Research Article - (2022) Volume 10, Issue 11
Efficacy and Safety of Systemic Corticosteroids among COVID-19 Patients
Isha Nirgun* and Swarupa chakole
*Correspondence: Isha Nirgun, Department of Community Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences, Sawangi, Wardha, Maharashtra, India, Email:
Abstract
For the sufferers of severe COVID-19, the advantages and risks of corticosteroids are unknown. The use of corticosteroids in corona virus illness patients has very little concrete evidence. As a result, conclusions about advantages and drawbacks must be made using indirect data from linked conditions. We looked into different studies showing the effects of Corticosteroids in patients with different severity groups like mild moderate and severe. Although a few studies have looked into the use of Steroids in the management of Coronavirus disease, they also included data from the SARS Coronavirus and MERS Coronavirus strains. Again, the majority of these trials only looked upon severe illness and only in terms of death rates. Corticosteroids have a role in cessation of inflammatory cascade due to which these are effective in respiratory disorders. Different studies and databases were looked to find out the effectiveness of Steroids in COVID sufferers.
Treatment with dexamethasone significantly reduced mortality in the Coronavirus patients who are on respiratory support (single study). Steroid administration was associated with a lower risk of death, a lower need for respiratory support, a lower need for ICU admission, a lower length of stay in the ICU (single study), and a lower duration of respiratory support in the serious and critically ill patients (two studies). Steroid therapy was linked to a longer length of hospital care” in the mild moderate population, but no differences were seen in other areas. Steroid treatment was related with "lower mechanical breathing requirements" in individuals at risk of developing "acute respiratory distress syndrome" (single study). This study recommends steroid treatment for corona virus sufferers who have different severity levels. Steroid medication may be advantageous in terms of lowering mortality in mechanically ventilated patients. Steroid medication was linked to lower mortality, less need for mechanical ventilation, and less time in the ICU among "serious and critical" patients.
In the "mild moderate" demographic, however, there was no advantage. To summarise, usage of steroids may be beneficial in individuals with coronavirus when correctly chosen patient groups (based on clinical severity and biomarker status).
Keywords
COVID-19, Meta-analysis, Steroid
Introduction
Corona virus disease is an on-going pandemic affecting millions of people around the globe [1]. It was originated in China in 2019. Many countries are still reeling under the pressure of disease and still high numbers of cases are being reported from countries. Two waves of the disease have passed and right now we are under the threat of third wave. As of June 4,2021,172,191,804 infection cases has been registered across the world and 3,702,736 deaths have been reported from all over the globe due to the disease and its related complications.
Corona virus spreads by droplets that have been infected with the virus [2]. When these droplets are breathed by a human, they cause sickness to develop. People are infected for around 20 days. Some people may be carriers yet not show symptoms [3]. These individuals are the ones that contribute the most to the virus's spread throughout the community. Because it is a very infectious disease, accurate diagnosis is critical. RT-PCR can be used to do this. A radiological diagnostic, such as a CT scan, can aid in the identification of a clinically suspect case. Early infection is characterised by bilateral multi lobar ground glass opacities in the lungs [4]. As the illness advances, sub pleural dominance and further progression to consolidation may occur [5]. Asymmetric peripheral ground glass opacities without pleural effusions are common imaging results on chest radiographs and CT of persons who are sick [6].
The importance of illness prevention cannot be overstated. Staying indoors, wearing a mask in public settings, having a safe distance from others, avoiding crowded places, and being vaccinated are some of the preventative measures performed [7]. People who have got the disease or those who experience symptoms are told to stay indoors and get the medical care. Masks are also strongly advised for anyone who may have been exposed to the virus and those caring for someone who may be affected [8]. When coughing or sneezing without a mask, cover the mouth and nose with a tissue and use the inside of the elbow if no tissue is available. It is recommended that you wash your hands after coughing or sneezing. In addition to additional personal protective equipment, healthcare practitioners who engage directly with persons who have infection should use respirators that are at least as protective as N95 or comparable [9]. Hands should be washed with soap and water, or sanitizers should be used if the former is not accessible. The spread of illness can be reduced by adopting appropriate hand hygiene.
Vaccination drives have been started all over the world to stop the spread of the disease. Many nations are first giving vaccines to people who are at risk of difficulties and have comorbid conditions, such as the elderly, as well as those most at risk of exposure and transmission, such as healthcare personnel. According to reports as of December 7, 2021, 8.24 billion doses of corona virus vaccination has been delivered across the globe [10]. Still USA, India, Brazil, France, Turkey, are among top chart of countries reporting more than half infection and most deaths.
Symptoms may occur 2 to 14 days after exposure. The common symptoms that a person experiences are fever, throat irritation, coryza, lethargy, loss of taste and anosmia, difficulty in breathing [11]. The other symptoms reported are not so common among people which are loose stools, erythema, and pain in chest.
At least one in three individuals affected do not show any signs or symptoms. Most of the people who have symptoms are mild to moderate which presents as mild pneumonia while in some the disease progresses and cause 50% of lung involvement with symptoms like dyspnoea, hypoxia. Rare cases progress to respiratory failure or multi organ damage [12]. People with low immunity, old age, comorbidities like Diabetes, Hypertension, CKD, malignancy have high chances of developing severe infection.
The course of the disease has three phases which comprises of early viral multiplication. The patient is asymptomatic during this phase of illness. The next come the immune response from the host and damage to the host cells. The final phase results in pulmonary or other organ failure [13].
Increased cytokine production is a key element in corona virus infection [14]. Cytokines are proteins that are produced in the body in reaction to the invasion of an external pathogen. They have a significant function in cell signalling. Interleukins 1 and 6, as well as tumour necrosis factor, are pro-inflammatory cytokines generated by macrophages, b lymphocytes, and t lymphocytes [15]. The release of the above leads to organ damage. Excessive inflammatory discharge is caused by an overzealous host immune system response to corona virus, and cytokine profiles are connected to tissue damage, such as lung failure and the development of multi organ failure [16].
The majority of instances are not significant and are treated with drugs such as Paracetamol or NSAIDS to alleviate symptoms. Diet, multi vitamins, and hydration intake are all recommended for control. In home care patients antibiotics are given to avoid secondary infections. Antivirals also used which were used in other corona strains. Steroids when used in early phase and home care patients have shown bag prognosis.
Steroids are frequently recommended for hospitalized patients because they minimize the chance of mortality. Respiratory assistance is provided, as well as ICU care.
Steroids are anti-inflammatory medications used to treat a variety of conditions such as autoimmune, inflammatory, rheumatic, and lung illness. Steroids reduce inflammation and immune system activity. Inflammation is a natural defence mechanism used by the body's white blood cells and chemicals to fight infection and external invaders like bacteria and viruses. The body's defensive system, on the other hand, doesn't work effectively in some disorders. Inflammation may then operate against the body's tissues, causing harm.
Steroids work by reducing the production of inflammatory molecules. This aids in minimizing tissue damage. Steroids also suppress the immune system's activity through altering the functions of white blood cells.
The pathophysiology of viral escape of cellular immune response and increased cytokines in corona virus is crucial. Lung inflammation and severe symptoms such as respiratory distress, respiratory failure, and sepsis originate from cytokine dysregulation and invasion of inflammatory myeloid cells. Steroids play a function in lowering pulmonary inflammation because they have anti-inflammatory and ant fibrotic properties. Steroid medication may also help to inhibit the advancement of COVID pneumonia related pulmonary fibrosis.
The illness has a severe course with long term consequences. Complications might include respiratory, neurological, cardiovascular, and other issues. Those with additional co-morbidities, such as diabetes, hypertension, or cancer, as well as pregnant women, are more likely to have problems. These problems are responsible for the majority of reported deaths.
Materials and Methods
The present study was carried out in accordance with the guidelines for studying reviews and meta-analysis.
Objectives
For sufferers treated with steroids the studies were done including following points:
• Death of sufferers
• Requirement of respiratory support
• ICU requirement
• Time to PCR negative (virological cure)
• Length of hospital stay
Patients were grouped as
• Mild moderate patients
• Serious and critical patients
• Individuals on respiratory support
Comparisons
• Steroid plus standard care compared to standard care alone
• Early steroid plus standard care compared to standard care
• High dosage compared to standard dosage
Results
Included study details: The following studies were looked to find out the outcome of steroids in different groups (Table 1 and Figure 1).
| Author, year/Country | Study design | Study population | Intervention | Control | Outcome | Comment |
|---|---|---|---|---|---|---|
| Nelson, et al. | Cohort study | Inclusion: RT-PCR+for COVID-19, requiring MV Exclusion: Patient expired <5 days after admission, patient receiving steroid other than MP | MP was initiated within 14 days of admission, patients received >24 h MP, Dose: 1 mg/kg/day, ceiling dose 80 mg/day MP: n=48 (overall) MP: n=42 (in propensity matched cohort) Median time from hospitalization to MP initiation: 4 (2-6) days, Median dose: 80 (78.8-80) mg | Propensity matched control, without steroid therapy C: n=69 (overall) C: n=42 (in propensity matched cohort) | Ventilator-free days at day 28th. extubation, death, discharge from hospital day 28th and day 60th | To ensure baseline comparability in terms of severity, data from propensity score matched cohort was used in the analysis (there were 42 well matched pairs |
| Keller, et al. | Observational study | All COVID-19 positive admitted patients, patients who died or discharged during 11th March to 13th April During the study period, some clinicians started prescribing steroids and some did not | Patients prescribed steroid within 48 h of admission were considered as early CS group and analysed n=140 | Patient never treated with CS n=1666 | Mortality, requirement of MV | Both the groups were comparable in terms of ferritin level, Charlson index, intubation rate, procalcitonin level |
| Horby, et al. | RCT | Clinically suspected or lab confirmed hospitalized COVID-19 patients | IV Dexamethasone (6 mg OD) for ten day or till discharge from hospital whichever earlier CS: n=2104 | SOC: n=4321 | Mortality within 28 days, time until discharge from hospital, need of MV | Patients who required either IMV or supplemental oxygen at baseline were considered severe disease |
| Chopra, et al.,2020 [37] | Retrospective analysis | "Mechanically ventilated COVID-19" related ARDS patients admitted between 20th March to 17th April Decision of administering CS was at discretion of clinician, however, many prescribed routinely, many after excluding other infections and many didn't use CS at all | Patients who were administered steroid n=38 (Dexamethasone=36, MP=2) Dexamethasone Average Cumulative Dose=143 mg MP=120 mg Mean duration=9.2 days | SOC n=23 | "Ventilator free days" at day 28, "ICU free days" at day 30. "hospital free days" at day 30 | Both the groups were comparable in terms of Charlson score, ferritin level, CRP, pro-calcitionin and lactate level |
| Monreal, et al. 1301/Spain/preprint | Controlled observational comparative study | Adult, "lab confirmed COVID-19 patients" with ARDS, who are treated with CS Minimum equivalent dose of CS to be considered as CS treatment was >0.5 mg/kg MP equivalent dose for >2 days Patients treated with remdesivir were excluded | High dose CS>250 mg/day of MP n=177 | Standard doses (1.5 mg/kg/day of MP n=396) | Mortality, need of MV | Both the groups were comparable at baseline in terms of "co-morbidities," "time from symptom onset to hospitalization," and "pattern of pneumonia at presentation," "bacterial co-infection" |
| Salton, et al. pre-print | Multicentre observational study | COVID-19 positive, 18-80 years, PaO, FIO, <250 mm Hg. B/L infiltrate, CRP >100 ARDS as per Berlin criteria. n=173 (CS=83, NCS=90) | MP Loading dose=80 mg IV. maintenance dose=80 mg/day until achieving either a PaO2: Fio, >350 mmHg or a CRP <20 mg/L | CS unexposed concurrent patients | "ICU admission, "need of IMV" or "all-cause death by day 28, "MV free days by day 28" and "change in the level of CRP" | Both the groups were comparable in terms of baseline occurrence of co-morbidities, respiratory rate, CRP, LDH level |
| Jeronimo, et al. (401/Brazil) | Double blind RCT | Clinical and/or radiological suspicion of COVID-19 hospitalized patients AND/OR ground glass opacity OR pulmonary consolidation on CT scan), age>18 years, with SpO2 S 94% at room air OR in use of supplementary oxygen OR under IMV | IV sodium succinct MP (0.5 mg/kg). twice daily for 5 days n=194 | Placebo (IV normal saline) n=199 | 28 days mortality, early mortality, IMV. oxygen index <100 by day 7 | As SpO2=94% at room air OR in use of supplementary oxygen OR under IMV comes under severe disease in NIH, the study was considered under the "severe disease" population |
| Ooi, et al. (411/Singapore/Preprint) | Retrospective cohort study | Adult, RT-PCR+, admitted, COVID-19 patients requiring only supportive care or MV at baseline were excluded | CS+SOC CS: Prednisolone 30 mg OD to 40 mg BD CS >3 days use considered in analysis n=35 | SOC n=57 | Death, clinical progression, mechanical ventilation | Stage 1 and 2 did not require supplemental oxygen were considered under mild to moderate category and stage 3 patients requiring supplemental oxygen were considered under severe category while analysis |
| Fadel, et al. (42//USA/observational study) | Multicentre Quasi experimental study | RT-PCR+, COVID-19 patient with B/L infiltrate (radiology), requiring supplemental oxygen in the form of nasal cannula, or MV | IV MP 0.5 to 1 mg/kg/day in 2 divided doses for 3-7 days Total n in MP=81 | Standard of care n=132 | Survival, MV need, ARDS, shock, AKD, hospital stay | Both the groups were comparable in terms of baseline severity as measured by QSOFA, NEWS score, number of patients requiring ICU and mechanical ventilation at baseline |
| Awasthi, et al. (431/USA/Cohort study) | Observational study | Critically ill COVID-19 patients | Dexamethasone prednisone, triamcinolone, MP. prednisolone betamethasone, hydrocortisone n=17 | Standard of care n=41 | ICU duration, plasma IL6 level pre-and post-CS treatment, survival | As all patients were critically ill. we can assume baseline comparability in terms of severity between the two groups |
| Li, et al. 44/China | Observational study | Patient with marked radiological progression However they did not enrol patients on mechanical ventilation at baseline | Early steroid group: 40-80 mg/day (0.75-1.5 mg/kg/day) of MP for 3 days tapered to 20 mg/d for <7 days duration n=47 | Control group: Patient with no CS treatment or Cs given at late phase (rescue treatment) n=21 | Requirement of IMV. safety, complications of CS therapy, viral clearance and blood glucose level | Considered severe category as population included are having extensive radiological Progression which falls into severe category of COVID-19 according to NIH guideline 4 |
| Wang, et al. Cohort study | Retrospective cohort | Severe COVID-19 patients Case definition meeting any one criteria according to NIH definition of severe COVID-194 n=46 CS=26 NCS=20 | Received MP 1-2 mg/kg/day for a period of 5-7 days via IV injection in addition to SOC | SOC | Requirement of MV. death, length of ICU stay and length of hospital stay | Comparison: Steroid versus Standard of care Considered under severe category |
| Zha, et al. Observational study | Observational study | Severe COVID-19 patients as defined by occurrence of respiratory distress, O2 saturation S 93% artery O2 partial pressure/O, concentration <300. rate of respiration>30 | 40 mg MP OD or BD per day n=11 | SOC n=20 | Recovered death, viral clearance. Duration of symptom, length of hospital stay. Kidney injury. liver injury | No difference was observed between the two treatment groups in terms of age, sex, co-morbidity, cytokine levels, and ferritin level |
| Yuan, et al. | Retrospective cohort study | RT-PCR+, non-severe COVID-19 pneumonia patients | CS group MP maximum dose 52.2 mg/day n=74 | None CS n=58 | Progression to severe disease, secondary infection, time for fever, hospital stay, duration of viral shredding after illness onset | Analysis was done on propensity score matched group data, which ensures baseline comparability among the groups |
| Corral, et at. RCT | Partially randomized preference, open-label trial | Moderate to severe COVID-19 patients Three arm Preference arm, intervention or control arm If preference than preferred arm, otherwise randomized into intervention or control arm | MP 40 mg IV every 12 h for 3 days than 20 mg every 12 h for 3 days n=34 | Standard of care n=29 | Death, ICU admission, requirement of non-invasive ventilation | Data collected only from the randomized two arms |
Table 1: The following studies were looked to find out the outcome of steroids in different groups.
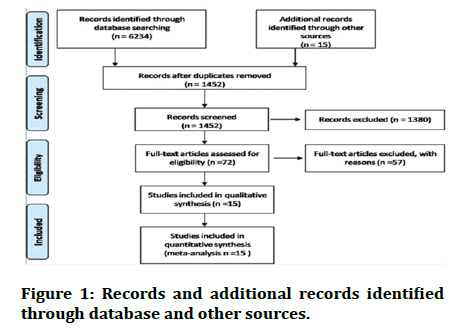
Figure 1: Records and additional records identified through database and other sources.
Risk of bias of the studies taken: The following table shows risk of bias among included trials and risk of bias in the observational studies (Figure 2).
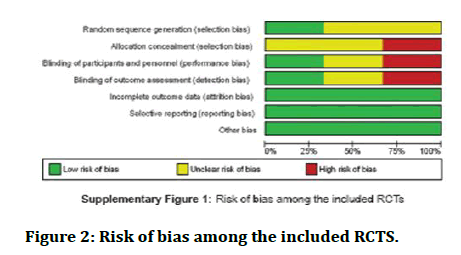
Figure 2: Risk of bias among the included RCTS.
Patients on respiratory support: Effectiveness of steroids in sufferers who needed respiratory support in terms of mortality.
The usages of dexamethasone in sufferers who are on respiratory support have shown some improvement [17].
The use of Methylprednisolone did not show any improvement as seen in the studies [18]. The dose of Methylprednisolone was 1 mg/kg/day. The following table shows the data (Figure 3).
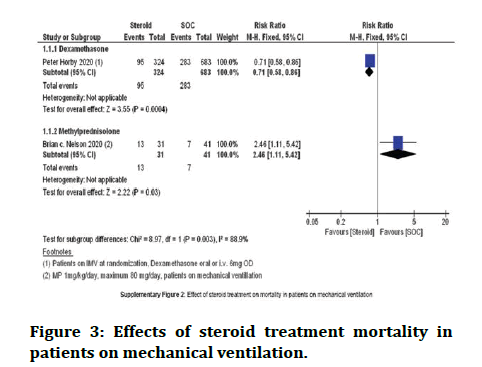
Figure 3: Effects of steroid treatment mortality in patients on mechanical ventilation.
Death among severe to critical patients combined: Studies showed that sufferers who are given steroid plus standard care had a lower death rate. Patients who were given standard care alone among this group did not show much benefit (Figure 4).
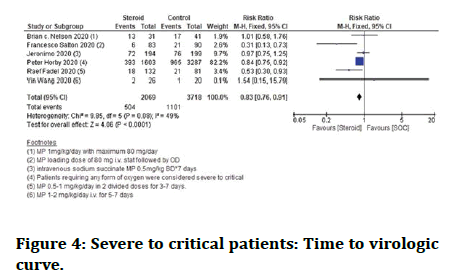
Figure 4: Severe to critical patients: Time to virologic curve.
Severe to critical patients: Need for respiratory support: When compared with standard care alone, steroid plus standard care together demonstrated a substantial improvement in lowering the "requirement of respiratory support”. Both dexamethasone and methylprednisolone administration were linked to a considerable decrease in the need for respiratory support in serious patients at the individual steroid level (Figure 5).
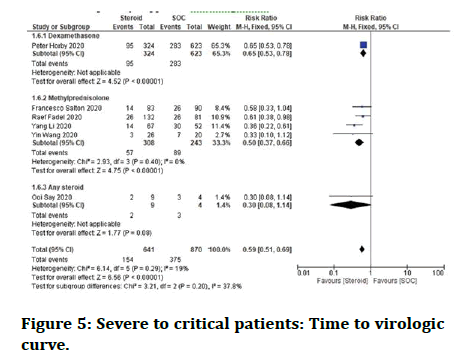
Figure 5: Severe to critical patients: Time to virologic curve.
Severe to critical patients need for ICU: Steroid therapy lowered the need for ICU in severe Corona virus patients (Figure 6).
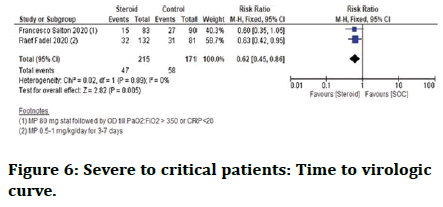
Figure 6: Severe to critical patients: Time to virologic curve.
Severe patients: Length of hospital stay: It was found that in sufferers on steroid plus standard care the time for which they stayed in hospital was less than standard care patients alone.
Time to PCR negative: In severe to critical corona virus patients, Francesco Salton 2020 research showed that the time for PCR negativity in patients treated with steroid plus standard care whereas standard care alone showed no difference (Figure 7).
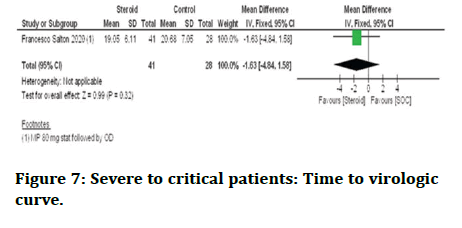
Figure 7: Severe to critical patients: Time to virologic curve.
Severe patients: Length of ICU stay: Among the trials considered, one found that the steroid with standard care had a substantially shorter ICU time in severe patients than the standard care alone [19].
Mild to moderate patients
Mortality: Two studies found that individuals treated with steroid and standard care had a higher death rate than those given standard care alone [20]. However, there was no distinction in death rate between the two groups in mild to moderate patients. The data is shown in figure below (Figure 8).
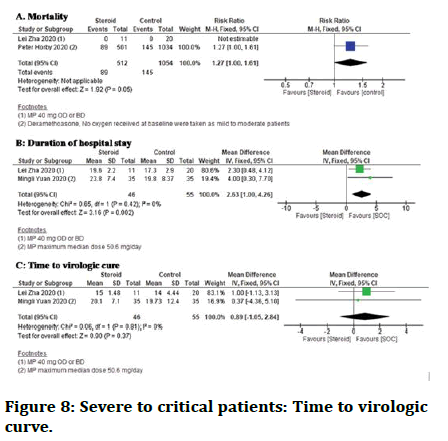
Figure 8: Severe to critical patients: Time to virologic curve.
Requirement of respiratory support: Ooi Say Tat, et al. [21], in a single research, reported the need for respiratory support in sufferers given steroids plus standard care with patients on standard care alone and had mild to average illness at the start of the study showed no difference.
Length of hospital care: The standard care with steroid needed a considerably longer hospital care than the standard care alone.
Time to PCR negativity: The time to PCR negativity in mild to moderate patients on steroid with standard care when compared with standard care only showed no difference (Table 2).
| Steroid compared to no steroid: All patients for COVID-19 Date August 3, 2020 | ||||||
| Patient or population: COVID-19 Date August 3, 2020 | ||||||
| Setting | ||||||
| Intervention: steroid | ||||||
| Comparison: No steroid: All patients | ||||||
| Outcomes | Anticipated absolute effects' (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (grade) | Comments | |
| Risk with no steroid: All patients | Risk with steroid | |||||
| Severe and critical: Mortality | 296 per 1000 | 246 per 1000 (225-269) | RR 0.83 (0.76-0.91) | 5787 (6 observational studies) | 0003 Moderate** | |
| Severe and critical: Requirement of MV | 431 per 1000 | 254 per 1000 (220-297) | RR 0.59 (0.51-0.69) | 1511 (6 observational studies) | 0000 LOW | |
| Severe and critical: Requirement of ICU | 339 per 1000 | 210 per 1000 (153292) | RR 0.62 (0.450.86) | 386 (2 observational studies) | 0033 Low | |
| Mild and moderate: Mortality | 138 per 1000 | 175 per 1000 (138-221) | RR 1.27 (1.00-1.61) | 1566 (2 observational studies) | 0000 Low | |
| Mild and moderate: Duration of hospital stay | The mean mild and moderate: Duration of hospital stay was 0 | MD 2.63 higher (1 higher to 4.26 higher) | 101 (2 observational studies) | 0000 Low | ||
| Mild and moderate: Time to virological cure | The mean mild and moderate: time to virological cure was 0 | MD 0.89 higher (1.05 lower to 2.84 higher) | 101 (2 observational studies) | 0000 Low | ||
*Risk of bias: As the studies included is mix of randomised and nonrandomised studies. RCT with t=high risk of bias at the level of allocation a concealment and binding and low risk with nonrandomized studies with certain limitations. We have downgraded at serious level considering these factors. Imprecision: The upper limit of the 95% CI is 0.76-0.91. The minimum expected risk reduction in mortality is 9%. We have not downgraded conserving the fast spread of this condition. Grade working group grades of evidence. High certainty: We are very confident that the true effect lies close to the estimate of the effect, Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate to the effect, but there is a possibility that it is substantially different, Low certainty: Our confidence in the limited: The true effect may be substantially different from the estimate of the effect, Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of the effect. The risk in the intervention group (and its 95% CI) is based on the assumed risk in the compassion group and the relative effect of the intervention (And its 95% CI). CI=Confidence interval, RR=Risk ratio, MD=Mean difference, RCT=Randomized controlled trial.
Table 2: Grade: Summary of findings table.
Discussion
The above studies shows that steroids are effective in corona virus patients but the category of patients must be chosen.
Usage of Dexamethasone significantly reduced death in patients requiring respiratory support. Treatment with low to moderate doses of Methylprednisolone, on the other hand, was linked to a considerable increase in death (single study). However, due to the small number of research, a definitive conclusion in this group of patients is not achievable at this time.
Overall steroid usage was linked to a lower death rate among patients in the severe to critical category. A single study made the most contribution to these findings. In terms of treatment with steroids plus standard care and standard care alone benefit was seen from usage of steroids.
In terms of other outcomes, steroid medication was linked to a considerable reduction in the need for respiratory support in clinical trial. Both Dexamethasone and Methylprednisolone were shown to have a benefit in subgroup analysis. Steroid administration was also linked to fewer ICU admissions (2 studies), shorter ICU stays (1 research), and shorter respiratory support (2 studies). However, when it came to the length of hospital care, there was no difference, and when it came to "time to PCR negativity, there was no difference (1 study). Dexamethasone was found to be helpful in additional sub-group analysis, however low to moderate dosage prednisolone failed to confirm its protective impact in terms of mortality.
In mild to moderate disease sufferers, steroid provided "no benefit" in terms of death (2 studies), the need for respiratory support (1 study), time to PCR negativity (2 studies), and duration of pyrexia (1 study). It was noticed that usage was linked to a significant increase in hospital care (2 studies). Patients treated with steroid and standard care have shown to increase in mortality
Safety consideration with treatment: Steroid usage has its own set of risks and problems, such as hyperglycaemia and infection. However, the studies gave minimal information on the corticosteroid's safety profile, and the majority of the studies did not identify any safety concerns [21]. As a result, typical safety precautions should always be addressed while using corticosteroids, especially among patients with additional co-morbidities, such as diabetes, as they are in other disorders. Control of Hyperglycaemia and a sepsis test may be required before starting corticosteroid medication, although the recommendations differ from person to person. Pre-screening and control of sugar is important when given steroids. Blood pressure monitoring should also be done when on steroids as they are known to cause hypertension. Other known side effects are weight gain, hyper pigmentation of skin, mood changes, swelling of face, retention of water causing swelling of feet, sleep disorders, blurred vision.
Continuous use of steroids can suppress our immune system which makes an individual prone to opportunistic infections. The infections may be caused by bacteria, fungus, protozoans etc. Various fungi are known to cause infection in people treated with steroids like candida, Mucor, Rhizopus, Cryptococcus.
Mucormycosis is the most dangerous and life threatening infection is caused by Rhizopus and Mucor which comes under Mucorales. Risk factors for this are steroids, diabetes, neutropenia, malignancy. The disease may spread from sinuses to orbit. If the infection is not controlled then it can spread to brain causing death in few days. Treatment of the condition is surgical debridement. Drugs like Amphotericin B are found to be effective.
Candida infection is also very common in long term steroid usage. It is gram positive yeast like fungus. Oral thrush, intertigo, nail infection, oesophageal candidiasis, and vaginal infection are all symptoms of a superficial infection. Patients may also develop systemic candidiasis. Joint discomfort, osteomyelitis, pericarditis, myocarditis,and lung infection are all symptoms of systemic candidiasis. Amphotericin B is used in the treatment.
Cryptococcus is true yeast and is reported to cause infections in these patients. The infection is acquired by inhalation of yeast. The primary site of infection is lung which can further spread in the body by haematological spread. Treatment comprise of fluconazoles and Amphotericin B.
Conclusion
Dexamethasone may reduce mortality in corona virus patients who need respiratory support; however, further research is needed to confirm this Corticosteroid treatment was linked to "reduced death," "reduced need for respiratory support", "reduced need for ICU," and "lower duration of respiratory support" in severe to critical patients. The steroid treatment had a longer hospital care among mild moderate patients. There was no difference between the steroid treatment and the standard care in other areas like as mortality, the need for respiratory support, the need for ICU, and the length of fever. Steroid therapy decreased the need for mechanical breathing in individuals at risk of developing ARDS in a single trial. These findings, however, need to be confirmed in larger investigations. When looked upon safety considerations steroids have reported side effects in patients who are on long term steroid treatment. People with co morbid conditions like diabetes, hypertension, and malignancy should be looked closely and monitored when under steroid therapy.
References
- Yam LY, Lau AC, Lai FY, et al. Corticosteroid treatment of severe acute respiratory syndrome in Hong Kong. J Infect 2007; 54:28-39.
- Centers for Disease Control and Prevention (CDCP)."Coronavirus disease 2019 (COVID-19)". U.S. Department of Health and Human Services.
- Australian Government. “Coronavirus disease 2019 (COVID-19): CDNA National guidelines for Public Health Units”.5.1. Communicable diseases network Australia/Australian Government Department of Health.
- Salehi S, Abedi A, Balakrishnan S, et al. "Coronavirus disease 2019 (COVID-19): A systematic review of imaging findings in 919 patients". AJR Am J Roentgenol 2020; 215:87–93.
- Li Y, Xia L. "Coronavirus disease 2019 (COVID-19): Role of chest CT in diagnosis and management". AJR Am J Roentgenol 2020; 214:1280–1286.
- Centers for Disease Control and Prevention (CDC). Viral Load Exposure Factors. Really Correct. 2020.
- Centers for Disease Control and Prevention (CDCP). Caring for someone sick at home advice for caregivers in non-healthcare settings. 2022.
- Centers for Disease Control and Prevention (CDCP). "Using Personal Protective Equipment (PPE)". U.S. 2020. U.S. Department of Health and Human Services.
- Richie H, Ortiz Ospina E, Beltekian D, et al. "Coronavirus (COVID-19) vaccinations statistics and research". Our World in Data, 2020.
- European Centre for Disease Prevention and Control. Clinical characteristics of COVID-19". 2022.
- Centers for Disease Control and Prevention (CDCP). "Interim clinical guidance for management of patients with confirmed Coronavirus Disease (COVID-19)". U.S. 2022
- Thompson MR, Kaminski JJ, Kurt Jones EA, et al. Pattern recognition receptors and the innate immune response to viral infection. Viruses 2011; 3:92040.
- Sinha P, Matthay MA, Calfee CS. Is a “Cytokine Storm” relevant to COVID-19? JAMA Intern Med 2020; 180:1152-1154.
- Ragab D, Salah Eldin H, Taeimah M, et al. The COVID-19 cytokine storm; what we know so far. Front Immunol 2020; 11:1446.
- Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19 Preliminary Report. N Engl J Med 2020.
- Nelson BC, Laracy J, Shoucri S, et al. Clinical outcomes associated with Methylprednisolone in mechanically ventilated patients with COVID-19. Clin Infect Dis 2020.
- Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther 2020; 5:57.
- Zha L, Li S, Pan L, et al. Corticosteroid treatment of patients with Coronavirus disease 2019 (COVID-19). Med J Aust 2020; 212:416-420.
- Ooi ST, Parthasarathy P, Lin Y, et al. Adjunctive Corticosteroids for COVID-19: A retrospective Cohort study. Open Forum Infect Dis 2020; 7.
- World Health Organization (WHO). Corticosteroids for COVID-19. 2020.
- Tamez Perez HE, Quintanilla Flores DL, Rodriguez Gutierrez R, et al. Steroid Hyperglycaemia: Prevalence, early detection and therapeutic recommendations: A narrative review. World J Diabetes 2015; 6:1073.
Author Info
Isha Nirgun* and Swarupa chakole
Department of Community Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences, Sawangi, Wardha, Maharashtra, IndiaCitation: Isha Nirgun, Swarupa chakole, Efficacy and Safety of Systemic Corticosteroids among COVID-19 Patients, J Res Med Dent Sci, 2022, 10 (11): 074-081.
Received: 05-Sep-2022, Manuscript No. JRMDS-22-64923; , Pre QC No. JRMDS-22-64923(PQ); Editor assigned: 08-Sep-2022, Pre QC No. JRMDS-22-64923(PQ); Reviewed: 22-Sep-2022, QC No. JRMDS-22-64923; Revised: 07-Nov-2022, Manuscript No. JRMDS-22-64923(R); Published: 15-Nov-2022
