocket depth values at three months (4.0) and nine months (3.0). The comparison between the two follow-up durations' pocket depth values was statistically significant (P.value 0.045).
Table 3: Differences in probing pocket depth at baseline, 3 months, and 9 months of treatment.
| Parameter | Baseline | After 3 months | After 9 months | p-values | ||
|---|---|---|---|---|---|---|
| Median (Min-Max; IQR) | Median (Min-Max; IQR) | Median (Min-Max; IQR) | 0-3 months | 0-9 months | 3-9 months | |
| PPD | ||||||
| Control | 4.00 (4.00-7.00; 1.00) | 4.0 (3.0-8.0; 0.5) | 4.0 (3.0-7.0; 1.0) | 0.005** | 0.004** | 0.411 ns |
| Test | 5.00 (4.00-7.00; 1.00) | 4.0 (3.0-7.0; 1.5) | 3.0 (3.0-7.0; 1) | 0.000** | 0.000** | 0.045* |
| p-values | 0.245ns | 0.826ns | 0.153ns | |||
|
PPD-probing pocket depth; SBI-sulcular bleeding index; IQR-inter quartile range; Min-Max-Minimum-Maximum; ns-not significant; *P<0.05 **p<0.005 |
|
|
|
|
|
|
Table 4 Explained the median values of the Sulcus Bleeding Index (SBI), at baseline, three, and nine-month. The difference in the measurements carried between three and nine months was not significant regarding the control group. However, the sulcus bleeding index calculated from the test group depicted highly significant results from 0-9 months (P.value 0.005) and significant values from 3- 9 months (P.value0.011).
Table 4: Differences in sulcus bleeding index at baseline, 3 months, and 9 months of treatment.
| Parameter | Baseline | After 3 months | After 9 months | p-values | ||
|---|---|---|---|---|---|---|
| Median (Min-Max; IQR) | Median (Min-Max; IQR) | Median (Min-Max; IQR) | 0-3 months | 0-9 months | 3-9 months | |
| SBI | ||||||
| Control | 2.00 (1.00-4.00; 0.00) | 2.0 (1.0-3.0;0.5) | 2.0 (1.0-3.0; 1.0) | 0.003** | 0.002** | 0.105 ns |
| Test | 2.00 (1.00-4.00; 0.00) | 2.0 (1.0-3.0; 0.0) | 1.0 (0.0-2.0; 1.0) | 0.035* | 0.005** | 0.011* |
| p-values | 1.000ns | 0.618 ns | 0.453 ns | |||
PPD-probing pocket depth; SBI-sulcular bleeding index; IQR-inter quartile range; Min-Max-Minimum-Maximum; ns-not significant; *P<0.05 **p<0.005
Table 5 shows the comparison of the clinical parameters (PLI, GI, PPD, and BOP) between groups were analyzed using the Wilcoxon test. The four clinical parameters showed a statistically significant difference (p<0.01) when the values between baseline to three months and three months to nine months were compared. However, evaluation of the results between three and nine months did not show a significant difference.
Table 5: Comparison of clinical parameters between control and test group.
| Clinical Parameters | p-values | |||||
|---|---|---|---|---|---|---|
| baseline | 3 months | 9 months | 0-3 months | 0-9 months | 3-9 months | |
| Plaque Index | 0.157ns | 0.157 ns | 1.000 ns | 0.001** | 0.000** | 0.295 ns |
| Gingival Index | 0.157 ns | 0.157 ns | 1.000 ns | 0.005** | 0.001** | 0.062 ns |
| PPD | 0.130 ns | 0.637 ns | 0.166 ns | 0.000** | 0.000** | 0.068 ns |
| SBI | 1.000 ns | 0.317 ns | 0.285 ns | 0.000** | 0.000** | 0.003** |
PPD-probing pocket depth; SBI-sulcular bleeding index; Minimum-Maximum; ns-not significant;**p<0.005
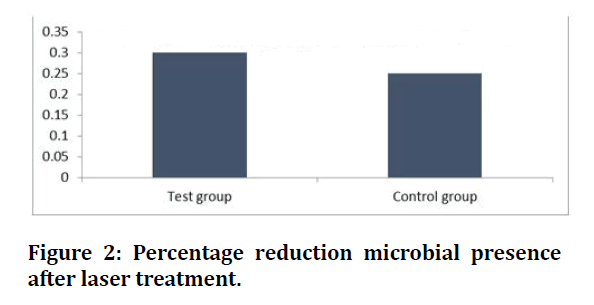
In terms of microbial reduction, a decrease in the microbial presence was observed after the treatment. The microbial reduction of 30% was kept for the test group (laser plus SRP), while only a 25% reduction was observed for the control group (SRP), as shown in Figure 2.
Figure 2: Percentage reduction microbial presence after laser treatment.
Discussion
Periodontitis is one of the major causes of tooth loss and principally caused by bacterial infection [17,18]. Conventional non-surgical therapy is the most effective path to eliminate the cause of the disease, and to decrease the periodontitis parameters [19,20]. The best protocol for eradicating the disease depends on eliminating or removing the periodontal pocket epithelium that allows better connective tissue formation. The current study reported the efficacy and the clinical benefit of using a diode laser in conjugation to conventional non-surgical therapy to treat periodontitis among Sudanese patients. The results indicate that non - surgical periodontal treatment alone or in conjugation with a diode laser (980 nm) provides a significant improvement in clinical periodontal parameters (GI, PLI, PPD, and BOP) of moderate and deep pockets for nine months follow-up next to treatment. Previous studies [21,22] documented the advantage of using conventional SRP combined with oral hygiene instructions to treat and control of periodontitis.
Our study is in agreement with researches carried out by Assaf et al. [23] and Dukic et al. [24] applied diode laser (980nm) in conjugation to conventional non-surgical therapy to reduce periodontal pockets. Both the test and control groups showed positive results following treatment. However, the test group (laser+SRP) showed better improvement in the mean difference of PPD reduction in moderate pockets during the baseline and three months and 3 to 9 months. Mortiz et al. [25] used different controls for comparison and reported that the clinical paraments were significantly affected by the addition of the diode laser in managing periodontitis.
In agreement with our results [5,26] conveyed that SRP plus diode laser therapy presented more effects in managing periodontitis than when SRP was used alone. Talat Gadri et al. [27], in a systematic review, showed that diode laser, when applied with wavelength range (808-980 Nm) and power of (9.8–2.5W), can be used safely as adjunctive therapy to SRP with excellent results. Some studies [24,26,28] reflected that if laser parameters are suitable, a good effect in up to 5mm pocket depth can be achieved. However, this finding contradicts the results obtained by Slots et al. [29], who reported no additional beneficial effects obtained by the addition of laser. On the other hand, some studies have shown positive results in clinical and microbiological findings using the same type of laser [30,31]. The divergence of the results may be due to the investigators different methods and laser parameters.
In contrast to the findings of the current study, Lai et al. [32] did not observe differences in the measured clinical parameters or radiographic findings between the test (He-Ne laser) and control sites 3,6,9,12 month. However, Lai et al. used laser therapy eight times within the first three months. Other studies [26,27] that contradict the present research findings showed no significant improvement in periodontal clinical parameters following treatment with SRP plus diode laser. However, Caruso et al. [33] reported a slight improvement, and results by Zingale et al. [34] described a moderate reduction in pocket depth. In a couple of studies, De Micheli et al. [31] and Caruso et al. [33] did not find any additional benefits using the diode laser during nonsurgical periodontal treatment.
The use of a Diode laser in the killing of a periodontal pathogen was not widely investigated. Several authors reported the use of Nd: YAG lasers, which destroyed periodontal pathogen through thermal effects. Alves et al. [30] reported a reduction in the total CFU six weeks following laser irradiation without significant difference between groups. However, after six months, the CFU returned to values similar to the baseline. The diode laser has a spectrum ranging from 655 to 980 nm and could represent a safer alternative in bacterial reduction [33]; however it could not remove or ablate calculus from the root surface [23,34]. Following bacterial count among this study's results, anaerobic periodontal pathogen showed a significant reduction in the laser group than the SRP group. Similarly, Kama et al. [5] demonstrated that the effect of 980 nm diode laser-assisted SRP was superior to SRP or laser therapy alone for clinical and microbial parameters.
Conclusion
When applied in conjugation to SRP, diode laser (980 nm) effectively reduces inflammation, periodontal pockets depth, and microbial presence. Thus, when combined with non-surgical periodontal therapy, the diode laser is helpful in the management of periodontitis than SRP alone.
Acknowledegements
Extended grateful acknowledgment to Dr. Salma Nageeb, Dr. Ahmed Bakri, Dr. Mohamed Alhadi, Osama Alameed, and Dr. Ahmed Yahia for preparing the patients and the clinic. Thanks to Dr. Mudathir (Almudathir Dental Clinic, Khartoum), who provided the 980 Nm Diode laser device free of charge. Thanks to all participating patients for their patience and cooperation.
Conflict of Interest
The author declared that no conflict of interest in this study.
References
- Caton JG, Armitage G, Berglundh T, et al. A new classification scheme for periodontal and peri-implant diseases and conditions–Introduction and key changes from the 1999 classification. J Periodontol 2018; 89:S1-S8.
- Cugini MA, Haffajee AD, Smith C, et al. The effect of scaling and root planing on the clinical and microbiological parameters of periodontal diseases: 12-Âmonth results. J Clin Periodontol 2000; 27:30-6.
- Roncati M, Gariffo A. Systematic review of the adjunctive use of diode and Nd: YAG lasers for nonsurgical periodontal instrumentation. Photomed Laser Surg 2014; 32:186-97.
- Adriaens PA, Adriaens LM. Effects of nonsurgical periodontal therapy on hard and soft tissues. Periodontology 2004; 36:121-45.
- Kamma JJ, Vasdekis VG, Romanos GE. The effect of diode laser (980 nm) treatment on aggressive periodontitis: evaluation of microbial and clinical parameters. Photomed Laser Surg 2009; 27:11-9.
- Schwarz F, Pütz N, Georg T, et al. Effect of an Er: YAG laser on periodontally involved root surfaces: an in vivo and in vitro SEM comparison. Lasers Surg Med 2001; 29:328-35.
- Theodoro LH, Haypek P, Bachmann L, et al. Effect of Er: YAG and diode laser irradiation on the root surface: Morphological and thermal analysis. J Periodontol 2003; 74:838-43.
- Borrajo JL, Varela LG, Castro GL, et al. Diode laser (980 nm) as adjunct to scaling and root planing. Photomed Laser Therapy 2004; 22:509-12.
- Mombelli A, Schmid B, Rutar A, et al. Persistence patterns of porphyromonas gingivalis, prevotella intermedia/nigrescens, and actinobacillus actinomycetemcomitans after mechanical therapy of periodontal disease. J Periodontol 2000; 71:14-21.
- Trombelli L, Rizzi A, Simonelli A, et al. Age-related treatment response following non-surgical periodontal therapy. J Clin Periodontol 2010; 37:346-52.
- Silness J, Löe H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scandinavica 1964; 22:121-35.
- Löe, H., The gingival index, the plaque index and the retention index systems. J Periodontol 1967; 38:610-616.
- Muhlemann HR. Gingival sulcus bleeding-a leading symptom in initial gingivitis. Helv Odontol Acta 1971; 15:107-13.
- Pandis N. Sample calculation for split-mouth designs. Am J Orthodont Dentofac Orthop 2012; 141:818-9.
- Kolbe MF, Ribeiro FV, Luchesi VH, Casarin RC, Sallum EA, Nociti Jr FH, Ambrosano GM, Cirano FR, Pimentel SP, Casati MZ. Photodynamic therapy during supportive periodontal care: clinical, microbiologic, immunoinflammatory, and patient-centered performance in a split-Âmouth randomized clinical trial. Journal of periodontology. 2014 Aug;85(8):e277-86.
- Pozos-Guillén A, Chavarría-Bolaños D, Garrocho-Rangel A. Split-mouth design in paediatric dentistry clinical trials. Eur J Paediatr Dent 2017; 18:61-5.
- Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J Clin Periodontol 1998; 25:134-44.
- Chitsazi MT, Pourabbas R, Shirmohammadi A, et al. Association of periodontal diseases with elevation of serum C-reactive protein and body mass index. J Dent Res 2008; 2:9.
- Slots J, Rams TE. New views on periodontal microbiota in special patient categories. J Clin Periodontol 1991; 18:411-20.
- Papaioannou W, van Steenberghe D, Cassiman JJ, et al. Adhesion of porphyromonas gingivalis to cultured pocket epithelium: Mono-and multi-layered. Clin Oral Investigations 2003; 7:162-6.
- Badersten A, Niveus R, Egelberg J. 4-Âyear observations of basic periodontal therapy. J Clin Periodontol 1987; 14:438-44.
- Badersten A, Nilveus R, Egelberg J. Effect of nonsurgical periodontal therapy: II. Severely advanced periodontitis. J Clin Periodontol 1984; 11:63-76.
- Assaf M, Yilmaz S, Kuru B, et al. Effect of the diode laser on bacteremia associated with dental ultrasonic scaling: A clinical and microbiological study. Photomed Laser Surge 2007; 25:250-6.
- Dukic W, Bago I, Aurer A, et al. Clinical effectiveness of diode laser therapy as an adjunct to nonâ?ÂÂsurgical periodontal treatment: A randomized clinical study. J Periodontol 2013; 84:1111-7.
- Moritz A, Schoop U, Goharkhay K, et al. Treatment of periodontal pockets with a diode laser. Lasers Surg Med 1998; 22:302-11.
- Saglam M, Kantarci A, Dundar N, et al. Clinical and biochemical effects of diode laser as an adjunct to nonsurgical treatment of chronic periodontitis: A randomized, controlled clinical trial. Lasers Med Sci 2014; 29:37-46.
- Qadri T, Javed F, Johannsen G, et al. Role of diode lasers (800–980 nm) as adjuncts to scaling and root planing in the treatment of chronic periodontitis: a systematic review. Photomed Laser Surg 2015; 33:568-75.
- Kreisler M, Al Haj H, d'Hoedt B. Clinical efficacy of semiconductor laser application as an adjunct to conventional scaling and root planing. Lasers Surg Med 2005; 37:350-5.
- Slot DE, Kranendonk AA, Paraskevas S, et al. The effect of a pulsed Nd: YAG laser in non-Âsurgical periodontal therapy. J Periodontol 2009; 80:1041-56.
- Alves VT, de Andrade AK, Toaliar JM, et al. Clinical and microbiological evaluation of high intensity diode laser adjutant to non-surgical periodontal treatment: A 6-month clinical trial. Clin Oral Investigations 2013; 17:87-95.
- De Micheli G, de Andrade AK, Alves VT, et al. Efficacy of high intensity diode laser as an adjunct to non-surgical periodontal treatment: A randomized controlled trial. Lasers Med Sci 2011; 26:43-8.
- Lai SM, Zee KY, Lai MK, et al. Clinical and radiographic investigation of the adjunctive effects of a low-power He-Ne laser in the treatment of moderate to advanced periodontal disease: A pilot study. Photomed Laser Surg 2009; 27:287-93.
- Caruso U, Nastri L, Piccolomini R, et al. Use of diode laser 980 nm as adjunctive therapy in the treatment of chronic periodontitis. A randomized controlled clinical trial. New Microbiol 2008; 31:513-8.
- Zingale J, Harpenau L, Chambers D, et al. Effectiveness of root planing with diode laser curettage for the treatment of periodontitis. J California Dent Assoc 2012; 40:786-93.
Author Info
Amel I Faragalla1*, Alhadi M Awooda2, Ahmed K Bolad3 and Ibrahim A Ghandour4
1Department of Periodontic, College of Dentistry, Al-Neelain University, Khartoum Sudan / King Khalid University College of Dentistry Saudi Arabia, Saudi Arabia2Guarantee Centre for Educational and Research, Khartoum, Sudan
3Department of Immunology, College of Medicine, Alneelain University, Khartoum, Sudan
4Medicine Khartoum University, Khartoum, Sudan
Citation: Efficacy of Diode Laser (980 Nm) and Non-Surgical Therapy on Management of Periodontitis-A Randomized Clinical Trial, J Res Med Dent Sci, 2021, 9(7): 166-172
Received: 15-Jun-2021 Accepted: 09-Jul-2021