Research Article - (2022) Volume 10, Issue 10
Effectiveness of COVISHIELD Vaccine in Healthcare Workers of AVBRH, Wardha
Rugved C Yasatwar* and Shiv Joshi
*Correspondence: Dr. Rugved C Yasatwar, Department of Community Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences (Deemed to be University), Sawangi (Meghe), Maharashtra, India, Email:
Abstract
Introduction: The objective of this study was to analyse the effectiveness of the COVISHIELD vaccine among the interns working in the Acharya Vinoba Bhave rural hospital (tertiary care hospital of central India).
Methods: We surveyed 71 M.B.B.S interns over the period of 1 month. Selected healthcare workers were invited to participate in the online survey. A pre-constructed, confidential, validated, self-administered online survey questionnaire inquiring about the history of COVID-19 and COVISHIELD vaccination was provided to selected interns. Online survey was based on the retrospective study with the questions related to the past medical history, COVID-19 infections in the past, vaccination history, symptoms during COVID-19 positive period, and presence of radiological evidence suggesting COVID-19 infection affecting the lungs. The prime question to calculate the analysis of this study was "Have you tested positive any time after taking 1st dose of COVISHIELD?
Results: According to survey analysis effectiveness of the COVISHIELD vaccine in interns working in AVBRH is found out to be 94.37 % after taking both doses of COVISHIELD vaccine. The most common symptoms were fever (13.33%), weakness/ fatigue (12.22%), cough (12.22%), and loss of taste (10%).
Conclusion: The study indicates that the COVISHIELD vaccine is found out to be highly effective in preventing COVID-19 infection among interns. However, this evidence should be further assessed in larger trials.
Keywords
Vaccination distribution, Acute respiratory, Preventing, Vulnerable population
Introduction
A new Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) appeared in late 2019, causing coronavirus illness in 2019 (COVID-19). COVID-19 was declared a pandemic by the World Health Organization on March 11, 2020. India had reported 33,935,309 cases and 450,408 deaths as of October 9, 2021 [1].
The COVID vaccination program in India integrates the suggestions of the world's leading specialists in immunization, public health, disease control, and information technology. The initiative prioritizes building the country's healthcare system by protecting the professionals, health, and frontline employees who staff it, as well as the most vulnerable population groups, based on scientific and epidemiological evidence [2-4].
COVISHIELD (Astra Zeneca) and COVAXIN (Bharat Biotech), two vaccine candidates in India, received emergency use permission on January 3, 2021. COVISHIELD (ChAdOx1 nCoV-19) is a non-replicating adenovirus vaccine containing recombinant SARS-CoV-2 spike protein. It exhibited an acceptable safety profile in phase I/II trials and a 74% efficacy in preventing infections in phase III interim analysis [5-7].
Understanding the risks and benefits of immunization programs requires assessing the real-world performance of the COVID-19 vaccine. Vaccination distribution and storage, as well as how patients are vaccinated, all have an impact on real world vaccine effectiveness. Furthermore, vaccination recipients in clinical trials are frequently young and healthy, and so different from those who would receive vaccines in the real world.
Real world vaccination efficacy studies can also address concerns like vaccine effectiveness by age group and risk factors, period of vaccine protection, transmission protection, the vaccine's efficiency against new SARSCoV- 2 strains, and the relative effectiveness of one vs. two doses.
The methodology of a retrospective cohort study to evaluate the efficiency of the COVISHIELD vaccine in health professionals, with an emphasis on MBBS interns, is outlined in this article.
Objectives
Primary objective: To determine the efficiency of the COVISHIELD vaccination against any laboratoryconfirmed COVID-19 infection among MBBS interns. And to determine the efficiency of the COVISHIELD vaccine in persons who have been partially vaccinated (single dose of COVISHIELD vaccine) versus those who have been fully immunized (two doses of COVISHIELD vaccines).
Secondary objectives: To measure COVISHIELD Vaccine effectiveness:
• Against confirmed and symptomatic COVID-19 infection.
• Against confirmed and asymptomatic COVID-19 infection.
• By different high-risk comorbidities.
• In persons with previous COVID-19 infection.
Materials and Methods
Study setting
Because of the convenience, the study will be done at the Acharya Vinoba Bhave Rural Hospital (AVBRH). With 1525 beds, it is Central India's largest teaching hospital. It provides services across the board. This hospital currently has 234 MBBS interns on duty [8].
Study design
Retrospective cohort design.
Study population
The sample population will be made up of MBBS interns working at AVBRH hospital who have no contraindications to receiving the COVISHIELD vaccination.
Contraindications are:
• Persons showing active symptoms of COVID-19
• Individuals who have had an adverse reaction to a previous dose of the COVID-19 vaccination.
• Persons who have taken 1st dose of COVID vaccines other than COVISHIELD.
Criteria for inclusion
Health workers include Interns working in the AVBRH hospital.
MBBS interns who have already been vaccinated (COVISHIELD).
Criteria for exclusion
The interns with the above-mentioned contraindications to the COVISHIELD vaccine were not included in the study.
Participants must provide the bare minimum of data
The following is a list of the minimum data that was gathered. An enrolment questionnaire was used to collect data.
• Age
• Sex
• Presence of chronic disease (s)
• COVID 19 infection in past (laboratory-confirmed)
• For symptomatic illness arising during the previous infection with COVID-19
• Symptoms
• Date of onset of symptoms.
• Date of PCR testing and PCR results.
Sample size
A list of 234 MBBS interns was prepared alphabetically from which a total of 80 MBBS interns were selected at random and was sent an online questionnaire through the means of WhatsApp and E-mail. A printed questionnaire was given to those interns who don't have access to these apps.
Study procedures
Study preparation: Selected Healthcare workers were invited to participate in the online survey. A preconstructed, confidential, validated, self-administered online survey questionnaire inquiring about the history of COVID-19 and COVISHIELD vaccination was provided to selected interns.
Data management and ensuring data confidentiality: At the time of enrolment, each participant was given a unique identity number, which was used as the identifier in all subsequent documents. In research databases, personal identifiers such as a person's name or national identity number were not maintained.
Baseline characteristics: It's a good idea to make a table of the participants' baseline characteristics.
The following are some of the baseline features to record:
• Age
• Sex
• Comorbidities
• vaccination history for COVID-19
• previously diagnosed with SARS-CoV-2 and test performed
Analysis plan
Vaccine effectiveness: Vaccination status can change over time, from one dose to two doses, making vaccination status a time varying exposure.
14 days after receiving the first vaccination dosage, a person should be considered fully vaccinated, and 14 days after receiving the second vaccine dose, they should be considered fully vaccinated.
Vaccination Efficacy (VE) was calculated using analytical data collected through the survey.
Ethical considerations
All healthcare workers approached for enrolment were told that participation is fully voluntary and that they can leave the study at any moment, for any motive, and with no repercussions. The fact that the participants' identities would be kept hidden during the study was clearly stated. To avoid data duplication during analysis, only the name and email address were required in the online survey.
Results
The survey was sent to 80 interns out of whom 71 filled the survey. Out of 71 interns, 50 were female and 21 were male (Table 1).
| Sex | ||
|---|---|---|
| Results | ||
| Options | % | count |
| Male | 29.58 | 21 |
| Female | 70.42 | 50 |
Table 1: Sex of participants.
The age group consists of interns ranging from 22 to 32 years of which maximum interns was of age 23 years (36.66%) and age 24 years (35.21%) (Table 2). 5 out of 71 of the interns have chronic systemic diseases such as hypertension (1 intern), diabetes (1 intern), and asthma (3 interns) and was on regular medication for the same.
| Age | No. of participants | Percentage |
|---|---|---|
| 22 | 6 | 8.45% |
| 23 | 26 | 36.66% |
| 24 | 25 | 35.21% |
| 25 | 10 | 14.08% |
| 26 | 2 | 2.81% |
| 27 | 1 | 1.40% |
| 32 | 1 | 1.40% |
Table 2: Age participants.
At the end of the survey, it was revealed that Out of 71 interns 69 interns (97.18%) had taken 1st dose of the COVISHIELD vaccine. The same number of interns also received 2nd dose of the COVISHIELD vaccine (97.18%).
Since the beginning of the pandemic in January 2020, 26 interns (36.11%) were tested positive for SARC-CoV 2 (Figure 1).
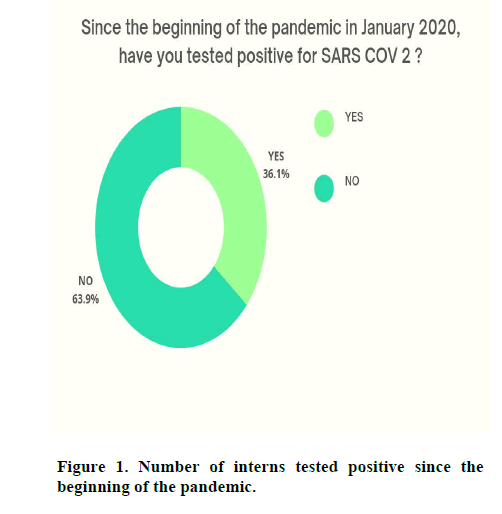
Figure 1: Number of interns tested positive since the beginning of the pandemic.
All the interns were diagnosed positive using the RT-PCR test, as it was the only reliable and accessible test available in the hospital. Chest X-ray/CT scan was done by all the COVID-19 positive interns, only 4 of them have radiological lesions compatible with COVID-19.
Response to symptoms during COVID-19 positive status was collected using a questionnaire with multiplechoice options. Predetermined symptoms included as options are fever, cough, cold, weakness/fatigue, headache, myalgia, dyspnoea, anorexia/ nausea/vomiting, diarrhoea, altered mental status, loss of smell, loss of taste, and 'others'. The 'other' option allows respondents to provide an answer that was outside of my predetermined list of symptoms with the help of a text box (Table 3). The most common symptoms were fever (13.33%), weakness/fatigue (12.22%), cough (12.22%), and loss of taste (10%).
| Symptoms of during COVID positive status | ||
|---|---|---|
| results | ||
| options | % | count |
| fever | 13.33 | 12 |
| cough | 12.22 | 11 |
| cold | 8.89 | 8 |
| weakness/fatigue | 12.22 | 11 |
| headache | 8.89 | 8 |
| myalgia | 8.89 | 8 |
| dyspnoea | 4.44 | 4 |
| diarrhoea | 3.33 | 3 |
| altered mental status | 1.11 | 1 |
| loss of smell | 8.89 | 8 |
| loss of taste | 10 | 9 |
| others | 3.33 | 3 |
Table 3. Symptoms during COVID positive status.
After a survey, it was found that 6 interns were tested COVID positive after taking 1st dose of the COVISHIELD vaccine. From which 2 interns were tested positive in the interval between 1st and 2nd dose COVISHIELD vaccine and remaining 4 were tested positive after completion of both doses of COVISHIELD vaccine.
Discussion
Our findings demonstrate that vaccination reduces the risk of COVID-19, a moderately severe infection requiring hospitalisation, notably among health care personnel in our facilities.
In another test-negative case-control study from Vellore, India [8], vaccination effectiveness was found to be 65% among health care workers who had received two doses of the COVID-19 vaccine, which was slightly lower than in our study. Disparities in results can be caused by a variety of reasons, including variances in the research population, virus strains, disease endemicity, and vaccine coverage. Vaccine effectiveness of the AstraZeneca vaccine, to which COVISHIELD is identical, has been studied in several studies from around the world. Vaccine Effectiveness was reported to be 65.9% (95% confidence interval: 65.2-66.6) among fully vaccinated patients in a cohort trial conducted in Chile between February and May 2021 [9].
Vaccine effectiveness in a cohort immunize conducted in Scotland was 88% between December 2020 and February 2021 (95% confidence interval: 75-94) [10].
Vaccine effectiveness against the B.1.617.2 variant was estimated to be 32.9% (95% confidence interval: 19.3-44.3) after one dose and 59.8% (95% confidence interval: 28.9-77.3) after two doses in the United Kingdom [11].
COVISHIELD's vaccine effectiveness against moderately severe disease was much higher than that against diseases of any severity, according to our research. This is critical because the fundamental goal of the COVID-19 vaccination is to avoid serious infections that require hospitalisation, ensuring that healthcare systems are not overburdened and that no lives are wasted. Testing the efficacy of COVID-19 vaccines in clinical research against mild to severe disease has been problematic due to the latter's rarity. Despite the fact that COVISHIELD contributed statistically notable immunity against relatively severe disease, the estimate's confidence intervals are relatively wide and further data on this association may be needed to strengthen our confidence in this finding.
In our study, we discovered two big flaws. First, in the study design, the RT-PCR testing is heavily relied on reporting. As a result, the advantage of vaccination may be overstated if vaccinated interns, whether asymptomatic or with symptoms implying COVID-19, believe they are unlikely to have COVID-19 and choose not to report for testing. Second, there were some shortcomings to choosing a retrospective study design. Researchers have little control over exposure or outcome evaluation, so they must rely on others to keep accurate records. Individual recall of earlier risk factor exposure can be inaccurate and biased when relying on individual memory. It can be difficult to make precise comparisons between the exposed and non-exposed.
To summarise, our findings reveal that COVISHIELD immunization, given once or twice, was successful in reducing the prevalence of COVID-19 infection among interns, and even more effective in preventing a more serious and clinically related form of the infection.
Conclusion
To find out the efficiency of the COVISHIELD vaccine among the interns working in Acharya Vinoba Bhave Rural Hospital (AVBRH) l, Wardha, Maharashtra, an online survey was done. It is found that the majority of the interns were not infected by the COVID-19. All interns took the safety precautions such as wearing face masks, washing hands with soap, proper use of hand sanitizers and disinfectants, maintaining social distancing, avoiding touching face nose, and mouth, avoiding unnecessary travel, and going to public places.
COVISHIELD vaccine is found out to be highly effective in preventing symptomatic COVID-19 among interns. According to survey analysis effectiveness of the COVISHIELD vaccine in interns working in AVBRH is found out to be 94.37 % after taking both doses of COVISHIELD vaccine. Interns who were infected with COVID-19 after taking both doses of COVISHIELD vaccine had only minor symptoms like fever, cold, and weakness without any radiological lesions suggestive of COVID 19 infection in the lungs. This finding should support efforts to maximize vaccine uptake with two doses among highrisk populations.
References
- Worldometer's. Coronavirus Cases. India, 2022.
- Press Information Bureau Government of India. Pregnant Women now eligible for COVID-19 Vaccination, Ministry of Health and Family Welfare has accepted the recommendations of NTAGI, Department of Ministry of Health and Family Welfare. Delhi, 2021.
- Indian Council of Medical Research. SARS-CoV-2 (COVID-19) Testing Status. 2022.
- Patel MM, Jackson ML, Ferdinands J, et al. Post licensure Evaluation of COVID-19 Vaccines. JAMA 2020; 324:1939-1940.
- Mohandas S, Yadav PD, Shete-Aich A, et al. Immunogenicity and protective efficacy of BBV152, whole virion inactivated SARS-CoV-2 vaccine candidates in the Syrian hamster model. Science 2021; 24:102054.
- Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020; 396:467-478.
- Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021; 397:99-111.
- Victor PJ, Mathews KP, Paul H, et al. Protective effect of COVID-19 vaccine among health care workers during the second wave of the pandemic in India. Mayo Clin Proc 2021; 96:2493-2494.
- Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med 2021; 385:875-884.
- Vasileiou E, Simpson CR, Shi T, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet 2021; 397:1646-1657.
- Bernal JL, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med 2021; 385:585-594.
Author Info
Rugved C Yasatwar* and Shiv Joshi
Department of Community Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences (Deemed to be University), Sawangi (Meghe), Maharashtra, IndiaCitation: Rugved C Yasatwar, Shiv Joshi, Effectiveness of COVISHIELD Vaccine in Healthcare Workers of AVBRH, Wardha, J Res Med Dent Sci, 2022, 10 (10): 200-205.
Received: 19-Aug-2022, Manuscript No. JRMDS-22-65507; , Pre QC No. JRMDS-22-65507(PQ); Editor assigned: 21-Aug-2022, Pre QC No. JRMDS-22-65507(PQ); Reviewed: 04-Oct-2022, QC No. JRMDS-22-65507; Revised: 21-Oct-2022, Manuscript No. JRMDS-22-65507(R); Published: 28-Oct-2022
