Research - (2023) Volume 11, Issue 6
Effects of the systemic administration and topical application of omega -3 polyunsaturated fatty acid on experimental gingivitis in rats.
Qayssar Riyadh Abdulrazzaq* and Athraa Mustafa Salih
*Correspondence: Qayssar Riyadh Abdulrazzaq, Department of Pedodontic and Preventive Dentistry, College of Dentistry, University of Baghdad, Iraq, Email:
Abstract
Introduction: Gingivitis is a reversible form of periodontal disease in which the inflammation is limited to the gingiva and no other supporting tissues are destroyed. Omega-3 PUFA has the most effective immune modulatory properties of all the fatty acids. Few studies have investigated topical usage of omega 3 poly unsaturated fatty acid in the treatment of gingivitis.
Aim of the study: The present study was aimed to evaluate the effects of omega-3 polyunsaturated fatty acid on ligature induced gingivitis in rats through the immunological analysis for rat’s gingival tissue level of IL-1β.
Materials and methods: The gingivitis was induced for rats by ligation around lower central incisors. Fifty animals with the induced gingivitis were divided into: Five animals were sacrificed at the first day after removal of ligature and fifteen animals as positive control group. Fifteen animals were used as 1st treatment group with orally gavage omega 3 polyunsaturated fatty acid and fifteen animals as 2nd treatment groups with topically applied omega 3 a five animals were used as negative control groups (-ve) group.
The rats were sacrificed and histological tissue samples were evaluated for the inflammatory tissue changes. Interleukin-1β (IL-1β) levels were measured by ELISA three hours after ligation removal (day zero), three days, one week and two weeks for immunological analysis.
Results: The result showed that the level of IL-1β were significantly lower in the treatment groups in comparison with positive control groups, and there were significant difference between the two treatment groups at the 3rd day after removal of ligature and non-significant difference between these groups in the 7th and 14th day. T-test was used for the statistical analyses, with P<0.05 regarded as significant.
Conclusion: Systemic and topical application of omega-3 fatty acids is effective in reducing inflammation in induced rat gingivitis, resulting in a decreased level of IL-1β.
Keywords
Interleukin-1β, Omega-3 fatty acids, Gingivitis, Immunological analysis, Immunomodulatory
Introduction
Gingivitis is a reversible form of periodontal disease in which the inflammation is limited to the gingiva and no other supporting tissues are destroyed. Gingivitis is a complex disease caused by the combination of pathogenic bacteria invasions and various degrees of host immune response. The most common kind of gingivitis is plaque induced gingivitis, which is caused by the deposition of microbial plaque comprising more than 300 different bacterial species and is characterized by gingival redness and edema [1]. However, due to a breakdown of symbiosis between the biofilm and the host's immune inflammatory response, and the development of an incipient dysbiosis, gingivitis will be developed if dental plaque accumulates for days or weeks without disruption or eradication [2].
IL1, TNF- and IL8 were the most common cytokines found in normal healthy gingiva as well as in induced gingival tissue inflammation in rats.
In response to infection, monocytes and macrophages produce IL-1, but too much of it can cause irreversible tissue damage [3].
Polyunsaturated Fatty Acids (PUFAs) are fatty acids that have more than one double bond between carbon and carbon. In human cells, both omega-3 and omega-6 have anti-inflammatory effects via the synthesis of nuclear transcription factors, enzymes and cytokines [4].
Omega-3, omega-6 and omega-9 fatty acids are the three main types of omega fatty acids. Omega-3 is found in foods like fish, walnuts, and green leafy vegetables, whereas omega-6 is found in grains and vegetable oils and omega-9 is found in animal fat and vegetable oil [5].
Omega-3 PUFA have the most effective immune modulatory properties of all the fatty acids, and those from fish oil, Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA), are more biologically potent than Linoleic Acid (ALA). DHA is more beneficial than EPA, according to dose response studies and diets combining the two omega-3 fatty acids are synergistic [6].
In periodontics, Host Modulatory Therapy (HMT) refers to medications that are administered systemically or locally as a supplement to traditional periodontal treatments [7].
There have been a few studies on the influence of omega-3 on IL-1, as well as the local effects of using these fatty acids for the treatment of periodontal disorders in both humans and animals, but the results have been contradictory [8].
As a result, the purpose of this study was to investigate and evaluate the effects of orally gavage omega-3 polyunsaturated fatty acid (60 mg/kg) and topically applied omega-3 polyunsaturated fatty acid on ligature induced gingivitis in rats using immunological analysis for tissue levels of IL-1.
Materials and Methods
Rats and housing: The research protocol was approved by the ethics committee of college of dentistry, Baghdad University (ref number 331 in 18-4-2021). The study was conducted according to the ethical guidelines for the care and usage of animals.
Fifty five male Sprague Dawley rats (8-9) weeks old (weighing 175-200 g) and cared in the animal house. The animals were housed for acclimatization, one week before the start of the experiment. One rat was housed in each wire cage in a temperature and humidity controlled room (23°C ± 1°C and 60% ± 5% relative humidity), under 12 hours light/dark cycle, with access to standard rat chow pellets and water available.
Induction of experimental gingivitis: An intramuscular injection of 0.12 ml/100 g body weight of a solution of 10% ketamine and 2% xylazine (2:1) was used to establish general anesthesia.
After administration, anesthesia was installed in 4-5 minutes. Each subject's body weight was determined. Non-resorbable 4/0 sterile silk thread were placed in figure “8” in the inferior frontal group.
This ligature worked as a gingival irritant for 7 days, promoting plaque accumulation and the progression of periodontal disease. The rats were kept in the same condition after the ligatures were placed, with one rat in each cage. The rats were transferred to a soft diet consisting of commercial food biscuits soaked in warm water for 10 minutes and then drained. Rats were monitored for seven days. The animals were assessed for proper diet and body weight on a daily basis and performed ligatures control [9].
3 Experimental procedures: A total of 55 males’ rats were divided randomly into two main groups as follow:
• 1st negative control group consist of (5 rats) without induced gingivitis and without any treatment just brushing the gingiva of the lower frontal teeth with distal water were fed with the normal food scarified after 7 days. • 2nd study groups fifty rats with induced gingivitis are divided into following groups: 5 anesthetized animals were sacrificed at the day of removal of the ligature for tissue IL-1β analysis and histological examination.
The (45 rats) was subdivided into 3 subdivision groups as Figure 1.
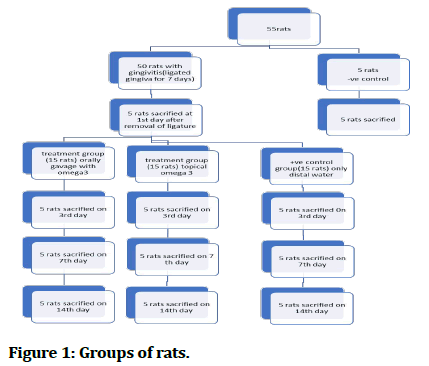
Figure 1: Groups of rats.
Cytokines analysis: These proteins antigens were identified using Enzyme Linked Immunosorbent Assay (ELISA) techniques. Tissue samples were obtained and coded from the lower labial gingiva of central incisor teeth. They were then placed in sterile plastic tubes and maintained at -70°C for one month to prevent bioactivity loss and contamination until IL-1 concentrations were determined using an immunoassay. IL-1β concentrations were measured using rat specific enzyme linked immunosorbent assay kits (CSB-E08055r) according to the manufacturer’s instructions. Tissue was homogenized in 1 ml of Phosphate Buffered Saline (PBS), the homogenates were centrifuged for 5 minutes at 5000 rpm. The supernatant was removed and assayed immediately.
Data were analysed using SPSS software version 23. The data were summarized using means and standard deviations. The T-test was performed to assess individual pair of groups for statistically significant finding. A p-value less than 0.05 were considered statistically significant and p-value less than 0.01 were considered highly significant.
Results
Tissue level of IL-1 β statistical analysis showed high significant increase in tissue IL-1 β level (p ≤ 0.01) in the (+ve) control group, systemic omega 3 treatment group and topical omega 3 treatment groups in relation with the (-ve) control groups in all durations studied (Table 1).
| Groups | 1st day | P value | 3rd day | P value | 7 days | P value | 14 days | P value |
|---|---|---|---|---|---|---|---|---|
| -ve/+ve | 1768.9 ± 98.71 | 0.0001** | 1768.9 ± 98.71 | 0.0001** | 1768.9 ± 98.71 | 0.0001** | 1768.9 ± 98.71 | 0.0001** |
| 5658.6 ± 174.50 | 5584.3 ± 144.85 | 5283.7 ± 127.49 | 4952.4 ± 135.06 | |||||
| -ve/systemic | - | - | 1768.9 ± 9871 | 0.0001** | 1768.9 ± 98.71 | 0.0006** | 1768.9 ± 98.71 | 0.0058** |
| 4653.7 ± 128.52 | 3932.7 ± 115.23 | 2873.2 ± 121.03 | ||||||
| -ve/topical | - | - | 1768.9 | 0.0001** | 1768.9 | 0.0001** | 1768.9 | 0.0033** |
| 5144.6 | 4346.9 | 3150.2 | ||||||
| **(P>0.01). | ||||||||
Table 1: The difference in IL-1β tissue levels in different groups with difference periods.
The systemic groups show significant decrease (p ≤ 0.05) in IL-1β level in the 3rd day and high significant decrease (0 ≤ 0.01) in the IL-1β level in the 7th and 14th days in comparison with (+ve) control groups.
The topical groups show significant decrease (p ≤ 0.05) in IL-1β level in the 3rd day and 7th day and high significant decrease (0 ≤ 0.01) in the IL-1β level in the 14th days in comparison with (+ve) control groups.
The systemic groups show significant decrease (p ≤ 0.05) in IL-1β level in the 3rd day and non-significant decrease in the IL-1β level in the 7th and 14th days in comparison with topical groups (Table 2).
| Groups | 3rd day | P value | 7 days | P value | 14 days | P value |
|---|---|---|---|---|---|---|
| +ve/systemic | 5658.6 ± 174.50 | 0.0327* | 5658.6 ± 174.50 | 0.0063** | 5658.6 ± 174.50 | 0.0003** |
| 4653.7 ± 128.52 | 3932.7 ± 115.23 | 2873.2 ± 121.03 | ||||
| +ve/topical | 5658.6 ± 174.50 | 0.0497* | 5658.6 ± 174.40 | 0.0371* | 5658.6 ± 174.50 | 0.0027** |
| 5144.6 ± 162.47 | 4346.9 ± 117.92 | 3150.2 ± 106.44 | ||||
| Systemic/topical | 4653.7 ± 128.52 | 0.0407* | 3932.7 ± 115.23 | 0.084 NS | 2873.2 ± 121.03 | 0.148 NS |
| 5144.6 ± 162.47 | 4346.9 ± 139.86 | 3150.2 ± 115.38 | ||||
| *(P>0.05), **(P>0.01), NS: Non-Significant. | ||||||
Table 2: The effect of group inIL-1β tissue levels with difference period.
Discussion
Gingivitis is one of the most common diseases in humans and many studies have looked at its pathogenesis using experimental animals. The use of ligatures in the teeth has been recommended as a way to achieve an experimental gingivitis condition faster than natural gingivitis [10]. Because of their morphological and histological similarities to the human cohort, rats are the most extensively researched animals for the development of periodontal disorders [11].
Most rodent studies have used ligatures in the gingival sulcus around the molar teeth to induce periodontal disease and increase biofilm build-up [12,13].
Because of the complexity and difficulty of performing experiments on rats and placing ligatures around molars, the present study proposed a modification of the existing model by placing ligatures around lower incisors instead of around molars. This modification was done to produce a reproducible experimental model for the induction of periodontal disease in wistar rats and this method was used in a recent study. Induction of periodontal disease in Wistar rats, this method was used in recent study.
The study showed that gingivitis causes a high significant increase in IL-1β in all durations studied in comparison with the negative control groups. Araghizadeh, et al. found that the level of IL-1 β in negative control group rats were (233.29 ± 28.20) and these levels were increased significantly in the experimental gingivitis group to (418.24 ± 40.52) after ligation.
Jalal, et al. showed that periodontitis causes a significant increase in IL-1β in all durations studied in comparison with the negative control/water treatment group.
The effect of orally gavage omega 3 PUFA on IL-1β in rats with gingivitis. The present study showed a high significant (P ≤ 0.01). Differences present between the IL-1β of the systemic omega 3 treatment groups and that of negative group in all durations studied.
The systemic groups show significant decrease (p ≤ 0.05) in IL-1β level in the 3rd day and high significant decrease (0 ≤ 0.01) in the IL-1β level in the 7th and 14th days in comparison with (+ve) control groups.
Elwakeel study showed that there is a significant reduction in level of interleukin 1β at 3 and 6 months in group gavage omega-3 plus low dose aspirin compared to the placebo group [14].
These results disagree with that of Araghizadeh, et al. study; they found that the level of IL-1β in the treatment group was similar to that in the positive control group which was orally gavage by saline.
Vardar, et al. on the other hand, found a significant increase in the level of IL-1 in omega-3 fish oil consuming rats as compared to the positive control group [15]. This disagreement could be attributed to discrepancies in the two studies' techniques for measuring IL-1 in tissue and serum, as well as variances in omega-3 dosage.
The effect of topical omega 3 PUFA on IL-1β and TNF-α in rat with gingivitis. The present study showed a high significant (P ≤ 0.01). Differences present between the IL-1β tissue levels of the topical omega 3 treatment group and that of negative group in all durations of the study.
The topical groups show significant decrease (p ≤ 0.05) in IL-1β level in the 3rd day and 7th day, and high significant decrease (0 ≤ 0.01) in the IL-1β level in the 14th days l in comparison with (+ve) control groups.
Local application of omega-3 and omega-6 PUFAs can prevent or treat experimental gingivitis in humans, according to a study by Eberhard, et al. In the test group, the main effect was an insignificant decrease in gingival crevicular fluid. The theory that rinsing with PUFAs could prevent gingivitis was disproved [16].
Hasturk, et al. studied the effects of RvE1 on periodontitis in rabbits and discovered that the anti-inflammatory benefits of this omega-3 bioactive product are related to a decrease in systemic inflammatory biomarkers such as C-reactive protein and interleukin-1 [17].
Changed activation of important transcription factors involved in controlling the expression of genes encoding inflammatory proteins, such as nuclear factor kappa light chain enhancer of activated B cells (NF-B), may be a mechanism through which omega-3 reduces IL-1 [18,19].
The metabolism of omega-3 fatty acids produces "proresolving lipid mediators," or SPMs, such as resolvins and protectins, which have anti-inflammatory and immune regulatory characteristics and limit immune cell passage while inhibiting the synthesis of pro-inflammatory cytokines [20,21].
Conclusion
The results of the current study indicated that the use of omega 3 PUFAs topically and gavage orally can reduce the gingival inflammation. Because of its anti-inflammatory and antibacterial effects. Omega 3 also showed reduced level of pro inflammatory cytokines (IL-1β) in gingival tissue.
References
- Balta MG, Loos BG, Nicu EA. Emerging concepts in the resolution of periodontal inflammation: A role for resolvin E1. Front Immunol 2017; 8:1682.
[Crossref] [Google Scholar] [PubMed]
- Chanda W, Joseph TP, Guo XF, et al. Effectiveness of omega-3 polyunsaturated fatty acids against microbial pathogens. J Zhejiang Univ Sci B 2018; 19:253.
[Crossref] [Google Scholar] [PubMed]
- Feng X, Liu J. Association between IL-1A (-889C/T) polymorphism and susceptibility of chronic periodontitis: A meta-analysis. Gene 2020; 729:144227.
[Crossref] [Google Scholar] [PubMed]
- Jalal AA, Al-Qassab ZA, AL-Rawi RA. Effects of the systemic administration of omega-3 polyunsaturated fatty acid on experimental periodontitis (immunological analysis for rat’s serum). Ann Trop Med Public Health 2020; 23.
- Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med 2008; 233:674-688.
[Crossref] [Google Scholar] [PubMed]
- Figueredo CM, Martinez GL, Koury JC, et al. Serum levels of long chain polyunsaturated fatty acids in patients with periodontal disease. J Periodontol 2013; 84:675-682.
[Crossref] [Google Scholar] [PubMed]
- Salvi GE, Lang NP. Host response modulation in the management of periodontal diseases. J Clin Periodontol 2005; 32:108-129.
[Crossref] [Google Scholar] [PubMed]
- Araghizadeh N, Paknejad M, Alaeddini M, et al. The efficacy and prophylactic characteristics of omega-3 fatty acids in experimental gingivitis in rats. Iran J Basic Med Sci 2014; 17:87.
[Google Scholar] [PubMed]
- Lourenco AL, Booij-Vrieling HE, Vossebeld CB, et al. The effect of dietary corn oil and fish oil supplementation in dogs with naturally occurring gingivitis. J Anim Physiol Anim Nutr 2018; 102:1382-1389.
[Crossref] [Google Scholar] [PubMed]
- Srivastava M, Neupane YR, Kumar P, et al. Nanoemulgel (NEG) of ketoprofen with eugenol as oil phase for the treatment of ligature induced experimental periodontitis in Wistar rats. Drug deliv 2016; 23:2228-2234.
[Crossref] [Google Scholar] [PubMed]
- Mester A, Ciobanu L, Taulescu M, et al. Periodontal disease may induce liver fibrosis in an experimental study on Wistar rats. J Periodontol 2019; 90:911-999.
[Crossref] [Google Scholar] [PubMed]
- Azuma MM, Castro dos Santos NC, Furukawa MV, et al. Does the use of omega-3 fatty acids as an adjunct to non-surgical periodontal therapy provide additional benefits in the treatment of periodontitis? A systematic review and meta-analysis. J Periodontal Res 2022; 57:435-447.
[Crossref] [Google Scholar] [PubMed]
- Saadi-Thiers K, Huck O, Simonis P, et al. Periodontal and systemic responses in various mice models of experimental periodontitis: Respective roles of inflammation duration and Porphyromonas gingivalis infection. J Periodontol 2013; 84:396-406.
[Crossref] [Google Scholar] [PubMed]
- Elwakeel NM, Hazaa HH. Effect of omega 3 fatty acids plus low dose aspirin on both clinical and biochemical profiles of patients with chronic periodontitis and type 2 diabetes: A randomized double blind placebo controlled study. J Periodontal Res 2015; 50:721-729.
[Crossref] [Google Scholar] [PubMed]
- Vardar-Sengul S, Buduneli N, Buduneli E, et al. Dietary supplementation of omega-3 fatty acid and circulating levels of interleukin-1β, osteocalcin and c-reactive protein in rats. J Periodontol 2006; 77:814-820.
[Crossref] [Google Scholar] [PubMed]
- Eberhard J, Heilmann F, Jepsen S, et al. Local application of n-3 or n-6 polyunsaturated fatty acids in the treatment of human experimental gingivitis. J Clin Periodontol 2002; 29:364-369.
[Crossref] [Google Scholar] [PubMed]
- Hasturk H, Kantarci A, van Dyke TE et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol 2007; 179:7021-7029.
[Crossref] [Google Scholar] [PubMed]
- Kruse AB, Kowalski CD, Leuthold S, et al. What is the impact of the adjunctive use of omega-3 fatty acids in the treatment of periodontitis? A systematic review and meta-analysis. Lipids Health Dis 2020; 19:1-6.
[Crossref] [Google Scholar] [PubMed]
- Chee B, Park B, Fitzsimmons T, et al. Omega-3 fatty acids as an adjunct for periodontal therapy: A review. Clin Oral Investig 2016; 20:879-894.
[Crossref] [Google Scholar] [PubMed]
- Vors C, Allaire J, Mejia SB, et al. Comparing the effects of docosahexaenoic and eicosapentaenoic acids on inflammation markers using pairwise and network meta-analyses of randomized controlled trials. Advan Nutr 2021; 12:128-140.
[Crossref] [Google Scholar] [PubMed]
- Chapple IL, Mealey BL, van Dyke TE, et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Clin Periodontol 2018; 89:S74-84.
[Crossref] [Google Scholar] [PubMed]
Author Info
Qayssar Riyadh Abdulrazzaq* and Athraa Mustafa Salih
Department of Pedodontic and Preventive Dentistry, College of Dentistry, University of Baghdad, IraqCitation: Qayssar Riyadh abdulrazzaq, Athraa Mustafa salih, Effects of the systemic administration and topical application of omega-3 polyunsaturated fatty acid on experimental gingivitis in rats, J Res Med Dent Sci, 2023, 11 (06): 039-043.
Received: 27-Aug-2022, Manuscript No. JRMDS-22-68515; , Pre QC No. JRMDS-22-68515 (PQ); Editor assigned: 29-Aug-2022, Pre QC No. JRMDS-22-68515 (PQ); Reviewed: 12-Sep-2022, QC No. JRMDS-22-68515; Revised: 12-Jun-2023, Manuscript No. JRMDS-22-68515 (R); Published: 19-Jun-2023
